19231484008de7a6cc957d6bdcc2fcdc.ppt
- Количество слайдов: 79
 1
1
 Cloning and Sequencing Explorer Series 2
Cloning and Sequencing Explorer Series 2
 Why Teach Cloning and Sequencing Series? • Students guide the research process and make decisions about their next steps • Encompasses a myriad of laboratory skills and techniques commonly used in research • Students generate original data that may lead to publications in Gen. Bank • Students formulate scientific explanations using data, logic, and evidence • Students understand research is a process rather than a single experiment giving students a real-life research experience with both its successes and challenges 3
Why Teach Cloning and Sequencing Series? • Students guide the research process and make decisions about their next steps • Encompasses a myriad of laboratory skills and techniques commonly used in research • Students generate original data that may lead to publications in Gen. Bank • Students formulate scientific explanations using data, logic, and evidence • Students understand research is a process rather than a single experiment giving students a real-life research experience with both its successes and challenges 3
 Appropriate courses • Molecular Biology • Recombinant DNA Techniques • Biotechnology • Molecular Evolution • Bioinformatics • Advanced Cell Biology • Advanced Genetics • Advanced Plant Biology • Independent Research 4
Appropriate courses • Molecular Biology • Recombinant DNA Techniques • Biotechnology • Molecular Evolution • Bioinformatics • Advanced Cell Biology • Advanced Genetics • Advanced Plant Biology • Independent Research 4
 Laboratory Overview Genomic DNA Extraction DNA Precipitation DNA Quantitation Bioinformatics Sequence Data Editing Contig Assembly Intron-Exon Prediction GAPDH PCR Nested PCR Degenerate primers Exonuclease Sequencing Automated sequencing Gel Electrophoresis DNA Gel Interpretation Band Identification Standard Curve Use Plasmid Miniprep Restriction Enzyme Digestion Gel Electrophoresis PCR Purification Size Exclusion Chromatography Microbial Culturing Antibiotic Selection Sterile Technique 5 Cloning Direct PCR cloning Transformation Ligation
Laboratory Overview Genomic DNA Extraction DNA Precipitation DNA Quantitation Bioinformatics Sequence Data Editing Contig Assembly Intron-Exon Prediction GAPDH PCR Nested PCR Degenerate primers Exonuclease Sequencing Automated sequencing Gel Electrophoresis DNA Gel Interpretation Band Identification Standard Curve Use Plasmid Miniprep Restriction Enzyme Digestion Gel Electrophoresis PCR Purification Size Exclusion Chromatography Microbial Culturing Antibiotic Selection Sterile Technique 5 Cloning Direct PCR cloning Transformation Ligation
