2e3cf259c116a9c5d5d65f7d7d865e90.ppt
- Количество слайдов: 54
 1 Correction of Artifacts in MR Image Analysis Jayaram K. Udupa Medical Image Processing Group Department of Radiology University of Pennsylvania Philadelphia, PA http: //www. mipg. upenn. edu/ Udupa
1 Correction of Artifacts in MR Image Analysis Jayaram K. Udupa Medical Image Processing Group Department of Radiology University of Pennsylvania Philadelphia, PA http: //www. mipg. upenn. edu/ Udupa
 2 CAVA: Computer-Aided Visualization and Analysis The science underlying computerized methods of image processing, analysis, and visualization to facilitate new therapeutic strategies, basic clinical research, education, and training.
2 CAVA: Computer-Aided Visualization and Analysis The science underlying computerized methods of image processing, analysis, and visualization to facilitate new therapeutic strategies, basic clinical research, education, and training.
 3 CAD vs CAVA CAD: Computer-Aided Diagnosis The science underlying computerized methods for the diagnosis of diseases via images
3 CAD vs CAVA CAD: Computer-Aided Diagnosis The science underlying computerized methods for the diagnosis of diseases via images
 4 Purpose of CAVA In: Multiple multimodality multidimensional images of an object system. Out: Qualitative/quantitative information about objects in the object system. Object system – a collection of rigid, deformable, static, or dynamic, physical or conceptual objects.
4 Purpose of CAVA In: Multiple multimodality multidimensional images of an object system. Out: Qualitative/quantitative information about objects in the object system. Object system – a collection of rigid, deformable, static, or dynamic, physical or conceptual objects.
 6 CAVA Operations Img Processing: for enhancing information about and defining object system. Visualization: for viewing and comprehending object system. Manipulation: for altering object system (virtual surgery). Analysis: for quantifying information about object system.
6 CAVA Operations Img Processing: for enhancing information about and defining object system. Visualization: for viewing and comprehending object system. Manipulation: for altering object system (virtual surgery). Analysis: for quantifying information about object system.
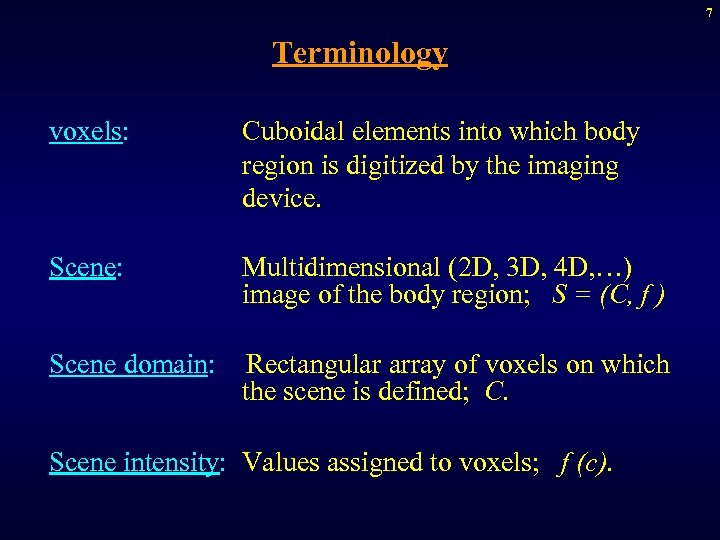 7 Terminology voxels: Cuboidal elements into which body region is digitized by the imaging device. Scene: Multidimensional (2 D, 3 D, 4 D, …) image of the body region; S = (C, f ) Scene domain: Rectangular array of voxels on which the scene is defined; C. Scene intensity: Values assigned to voxels; f (c).
7 Terminology voxels: Cuboidal elements into which body region is digitized by the imaging device. Scene: Multidimensional (2 D, 3 D, 4 D, …) image of the body region; S = (C, f ) Scene domain: Rectangular array of voxels on which the scene is defined; C. Scene intensity: Values assigned to voxels; f (c).
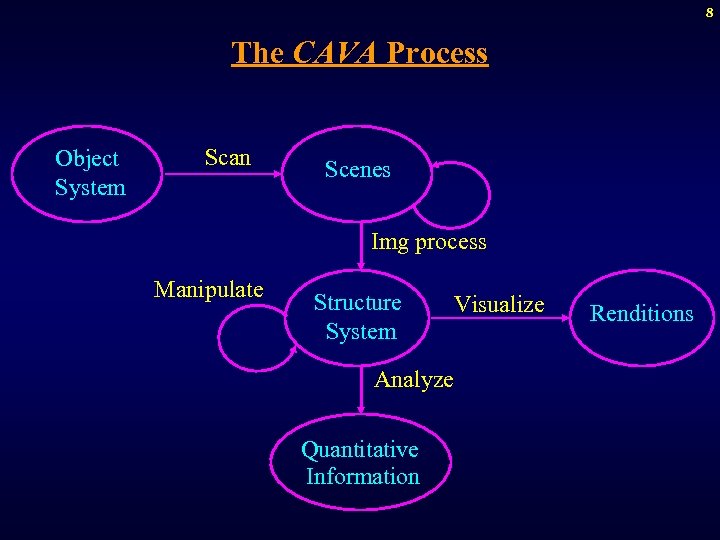 8 The CAVA Process Object System Scan Scenes Img process Manipulate Structure System Analyze Quantitative Information Visualize Renditions
8 The CAVA Process Object System Scan Scenes Img process Manipulate Structure System Analyze Quantitative Information Visualize Renditions
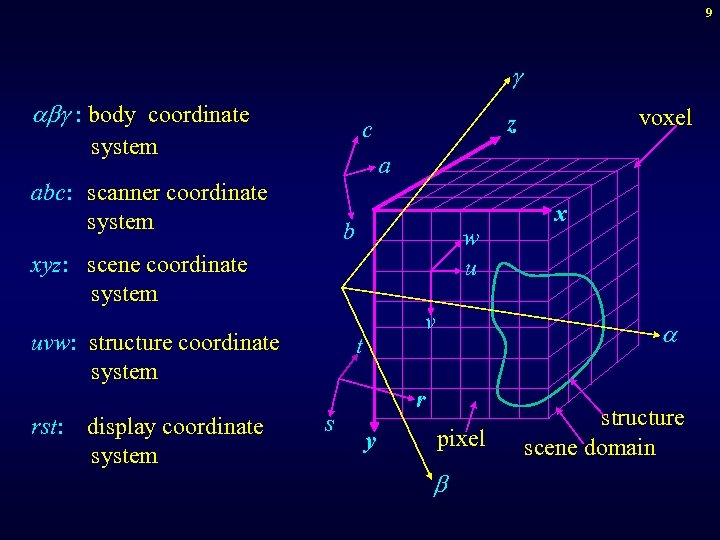 9 g a g : body coordinate system a abc: scanner coordinate system b w u xyz: scene coordinate system uvw: structure coordinate system rst: display coordinate system voxel z c v t s a r y x pixel structure scene domain
9 g a g : body coordinate system a abc: scanner coordinate system b w u xyz: scene coordinate system uvw: structure coordinate system rst: display coordinate system voxel z c v t s a r y x pixel structure scene domain
 10 CAVA Operations Img processing: Visualization Manipulation Analysis Volume of interest Filtering Interpolation Registration Segmentation
10 CAVA Operations Img processing: Visualization Manipulation Analysis Volume of interest Filtering Interpolation Registration Segmentation
 Scale in CAVA Scale represents level of detail of object information in scenes. Scale is needed to handle variable object size in different parts of the scene. Global scale: Process the scene at each of various fixed scales and then combine the results – scale space approach. Local scale: At each voxel, define largest homogeneous region, and treat these as fundamental units in the scene. 11
Scale in CAVA Scale represents level of detail of object information in scenes. Scale is needed to handle variable object size in different parts of the scene. Global scale: Process the scene at each of various fixed scales and then combine the results – scale space approach. Local scale: At each voxel, define largest homogeneous region, and treat these as fundamental units in the scene. 11
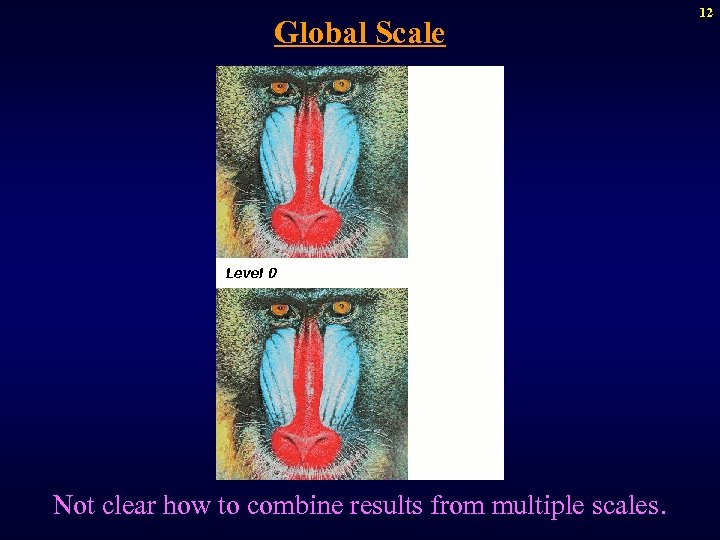 Global Scale Not clear how to combine results from multiple scales. 12
Global Scale Not clear how to combine results from multiple scales. 12
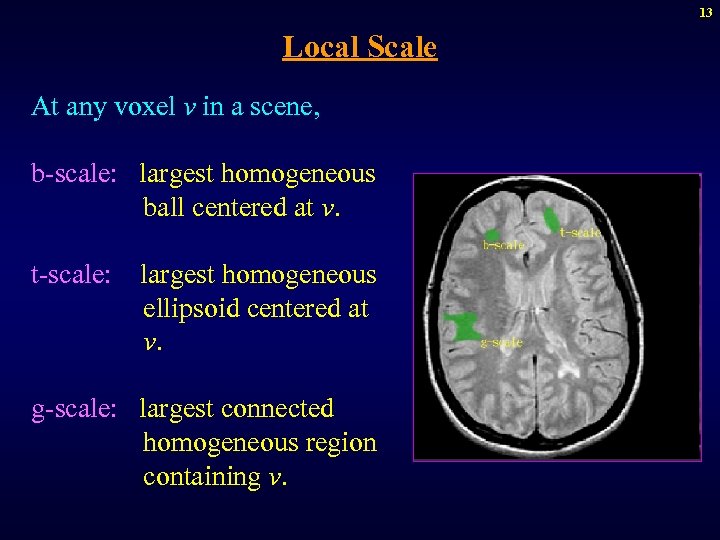 13 Local Scale At any voxel v in a scene, b-scale: largest homogeneous ball centered at v. t-scale: largest homogeneous ellipsoid centered at v. g-scale: largest connected homogeneous region containing v.