 Student use the following techniques 6 • • • • Micropipetting DNA extraction Gel electrophoresis & interpretation Polymerase chain reaction DNA purification Restriction enzyme digests Microbiological sterile technique Preparing competent bacteria DNA ligation Heat-shock transformation Plasmid DNA isolation Sequence analysis BLAST searching Gen. Bank submission
Student use the following techniques 6 • • • • Micropipetting DNA extraction Gel electrophoresis & interpretation Polymerase chain reaction DNA purification Restriction enzyme digests Microbiological sterile technique Preparing competent bacteria DNA ligation Heat-shock transformation Plasmid DNA isolation Sequence analysis BLAST searching Gen. Bank submission
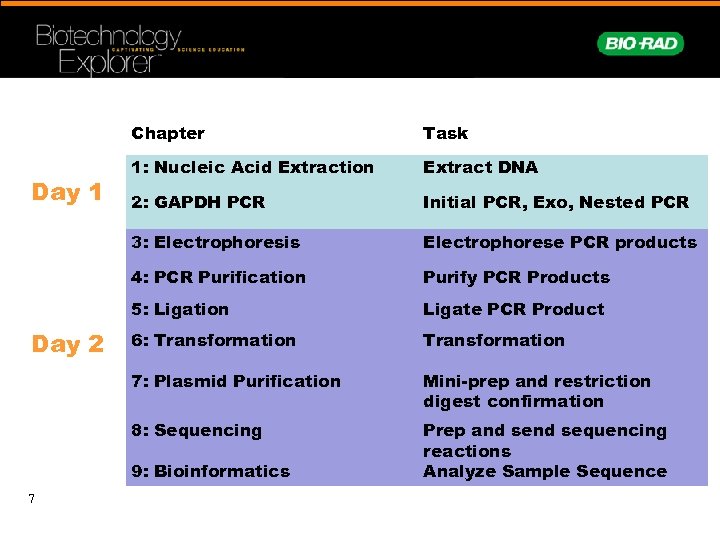 Chapter Extract DNA 2: GAPDH PCR Initial PCR, Exo, Nested PCR Electrophorese PCR products 4: PCR Purification Purify PCR Products 5: Ligation Ligate PCR Product 6: Transformation 7: Plasmid Purification Mini-prep and restriction digest confirmation 8: Sequencing Day 2 1: Nucleic Acid Extraction 3: Electrophoresis Day 1 Task Prep and sequencing reactions Analyze Sample Sequence 9: Bioinformatics 7
Chapter Extract DNA 2: GAPDH PCR Initial PCR, Exo, Nested PCR Electrophorese PCR products 4: PCR Purification Purify PCR Products 5: Ligation Ligate PCR Product 6: Transformation 7: Plasmid Purification Mini-prep and restriction digest confirmation 8: Sequencing Day 2 1: Nucleic Acid Extraction 3: Electrophoresis Day 1 Task Prep and sequencing reactions Analyze Sample Sequence 9: Bioinformatics 7
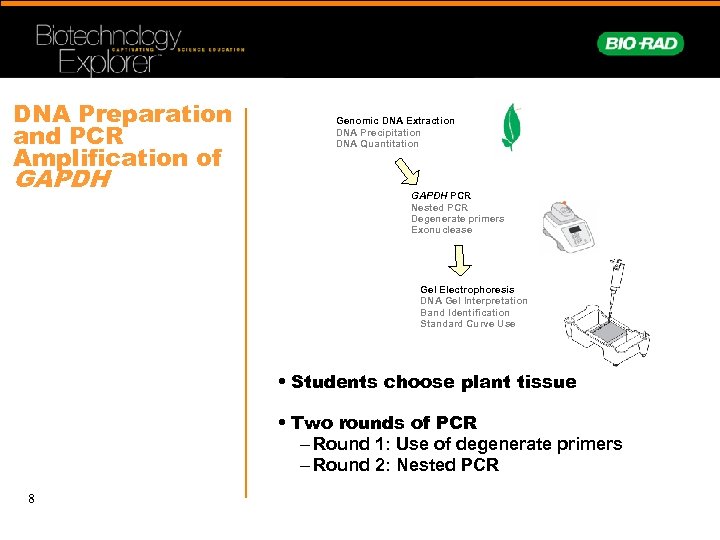 DNA Preparation and PCR Amplification of GAPDH Genomic DNA Extraction DNA Precipitation DNA Quantitation GAPDH PCR Nested PCR Degenerate primers Exonuclease Gel Electrophoresis DNA Gel Interpretation Band Identification Standard Curve Use • Students choose plant tissue • Two rounds of PCR – Round 1: Use of degenerate primers – Round 2: Nested PCR 8
DNA Preparation and PCR Amplification of GAPDH Genomic DNA Extraction DNA Precipitation DNA Quantitation GAPDH PCR Nested PCR Degenerate primers Exonuclease Gel Electrophoresis DNA Gel Interpretation Band Identification Standard Curve Use • Students choose plant tissue • Two rounds of PCR – Round 1: Use of degenerate primers – Round 2: Nested PCR 8