13 Local Scale At any voxel v in a scene, b-scale: largest homogeneous ball centered at v. t-scale: largest homogeneous ellipsoid centered at v. g-scale: largest connected homogeneous region containing v.
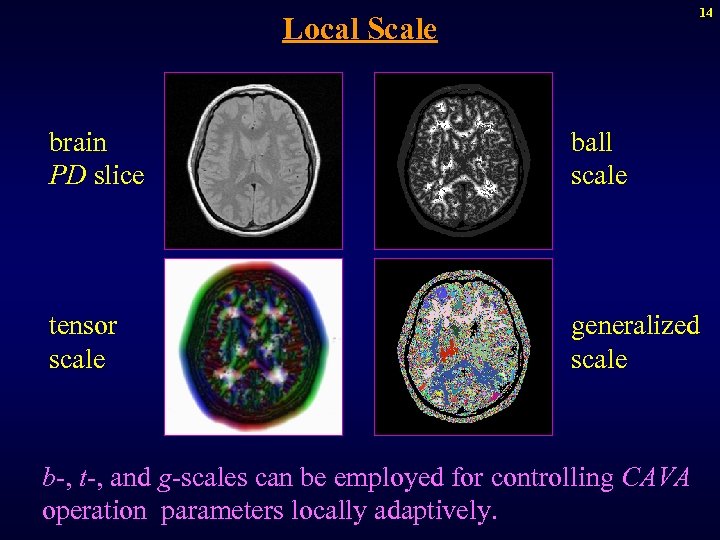 14 Local Scale brain PD slice ball scale tensor scale generalized scale b-, t-, and g-scales can be employed for controlling CAVA operation parameters locally adaptively.
14 Local Scale brain PD slice ball scale tensor scale generalized scale b-, t-, and g-scales can be employed for controlling CAVA operation parameters locally adaptively.
 15 Filtering Scene Purpose: Scene To suppress unwanted (non-object) information. To enhance wanted (object) information. Suppressive: Mainly for suppressing random noise. Enhancive: For enhancing edges, regions. For correcting background variation. For intensity scale standardization.
15 Filtering Scene Purpose: Scene To suppress unwanted (non-object) information. To enhance wanted (object) information. Suppressive: Mainly for suppressing random noise. Enhancive: For enhancing edges, regions. For correcting background variation. For intensity scale standardization.
 16 Suppressive Filtering – Gaussian, Median Gaussian: f. F(v) is a Gaussian weighted average of f(v) in a neighborhood of v. This neighborhood may be a b-, t-, or g-scale region of v. Median: f. F(v) is the median of the intensities f(u) in a neighborhood of v. This neighborhood may be a b-, t-, or g-scale region of v.
16 Suppressive Filtering – Gaussian, Median Gaussian: f. F(v) is a Gaussian weighted average of f(v) in a neighborhood of v. This neighborhood may be a b-, t-, or g-scale region of v. Median: f. F(v) is the median of the intensities f(u) in a neighborhood of v. This neighborhood may be a b-, t-, or g-scale region of v.
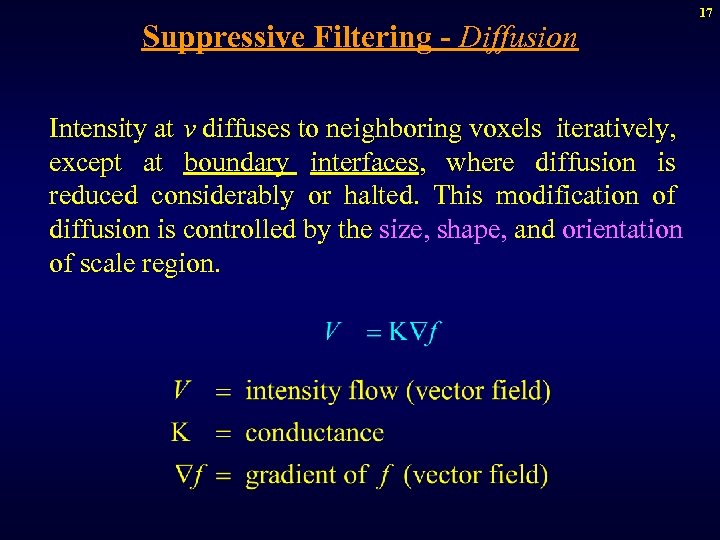 Suppressive Filtering - Diffusion Intensity at v diffuses to neighboring voxels iteratively, except at boundary interfaces, where diffusion is reduced considerably or halted. This modification of diffusion is controlled by the size, shape, and orientation of scale region. 17
Suppressive Filtering - Diffusion Intensity at v diffuses to neighboring voxels iteratively, except at boundary interfaces, where diffusion is reduced considerably or halted. This modification of diffusion is controlled by the size, shape, and orientation of scale region. 17
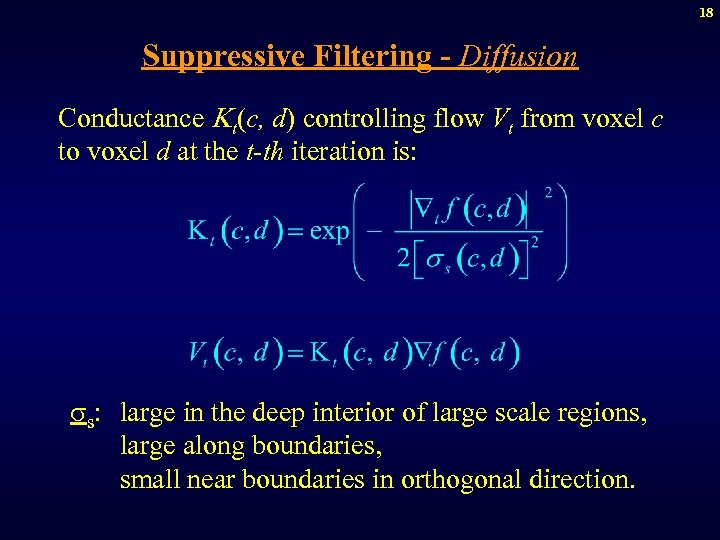 18 Suppressive Filtering - Diffusion Conductance t(c, d) controlling flow Vt from voxel c to voxel d at the t-th iteration is: s: large in the deep interior of large scale regions, large along boundaries, small near boundaries in orthogonal direction.
18 Suppressive Filtering - Diffusion Conductance t(c, d) controlling flow Vt from voxel c to voxel d at the t-th iteration is: s: large in the deep interior of large scale regions, large along boundaries, small near boundaries in orthogonal direction.
 19 Suppressive Filtering - Diffusion The iterative process is defined as follows: A - constant (depends on adjacency) D(c, d) - unit vector from c to d. Nc - neighborhood of c.
19 Suppressive Filtering - Diffusion The iterative process is defined as follows: A - constant (depends on adjacency) D(c, d) - unit vector from c to d. Nc - neighborhood of c.
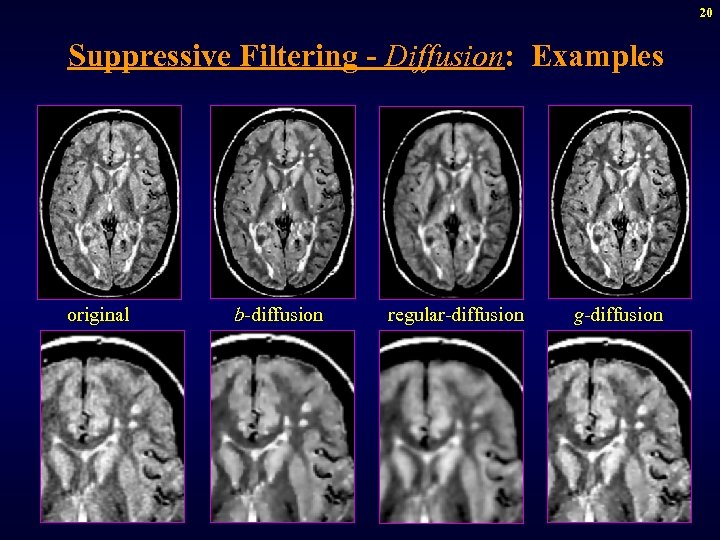 20 Suppressive Filtering - Diffusion: Examples original b-diffusion regular-diffusion g-diffusion
20 Suppressive Filtering - Diffusion: Examples original b-diffusion regular-diffusion g-diffusion
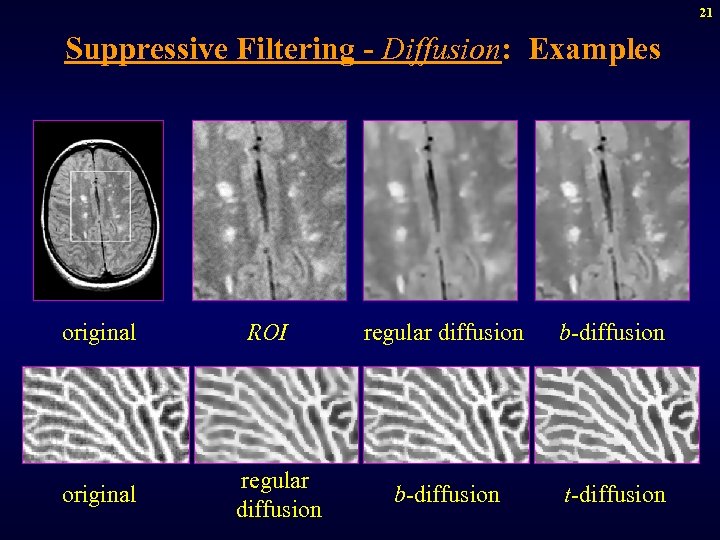 21 Suppressive Filtering - Diffusion: Examples original ROI regular diffusion b-diffusion t-diffusion
21 Suppressive Filtering - Diffusion: Examples original ROI regular diffusion b-diffusion t-diffusion
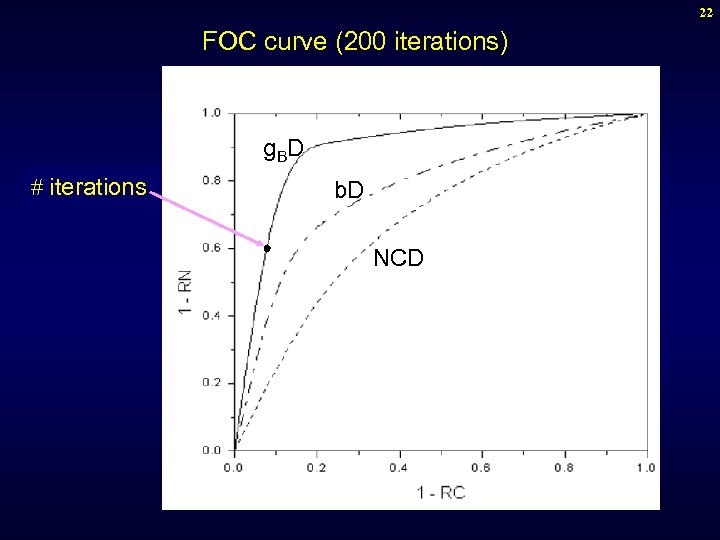 22 FOC curve (200 iterations) g. B D # iterations b. D NCD
22 FOC curve (200 iterations) g. B D # iterations b. D NCD
 23 Enhancive Filtering Enhancing edges: Edge detection. Enhancing regions: Histogram equalization. Intensity scale standardization: For MRI – to make sure that intensity values have the same tissue specific meaning. Inhomogeneity correction: For correcting background intensity variation.
23 Enhancive Filtering Enhancing edges: Edge detection. Enhancing regions: Histogram equalization. Intensity scale standardization: For MRI – to make sure that intensity values have the same tissue specific meaning. Inhomogeneity correction: For correcting background intensity variation.
 24 Enhancive Filtering: Intensity Standardization Problem: • MRI intensities do not have a fixed meaning, even for the same protocol, body region, patient, scanner. • Poses problems for image operations (segmentation). • Simple linear scaling does not help.
24 Enhancive Filtering: Intensity Standardization Problem: • MRI intensities do not have a fixed meaning, even for the same protocol, body region, patient, scanner. • Poses problems for image operations (segmentation). • Simple linear scaling does not help.
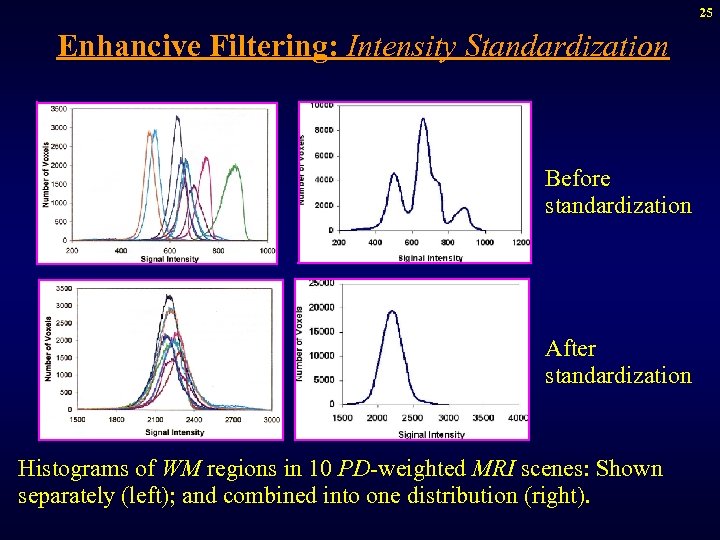 25 Enhancive Filtering: Intensity Standardization Before standardization After standardization Histograms of WM regions in 10 PD-weighted MRI scenes: Shown separately (left); and combined into one distribution (right).
25 Enhancive Filtering: Intensity Standardization Before standardization After standardization Histograms of WM regions in 10 PD-weighted MRI scenes: Shown separately (left); and combined into one distribution (right).
 26 Enhancive Filtering: Intensity Standardization Approach consists of: Training Transformation Training: (1) Identify tissue specific landmarks LM 1, …. , LMn on each of a set of images. (2) Choose a standard scale, say [0, 4000]. (3) Map LM 1, …. , LMn from each input image on to standard scale. (4) Find average location each landmark. on standard scale for
26 Enhancive Filtering: Intensity Standardization Approach consists of: Training Transformation Training: (1) Identify tissue specific landmarks LM 1, …. , LMn on each of a set of images. (2) Choose a standard scale, say [0, 4000]. (3) Map LM 1, …. , LMn from each input image on to standard scale. (4) Find average location each landmark. on standard scale for
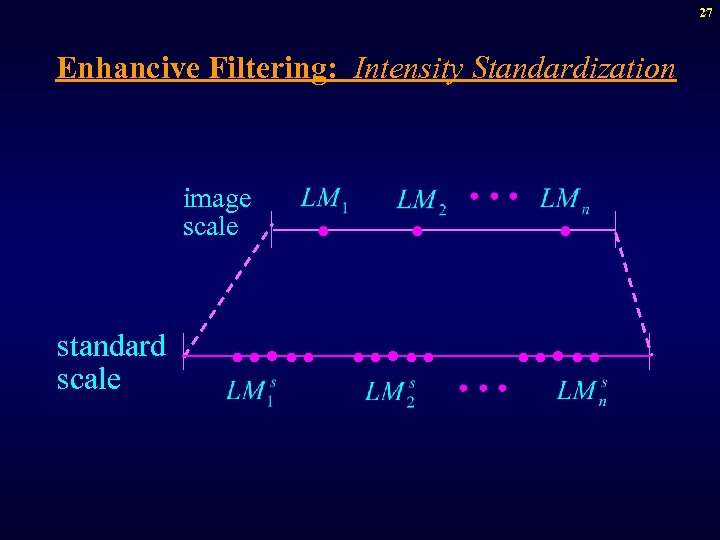 27 Enhancive Filtering: Intensity Standardization image scale standard scale ·····
27 Enhancive Filtering: Intensity Standardization image scale standard scale ·····
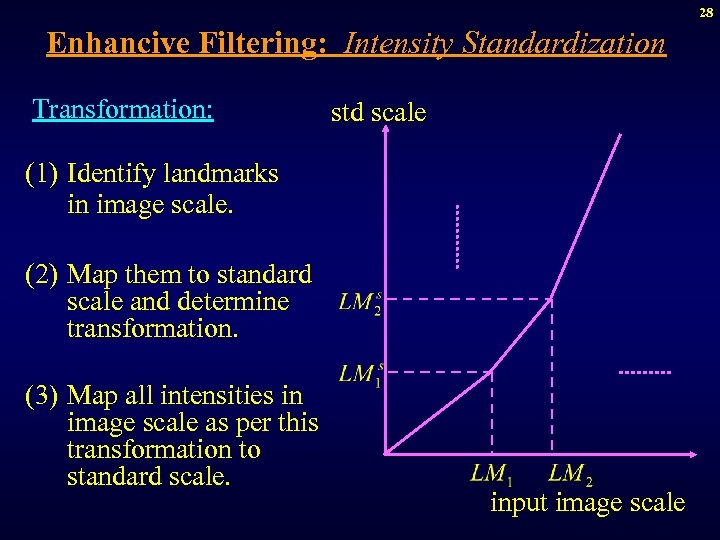 28 Enhancive Filtering: Intensity Standardization Transformation: std scale (1) Identify landmarks in image scale. (2) Map them to standard scale and determine transformation. (3) Map all intensities in image scale as per this transformation to standard scale. input image scale
28 Enhancive Filtering: Intensity Standardization Transformation: std scale (1) Identify landmarks in image scale. (2) Map them to standard scale and determine transformation. (3) Map all intensities in image scale as per this transformation to standard scale. input image scale
 29 Enhancive Filtering: Intensity Standardization Choosing landmarks on intensity scale: (1) On image histogram – median, mode, quartiles, (2) deciles, … (2) Using local scales – largest b-scale or g-scale (3) Interactively – paint regions corresponding to different tissues where mean intensities are used as LMi.
29 Enhancive Filtering: Intensity Standardization Choosing landmarks on intensity scale: (1) On image histogram – median, mode, quartiles, (2) deciles, … (2) Using local scales – largest b-scale or g-scale (3) Interactively – paint regions corresponding to different tissues where mean intensities are used as LMi.