 Chapter 1 1: Nucleic Acid Extraction Optional: Dependent on time constraints, analyze samples prior to the next steps. This may include agarose gel electrophoresis, fluorometry, or spectrometry. Optional: If time permits proceed directly to Step 1– 2 and set up PCR reactions using freshly extracted genomic DNA. 9
Chapter 1 1: Nucleic Acid Extraction Optional: Dependent on time constraints, analyze samples prior to the next steps. This may include agarose gel electrophoresis, fluorometry, or spectrometry. Optional: If time permits proceed directly to Step 1– 2 and set up PCR reactions using freshly extracted genomic DNA. 9
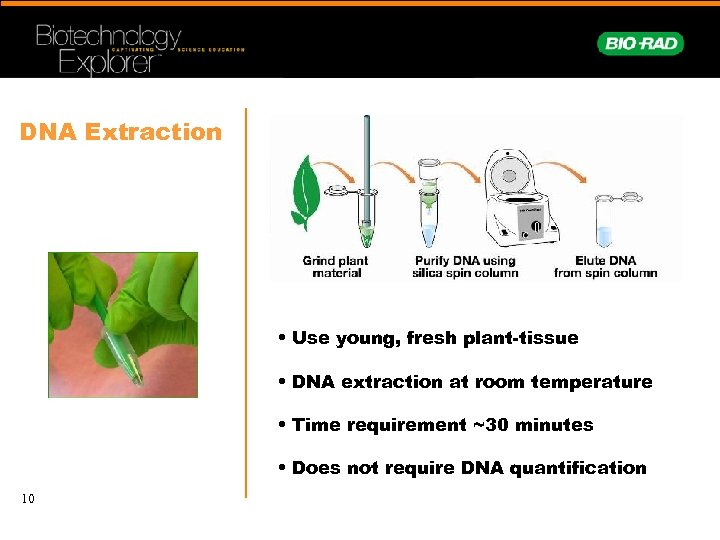 DNA Extraction • Use young, fresh plant-tissue • DNA extraction at room temperature • Time requirement ~30 minutes • Does not require DNA quantification 10
DNA Extraction • Use young, fresh plant-tissue • DNA extraction at room temperature • Time requirement ~30 minutes • Does not require DNA quantification 10
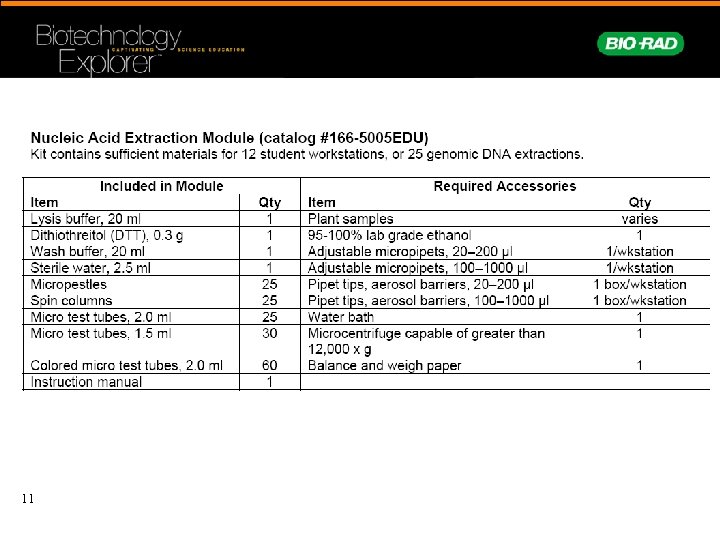 11
11
 Benefits of using plants • Large number of species • Lots of diversity • Phylogenetic approaches • Avoid ethical concerns associated with animals • No pre-approval 12
Benefits of using plants • Large number of species • Lots of diversity • Phylogenetic approaches • Avoid ethical concerns associated with animals • No pre-approval 12
 Nucleic Acid Extraction Quick Guide 13
Nucleic Acid Extraction Quick Guide 13
 Chapter 2 2: GAPDH PCR 14
Chapter 2 2: GAPDH PCR 14
 15 Optional: Although this protocol recommends analyzing the PCR products after both rounds of PCR have been completed, PCR results can be assessed using electrophoresis directly after this reaction is complete. Positive controls should yield visible bands. It is possible that some plant genomic DNA will not yield a visible band during the initial round of PCR and yet still be amplified after the second round of nested PCR. Note: If this is done — DO NOT add loading dye directly to the PCR reactions as loading dye may interfere with the subsequent round of PCR.
15 Optional: Although this protocol recommends analyzing the PCR products after both rounds of PCR have been completed, PCR results can be assessed using electrophoresis directly after this reaction is complete. Positive controls should yield visible bands. It is possible that some plant genomic DNA will not yield a visible band during the initial round of PCR and yet still be amplified after the second round of nested PCR. Note: If this is done — DO NOT add loading dye directly to the PCR reactions as loading dye may interfere with the subsequent round of PCR.