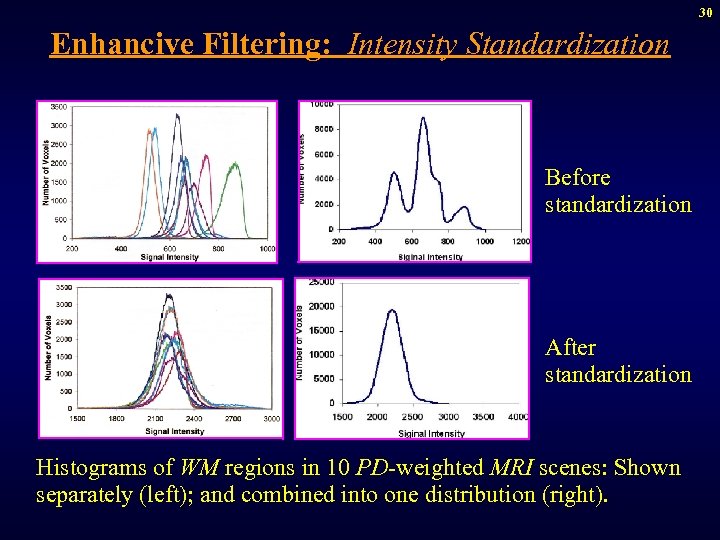 30 Enhancive Filtering: Intensity Standardization Before standardization After standardization Histograms of WM regions in 10 PD-weighted MRI scenes: Shown separately (left); and combined into one distribution (right).
30 Enhancive Filtering: Intensity Standardization Before standardization After standardization Histograms of WM regions in 10 PD-weighted MRI scenes: Shown separately (left); and combined into one distribution (right).
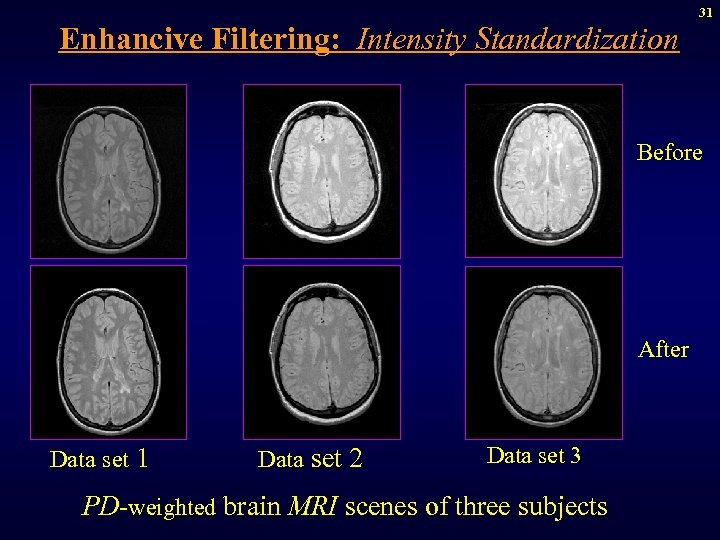 31 Enhancive Filtering: Intensity Standardization Before After Data set 1 Data set 2 Data set 3 PD-weighted brain MRI scenes of three subjects
31 Enhancive Filtering: Intensity Standardization Before After Data set 1 Data set 2 Data set 3 PD-weighted brain MRI scenes of three subjects
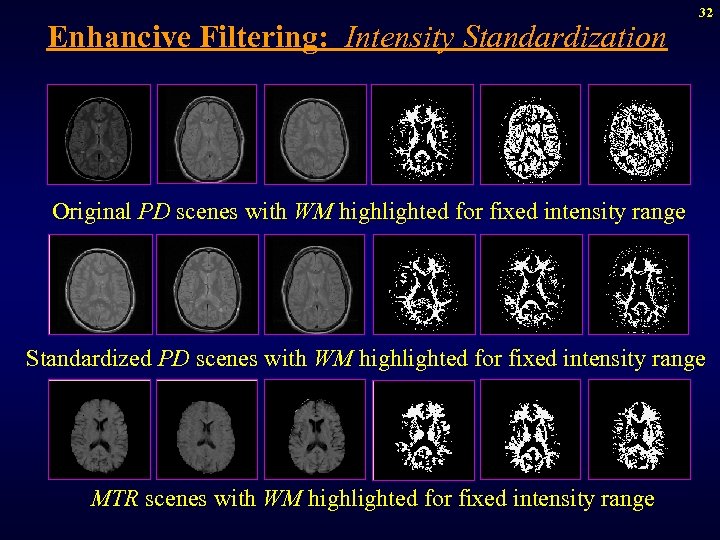 Enhancive Filtering: Intensity Standardization 32 Original PD scenes with WM highlighted for fixed intensity range Standardized PD scenes with WM highlighted for fixed intensity range MTR scenes with WM highlighted for fixed intensity range
Enhancive Filtering: Intensity Standardization 32 Original PD scenes with WM highlighted for fixed intensity range Standardized PD scenes with WM highlighted for fixed intensity range MTR scenes with WM highlighted for fixed intensity range
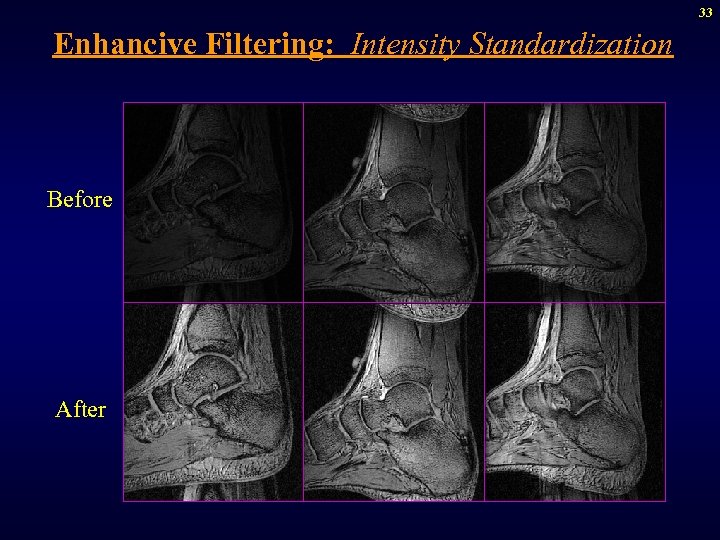 33 Enhancive Filtering: Intensity Standardization Before After
33 Enhancive Filtering: Intensity Standardization Before After
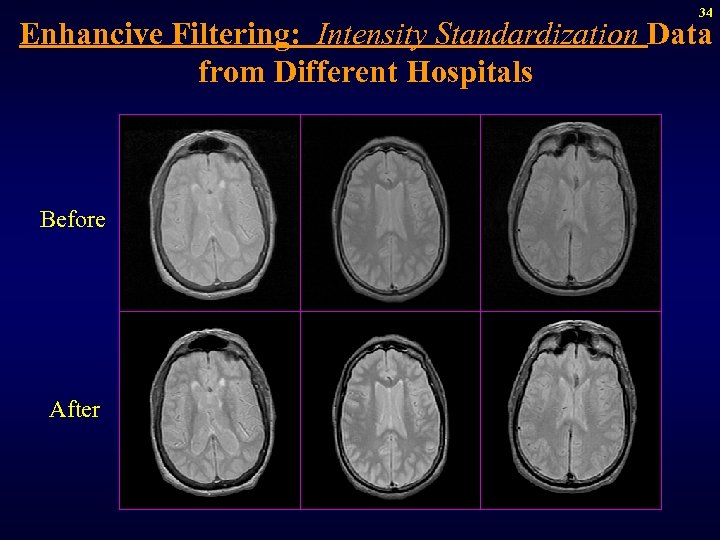 34 Enhancive Filtering: Intensity Standardization Data from Different Hospitals Before After
34 Enhancive Filtering: Intensity Standardization Data from Different Hospitals Before After
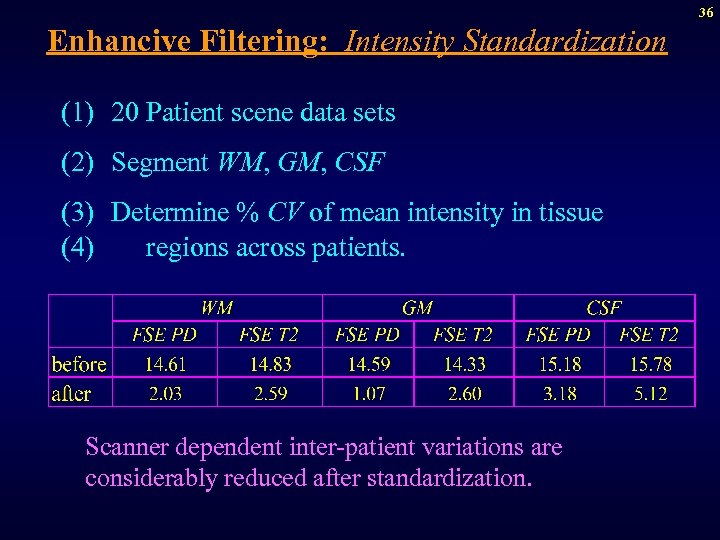 36 Enhancive Filtering: Intensity Standardization (1) 20 Patient scene data sets (2) Segment WM, GM, CSF (3) Determine % CV of mean intensity in tissue (4) regions across patients. Scanner dependent inter-patient variations are considerably reduced after standardization.
36 Enhancive Filtering: Intensity Standardization (1) 20 Patient scene data sets (2) Segment WM, GM, CSF (3) Determine % CV of mean intensity in tissue (4) regions across patients. Scanner dependent inter-patient variations are considerably reduced after standardization.
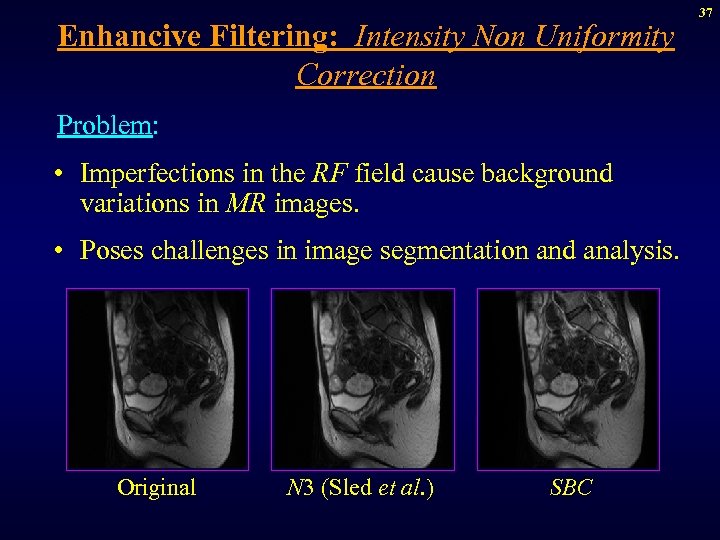 Enhancive Filtering: Intensity Non Uniformity Correction Problem: • Imperfections in the RF field cause background variations in MR images. • Poses challenges in image segmentation and analysis. Original N 3 (Sled et al. ) SBC 37
Enhancive Filtering: Intensity Non Uniformity Correction Problem: • Imperfections in the RF field cause background variations in MR images. • Poses challenges in image segmentation and analysis. Original N 3 (Sled et al. ) SBC 37
 38 Enhancive Filtering: Intensity Non Uniformity Correction Goal: To develop a general method for correcting the variations that fulfills: (R 1) no need for user help per scene (R 2) no need for accurate prior segmentation (R 3) no need for prior knowledge of tissue intensity distribution A standardization Based Correction (SBC) method is described.
38 Enhancive Filtering: Intensity Non Uniformity Correction Goal: To develop a general method for correcting the variations that fulfills: (R 1) no need for user help per scene (R 2) no need for accurate prior segmentation (R 3) no need for prior knowledge of tissue intensity distribution A standardization Based Correction (SBC) method is described.
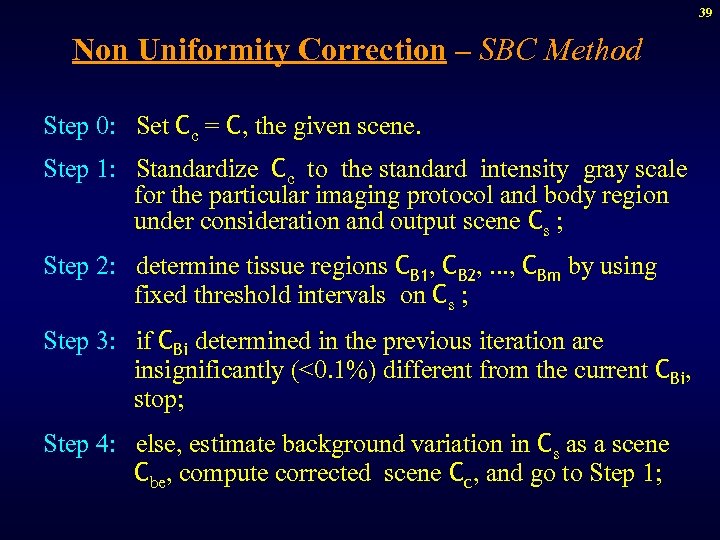 39 Non Uniformity Correction – SBC Method Step 0: Set Cc = C, the given scene. Step 1: Standardize Cc to the standard intensity gray scale for the particular imaging protocol and body region under consideration and output scene Cs ; Step 2: determine tissue regions CB 1, CB 2, . . . , CBm by using fixed threshold intervals on Cs ; Step 3: if CBi determined in the previous iteration are insignificantly (<0. 1%) different from the current CBi, stop; Step 4: else, estimate background variation in Cs as a scene Cbe, compute corrected scene Cc, and go to Step 1;
39 Non Uniformity Correction – SBC Method Step 0: Set Cc = C, the given scene. Step 1: Standardize Cc to the standard intensity gray scale for the particular imaging protocol and body region under consideration and output scene Cs ; Step 2: determine tissue regions CB 1, CB 2, . . . , CBm by using fixed threshold intervals on Cs ; Step 3: if CBi determined in the previous iteration are insignificantly (<0. 1%) different from the current CBi, stop; Step 4: else, estimate background variation in Cs as a scene Cbe, compute corrected scene Cc, and go to Step 1;
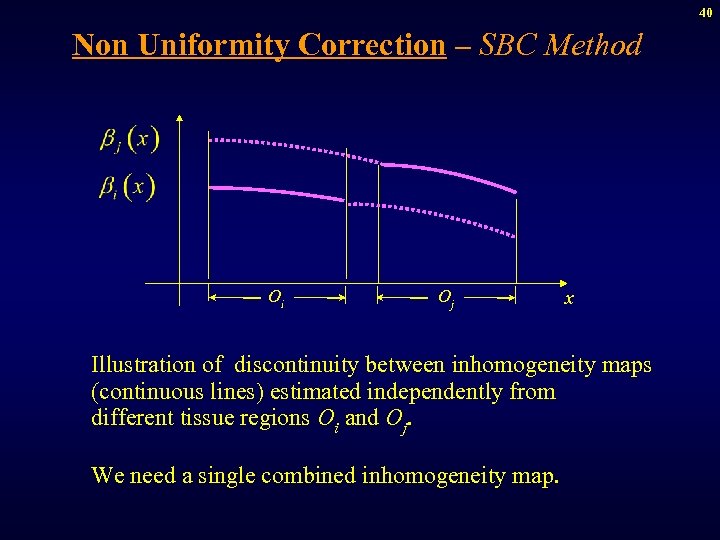 40 Non Uniformity Correction – SBC Method Oi Oj x Illustration of discontinuity between inhomogeneity maps (continuous lines) estimated independently from different tissue regions Oi and Oj. We need a single combined inhomogeneity map.
40 Non Uniformity Correction – SBC Method Oi Oj x Illustration of discontinuity between inhomogeneity maps (continuous lines) estimated independently from different tissue regions Oi and Oj. We need a single combined inhomogeneity map.
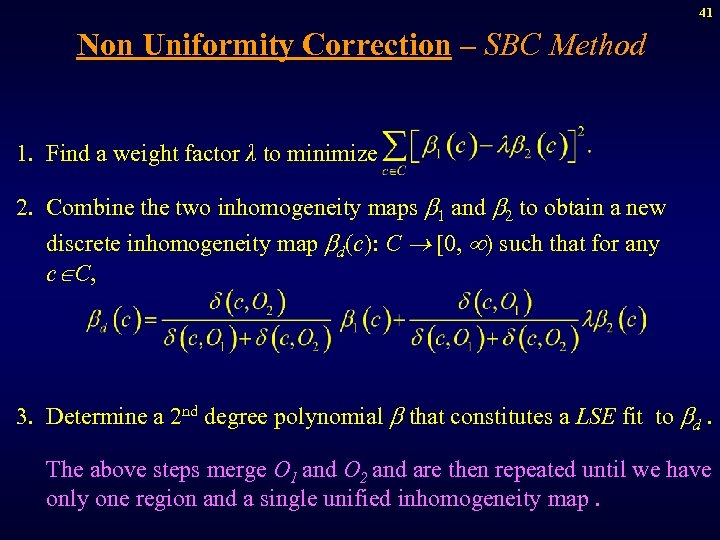 41 Non Uniformity Correction – SBC Method 1. Find a weight factor λ to minimize 2. Combine the two inhomogeneity maps 1 and 2 to obtain a new discrete inhomogeneity map d(c): C [0, ) such that for any c C, 3. Determine a 2 nd degree polynomial that constitutes a LSE fit to d. The above steps merge O 1 and O 2 and are then repeated until we have only one region and a single unified inhomogeneity map.
41 Non Uniformity Correction – SBC Method 1. Find a weight factor λ to minimize 2. Combine the two inhomogeneity maps 1 and 2 to obtain a new discrete inhomogeneity map d(c): C [0, ) such that for any c C, 3. Determine a 2 nd degree polynomial that constitutes a LSE fit to d. The above steps merge O 1 and O 2 and are then repeated until we have only one region and a single unified inhomogeneity map.
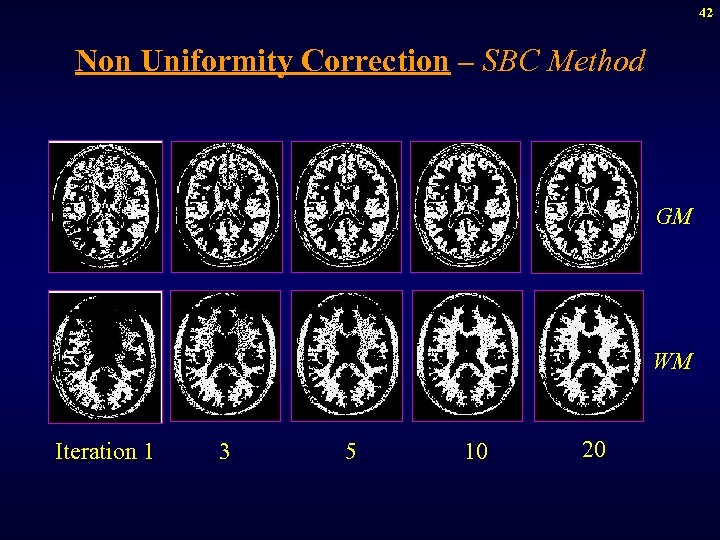 42 Non Uniformity Correction – SBC Method GM WM Iteration 1 3 5 10 20
42 Non Uniformity Correction – SBC Method GM WM Iteration 1 3 5 10 20
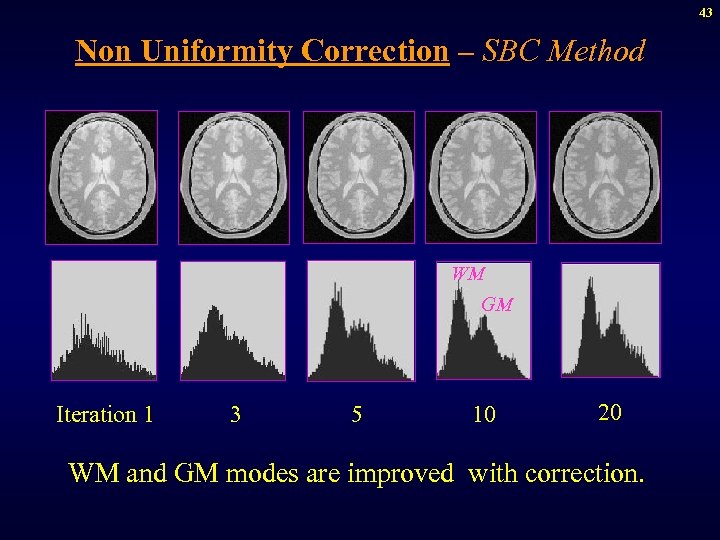 43 Non Uniformity Correction – SBC Method WM GM Iteration 1 3 5 10 20 WM and GM modes are improved with correction.
43 Non Uniformity Correction – SBC Method WM GM Iteration 1 3 5 10 20 WM and GM modes are improved with correction.
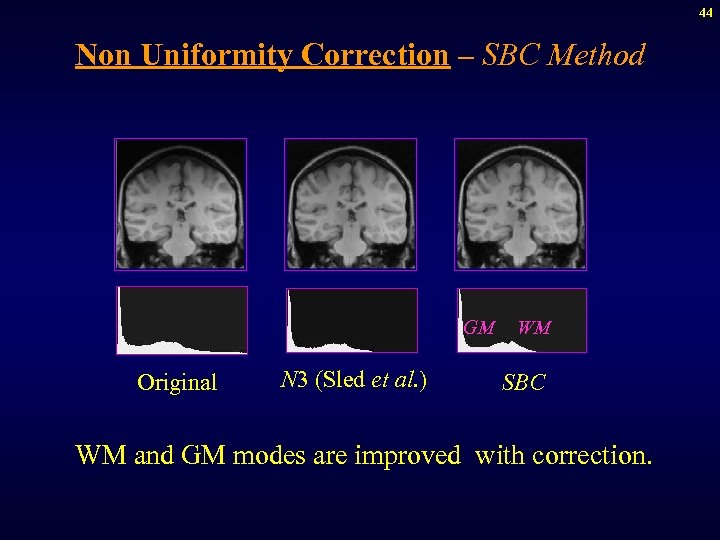 44 Non Uniformity Correction – SBC Method GM Original N 3 (Sled et al. ) WM SBC WM and GM modes are improved with correction.
44 Non Uniformity Correction – SBC Method GM Original N 3 (Sled et al. ) WM SBC WM and GM modes are improved with correction.
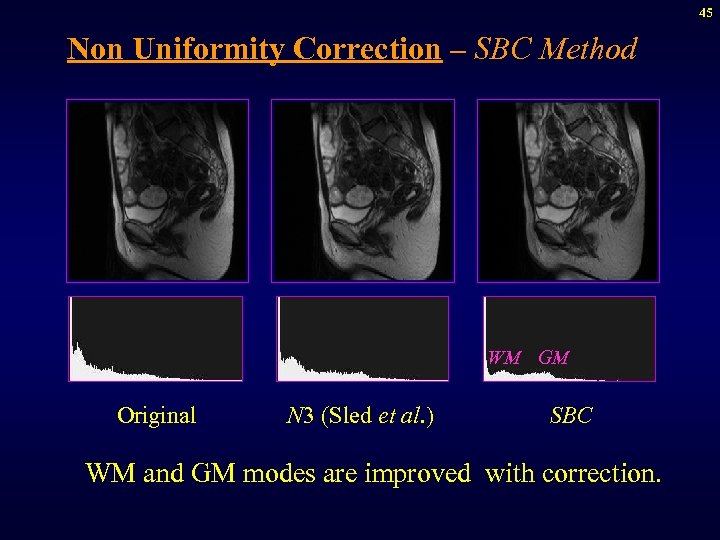 45 Non Uniformity Correction – SBC Method WM GM Original N 3 (Sled et al. ) SBC WM and GM modes are improved with correction.
45 Non Uniformity Correction – SBC Method WM GM Original N 3 (Sled et al. ) SBC WM and GM modes are improved with correction.
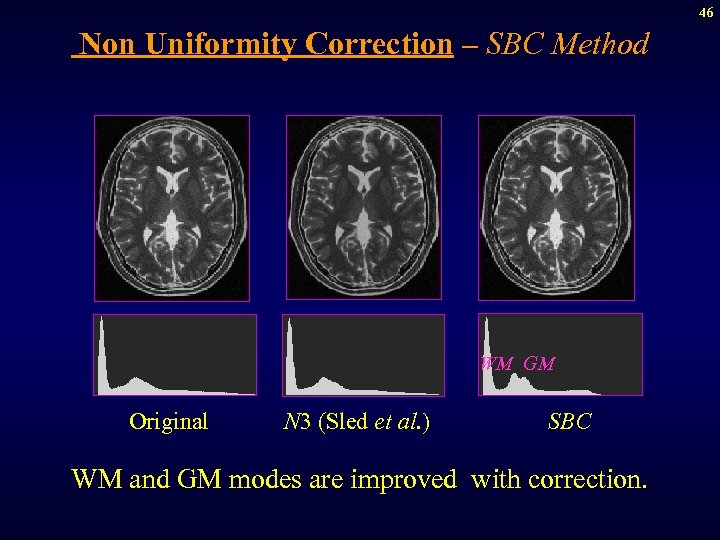 46 Non Uniformity Correction – SBC Method WM GM Original N 3 (Sled et al. ) SBC WM and GM modes are improved with correction.
46 Non Uniformity Correction – SBC Method WM GM Original N 3 (Sled et al. ) SBC WM and GM modes are improved with correction.
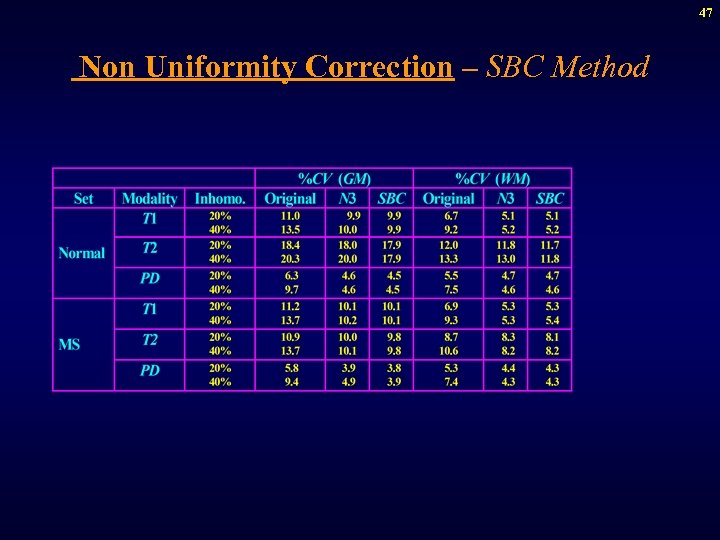 47 Non Uniformity Correction – SBC Method
47 Non Uniformity Correction – SBC Method
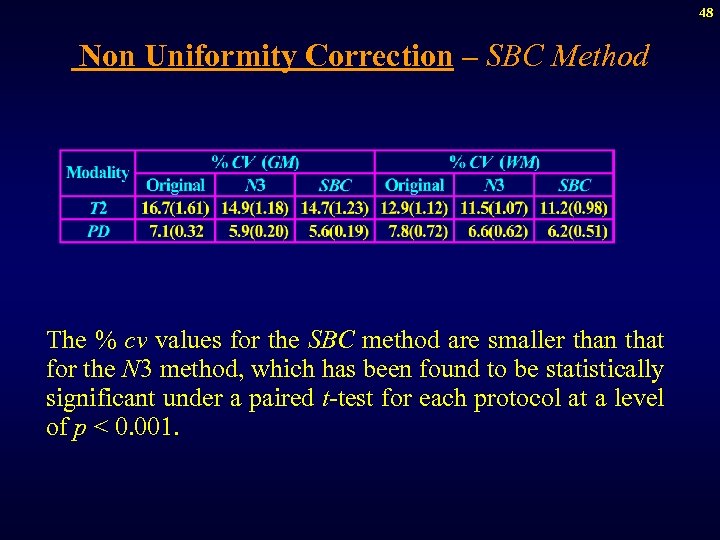 48 Non Uniformity Correction – SBC Method The % cv values for the SBC method are smaller than that for the N 3 method, which has been found to be statistically significant under a paired t-test for each protocol at a level of p < 0. 001.
48 Non Uniformity Correction – SBC Method The % cv values for the SBC method are smaller than that for the N 3 method, which has been found to be statistically significant under a paired t-test for each protocol at a level of p < 0. 001.
 49 Non Uniformity Correction – Interplay Between Standardization and Correction • What order to apply correction and standardization? Cor Std Cor or Cor Std. . . or Std Cor… • Does correction affect standardness or vice versa? • How does noise filtering affect correction/ standardization and vice versa? Cor Std Flt Cor or ….
49 Non Uniformity Correction – Interplay Between Standardization and Correction • What order to apply correction and standardization? Cor Std Cor or Cor Std. . . or Std Cor… • Does correction affect standardness or vice versa? • How does noise filtering affect correction/ standardization and vice versa? Cor Std Flt Cor or ….
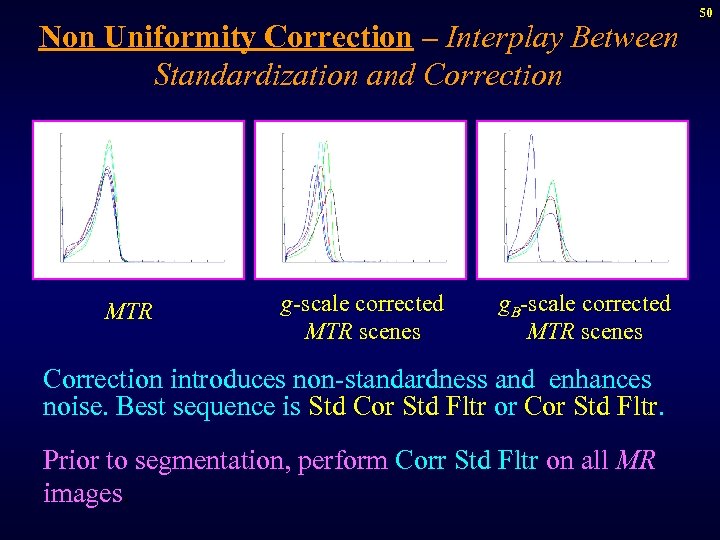 Non Uniformity Correction – Interplay Between Standardization and Correction MTR g-scale corrected MTR scenes g. B-scale corrected MTR scenes Correction introduces non-standardness and enhances noise. Best sequence is Std Cor Std Fltr or Cor Std Fltr. Prior to segmentation, perform Corr Std Fltr on all MR images. 50
Non Uniformity Correction – Interplay Between Standardization and Correction MTR g-scale corrected MTR scenes g. B-scale corrected MTR scenes Correction introduces non-standardness and enhances noise. Best sequence is Std Cor Std Fltr or Cor Std Fltr. Prior to segmentation, perform Corr Std Fltr on all MR images. 50
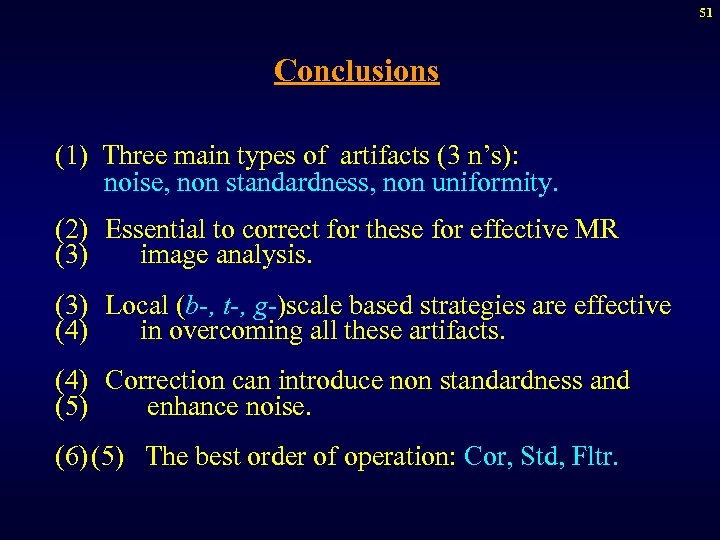 51 Conclusions (1) Three main types of artifacts (3 n’s): noise, non standardness, non uniformity. (2) Essential to correct for these for effective MR (3) image analysis. (3) Local (b-, t-, g-)scale based strategies are effective (4) in overcoming all these artifacts. (4) Correction can introduce non standardness and (5) enhance noise. (6) (5) The best order of operation: Cor, Std, Fltr.
51 Conclusions (1) Three main types of artifacts (3 n’s): noise, non standardness, non uniformity. (2) Essential to correct for these for effective MR (3) image analysis. (3) Local (b-, t-, g-)scale based strategies are effective (4) in overcoming all these artifacts. (4) Correction can introduce non standardness and (5) enhance noise. (6) (5) The best order of operation: Cor, Std, Fltr.
 52 Key References Background, Classification of Methods 1) Udupa, “Three-Dimensional J. K. : Image Processing, Analysis, Visualization: and Methods Techniques, ” and Categorical Course in Diagnostic Radiology Physics: Multidimensional Image Processing, Analysis and Display. Editors: S. G. Armato III and M. S. Brown, Radiological Society of North America, Inc. Oak Brook, Illinois, pp. 9 -26, 2005. 2) Udupa, Florida, 2000. Scale Cybernetics, 55: 367 -375, 1987. 4) Wang, Machine Intelligence, 20(10): 1040 -1055, 1998. 5) Burt, 1981. 6) Burt, Communications, 31(4): 532 -540, 1983. 7) Witkin, Intelligence , pp. 1019 -1022, 1983. 8) Lindeberg, Proceedings of Fundamental Structural Properties in Image and Pattern Analysis, 130: 9 -23, 1999.
52 Key References Background, Classification of Methods 1) Udupa, “Three-Dimensional J. K. : Image Processing, Analysis, Visualization: and Methods Techniques, ” and Categorical Course in Diagnostic Radiology Physics: Multidimensional Image Processing, Analysis and Display. Editors: S. G. Armato III and M. S. Brown, Radiological Society of North America, Inc. Oak Brook, Illinois, pp. 9 -26, 2005. 2) Udupa, Florida, 2000. Scale Cybernetics, 55: 367 -375, 1987. 4) Wang, Machine Intelligence, 20(10): 1040 -1055, 1998. 5) Burt, 1981. 6) Burt, Communications, 31(4): 532 -540, 1983. 7) Witkin, Intelligence , pp. 1019 -1022, 1983. 8) Lindeberg, Proceedings of Fundamental Structural Properties in Image and Pattern Analysis, 130: 9 -23, 1999.
 53
53
 21) Shi, J. Y. and H. T. Tsui: “Scale Space Filtering by Fejér Kernel, ” International Symposium on Speech Image Process and Neural Networks, 2: 638 -641, 1994. 22) Saha, P. K. and J. K. Udupa: “Scale-Based Image Filtering Preserving Boundary Sharpness and Fine Structures, ” IEEE Transactions on Medical Imaging, 20(11): 1140 -1155, 2001. 23) Perona, P. and J. Malik: “Scale-Space and Edge Detection Using Anisotropic Diffusion, ” IEEE Transactions on Pattern Analysis and Machine Intelligence, 12(7): 629 -639, 1990. 24) Whitaker, R. T. and S. M. Pizer: “A Multi-Scale Approach to Nonuniform Diffusion, ” Computer Vision Graphic and Image Processing: Image Understanding, 57(1): 99 -110, 1993. 25) Weikert, J. , et al. : “Theoretical Foundations of Anisotropic Diffusion in Image Processing, ” Theoretical Foundations of Computer Vision, Computing, Supplement 11: 221 -236, 1996. 26) Parker, G. J. M. and J. A. Schnabel: “Enhancement of Anisotropic Diffusive Filtering of MR Images Using Approximate Entropy, ” in Proceedings of International Society for Magnetic Resonance in Medicine, 7: 175, 1999. 27) Gerig, G. , O. Kubler, R. Kikinis and F. A. Jolesz: “Nonlinear Anisotropic Filtering of MRI Data, ” IEEE Transaction on Medical Imaging, 11(2): 221 -232, 1992. 28) Souza, A. , J. K. Udupa and A. Madabhushi: “Generalized Scale-Based Image Filtering, ” SPIE Proceedings, 5747(2): 732 -742, 2005. 29) Inhomogeneity Correction 29) Axel, L. , J. Costantini and J. Listerud: “Intensity Corrections in Surface-Coil MR Imaging, ” American Journal of Roentgenology, 148: 418 -420, 1987. 30) Haselgrove, J. and M. Prammer: “An Algorithm for Compensation of Surface-Coil Images for Sensitivity of the Surface Coil, ” Magnetic Resonance Imaging, 4: 469 -472, 1986. 31) Meyer, C. R. , P. H. Bland J. Pipe: “Retrospective Correction of Intensity Inhomogeneities in MRI, ” IEEE Transactions on Medical Imaging, 14: 36 -41, 1995. 54
21) Shi, J. Y. and H. T. Tsui: “Scale Space Filtering by Fejér Kernel, ” International Symposium on Speech Image Process and Neural Networks, 2: 638 -641, 1994. 22) Saha, P. K. and J. K. Udupa: “Scale-Based Image Filtering Preserving Boundary Sharpness and Fine Structures, ” IEEE Transactions on Medical Imaging, 20(11): 1140 -1155, 2001. 23) Perona, P. and J. Malik: “Scale-Space and Edge Detection Using Anisotropic Diffusion, ” IEEE Transactions on Pattern Analysis and Machine Intelligence, 12(7): 629 -639, 1990. 24) Whitaker, R. T. and S. M. Pizer: “A Multi-Scale Approach to Nonuniform Diffusion, ” Computer Vision Graphic and Image Processing: Image Understanding, 57(1): 99 -110, 1993. 25) Weikert, J. , et al. : “Theoretical Foundations of Anisotropic Diffusion in Image Processing, ” Theoretical Foundations of Computer Vision, Computing, Supplement 11: 221 -236, 1996. 26) Parker, G. J. M. and J. A. Schnabel: “Enhancement of Anisotropic Diffusive Filtering of MR Images Using Approximate Entropy, ” in Proceedings of International Society for Magnetic Resonance in Medicine, 7: 175, 1999. 27) Gerig, G. , O. Kubler, R. Kikinis and F. A. Jolesz: “Nonlinear Anisotropic Filtering of MRI Data, ” IEEE Transaction on Medical Imaging, 11(2): 221 -232, 1992. 28) Souza, A. , J. K. Udupa and A. Madabhushi: “Generalized Scale-Based Image Filtering, ” SPIE Proceedings, 5747(2): 732 -742, 2005. 29) Inhomogeneity Correction 29) Axel, L. , J. Costantini and J. Listerud: “Intensity Corrections in Surface-Coil MR Imaging, ” American Journal of Roentgenology, 148: 418 -420, 1987. 30) Haselgrove, J. and M. Prammer: “An Algorithm for Compensation of Surface-Coil Images for Sensitivity of the Surface Coil, ” Magnetic Resonance Imaging, 4: 469 -472, 1986. 31) Meyer, C. R. , P. H. Bland J. Pipe: “Retrospective Correction of Intensity Inhomogeneities in MRI, ” IEEE Transactions on Medical Imaging, 14: 36 -41, 1995. 54
 32) Dawant, B. M. , A. P. Zjidenbos and R. A. Margolin: “Correction of Intensity Variations in MR Images for Computer-Aided Tissue Classification, ” IEEE Transactions on Medical Imaging, 12: 770 -781, 1993. 33) Tincher, M. , C. R. Meyer, G. Gupta and D. M. Williams: “Polynomial Modelling and Reduction of RF Body Coil Spatial Inhomogeneity in MRI, ” IEEE Transactions on Medical Imaging, 12: 361 -365, 1993. 34) Lai, S. and M. Fang: “A New Variational Shape-from-Orientation Approach to Correcting Intensity Inhomogeneity in MR Images, ” Medical Image Analysis, 3(4): 409 -424. 1999. 35) Wells, W. M. , E. L. Grimson, R. Kikinis and F. A. Jolesz: “Adaptive Segmentation of MRI Data, ” IEEE Transactions on Medical Imaging, 15: 429 -442, 1996. 36) Guillemaud, R. and M. Brady: “Estimating the Bias Field of MR Images, ” IEEE Transactions on Medical Imaging, 16: 238 -251, 1997. 37) Van Leemput, K. , F. Maes, D. Vandermeulen and P. Suetens: “Automated Model-Based Bias Field Correction of MR Images of the Brain, ” IEEE Transactions on Medical Imaging, 18: 885 -896, 1999. 38) Sled, J. G. , A. P. Zjidenbos and A. C. Evans: “A Nonparametric Method for Automatic Correction of Intensity Nonuniformity in MRI Data, ” IEEE Transactions on Medical Imaging, 17: 87 -97, 1998. 39) Styner, M. , C. Brechbuhler, G. Szekely and G. Gerig: “Parametric Estimate of Intensity Inhomogeneities Applied to MRI, ” IEEE Transactions on Medical Imaging, 19: 153 -165, 2000. 40) Likar, B. , M. A. Viergever and F. Pernus: “Retrospective Correction of MR Intensity Inhomogeneity by Information Minimization, ” IEEE Transaction on Medical Imaging, 20: 1398 -1410, 2001. 41) Madabhushi, A. and J. K. Udupa: “The Interplay Between Intensity Standardization and Inhomogeneity Correction in MR Image Processing, ” IEEE Transactions on Medical Imaging, 24(5): 561 -576, 2005. 42) Zhuge, Y. , J. K. Udupa, J. Liu and P. K. Saha: “An Intensity Standardization-Based Method for Image Inhomogeneity Correction in MRI, ” in Proceedings of SPIE: Medical Imaging, 6143: 658 -668, 2006. 43) Madabhushi, A. , J. K. Udupa and A. Souza: “Generalized Scale: Theory, Algorithms, and Application to Image Inhomogeneity Correction, ” SPIE Proceedings, 5370(2): 765 -776, 2004. 55
32) Dawant, B. M. , A. P. Zjidenbos and R. A. Margolin: “Correction of Intensity Variations in MR Images for Computer-Aided Tissue Classification, ” IEEE Transactions on Medical Imaging, 12: 770 -781, 1993. 33) Tincher, M. , C. R. Meyer, G. Gupta and D. M. Williams: “Polynomial Modelling and Reduction of RF Body Coil Spatial Inhomogeneity in MRI, ” IEEE Transactions on Medical Imaging, 12: 361 -365, 1993. 34) Lai, S. and M. Fang: “A New Variational Shape-from-Orientation Approach to Correcting Intensity Inhomogeneity in MR Images, ” Medical Image Analysis, 3(4): 409 -424. 1999. 35) Wells, W. M. , E. L. Grimson, R. Kikinis and F. A. Jolesz: “Adaptive Segmentation of MRI Data, ” IEEE Transactions on Medical Imaging, 15: 429 -442, 1996. 36) Guillemaud, R. and M. Brady: “Estimating the Bias Field of MR Images, ” IEEE Transactions on Medical Imaging, 16: 238 -251, 1997. 37) Van Leemput, K. , F. Maes, D. Vandermeulen and P. Suetens: “Automated Model-Based Bias Field Correction of MR Images of the Brain, ” IEEE Transactions on Medical Imaging, 18: 885 -896, 1999. 38) Sled, J. G. , A. P. Zjidenbos and A. C. Evans: “A Nonparametric Method for Automatic Correction of Intensity Nonuniformity in MRI Data, ” IEEE Transactions on Medical Imaging, 17: 87 -97, 1998. 39) Styner, M. , C. Brechbuhler, G. Szekely and G. Gerig: “Parametric Estimate of Intensity Inhomogeneities Applied to MRI, ” IEEE Transactions on Medical Imaging, 19: 153 -165, 2000. 40) Likar, B. , M. A. Viergever and F. Pernus: “Retrospective Correction of MR Intensity Inhomogeneity by Information Minimization, ” IEEE Transaction on Medical Imaging, 20: 1398 -1410, 2001. 41) Madabhushi, A. and J. K. Udupa: “The Interplay Between Intensity Standardization and Inhomogeneity Correction in MR Image Processing, ” IEEE Transactions on Medical Imaging, 24(5): 561 -576, 2005. 42) Zhuge, Y. , J. K. Udupa, J. Liu and P. K. Saha: “An Intensity Standardization-Based Method for Image Inhomogeneity Correction in MRI, ” in Proceedings of SPIE: Medical Imaging, 6143: 658 -668, 2006. 43) Madabhushi, A. , J. K. Udupa and A. Souza: “Generalized Scale: Theory, Algorithms, and Application to Image Inhomogeneity Correction, ” SPIE Proceedings, 5370(2): 765 -776, 2004. 55
 44) Montillo, A. , J. K. Udupa, L. Axel and D. N. Metaxas: “ Integrated Approach for the Removal of Intensity Inhomogeneity and Thermal Noise in SPAMM-MRI Using Scale-Based Fuzzy Connectedness and Multiple Nonlinear Adaptive Filters, ” in Proceedings of SPIE: Medical Imaging, 5032: 1025 -1036, 2003. 45) Intensity Standardization 45) Nyul, L. G. and J. K. Udupa: “On Standardizing the MR Image Intensity Scale, ” Magnetic Resonance in Medicine, 42: 1072 -1081, 1999. 46) Nyul, L. G. , J. K. Udupa and X. Zhang: “New Variants of a Method of MRI Scale Standardization, ” IEEE Transactions on Medical Imaging, 19: 143 -150, 2000. 47) Ge, Y. , J. K. Udupa, L. G. Nyul, L. Wei and R. I. Grossman: “Numerical Tissue Characterization in MS via Standardization of the MR Image Intensity Scale, ” Journal of Magnetic Resonance Imaging, 12: 715 -721, 2000. 48) Madabhushi, A. and J. K. Udupa: “New Methods of MR Image Intensity Standardization via Generalized Scale, ” SPIE Proceedings, 5747(2): 1143 -1154, 2005. 49) Madabhushi, A. : “ Generalized Scale: Theory, Algorithms, and Applications in Image Analysis, ” Ph. D. Thesis, Department of Bioengineering - University of Pennsylvania, Philadelphia, Pennsylvania, 2004. 50) 56
44) Montillo, A. , J. K. Udupa, L. Axel and D. N. Metaxas: “ Integrated Approach for the Removal of Intensity Inhomogeneity and Thermal Noise in SPAMM-MRI Using Scale-Based Fuzzy Connectedness and Multiple Nonlinear Adaptive Filters, ” in Proceedings of SPIE: Medical Imaging, 5032: 1025 -1036, 2003. 45) Intensity Standardization 45) Nyul, L. G. and J. K. Udupa: “On Standardizing the MR Image Intensity Scale, ” Magnetic Resonance in Medicine, 42: 1072 -1081, 1999. 46) Nyul, L. G. , J. K. Udupa and X. Zhang: “New Variants of a Method of MRI Scale Standardization, ” IEEE Transactions on Medical Imaging, 19: 143 -150, 2000. 47) Ge, Y. , J. K. Udupa, L. G. Nyul, L. Wei and R. I. Grossman: “Numerical Tissue Characterization in MS via Standardization of the MR Image Intensity Scale, ” Journal of Magnetic Resonance Imaging, 12: 715 -721, 2000. 48) Madabhushi, A. and J. K. Udupa: “New Methods of MR Image Intensity Standardization via Generalized Scale, ” SPIE Proceedings, 5747(2): 1143 -1154, 2005. 49) Madabhushi, A. : “ Generalized Scale: Theory, Algorithms, and Applications in Image Analysis, ” Ph. D. Thesis, Department of Bioengineering - University of Pennsylvania, Philadelphia, Pennsylvania, 2004. 50) 56