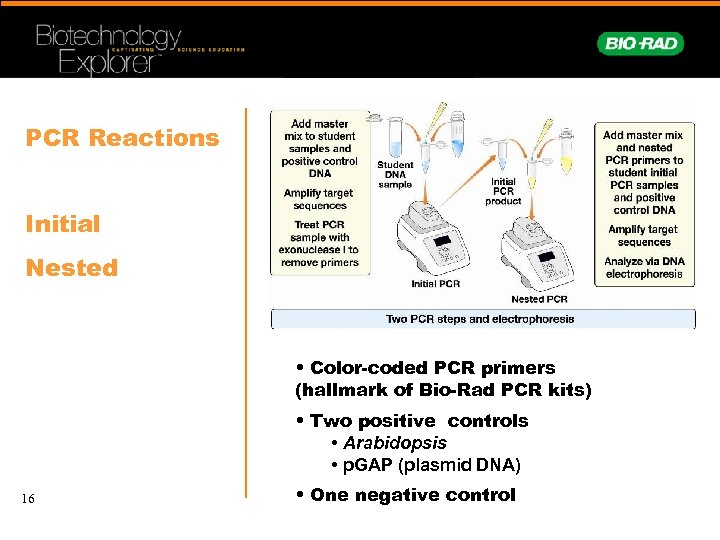 PCR Reactions Initial Nested • Color-coded PCR primers (hallmark of Bio-Rad PCR kits) • Two positive controls • Arabidopsis • p. GAP (plasmid DNA) 16 • One negative control
PCR Reactions Initial Nested • Color-coded PCR primers (hallmark of Bio-Rad PCR kits) • Two positive controls • Arabidopsis • p. GAP (plasmid DNA) 16 • One negative control
 What is a Housekeeping Gene? Highly conserved genes that must be continually expressed in all tissues of organisms to maintain essential cellular functions. Examples: • GAPDH • Cytochrome C • ATPase • ß-actin 17
What is a Housekeeping Gene? Highly conserved genes that must be continually expressed in all tissues of organisms to maintain essential cellular functions. Examples: • GAPDH • Cytochrome C • ATPase • ß-actin 17
 Why use GAPDH? Glyceraldehyde 3 -Phosphate Dehydrogenase (GAPDH) • Enzyme of glycolysis • Structure and reaction mechanism well-studied • Multitude of sequences • Highly conserved 18
Why use GAPDH? Glyceraldehyde 3 -Phosphate Dehydrogenase (GAPDH) • Enzyme of glycolysis • Structure and reaction mechanism well-studied • Multitude of sequences • Highly conserved 18
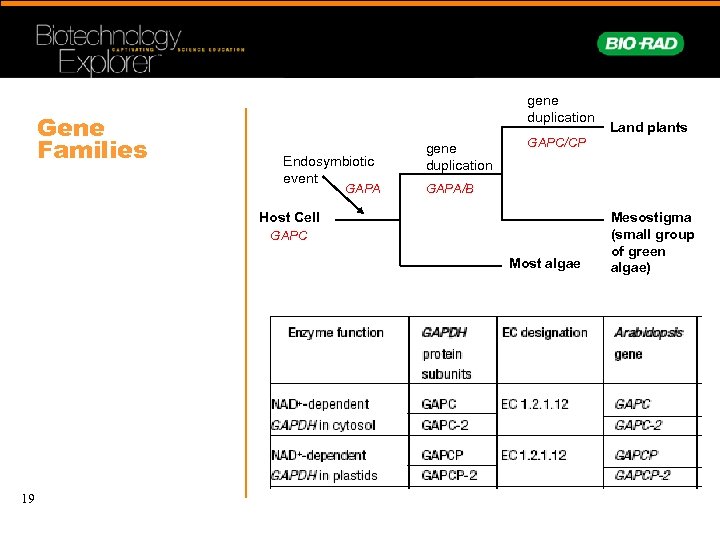 Gene Families gene duplication Endosymbiotic event GAPA gene duplication GAPC/CP GAPA/B Host Cell GAPC Most algae 19 Land plants Mesostigma (small group of green algae)
Gene Families gene duplication Endosymbiotic event GAPA gene duplication GAPC/CP GAPA/B Host Cell GAPC Most algae 19 Land plants Mesostigma (small group of green algae)
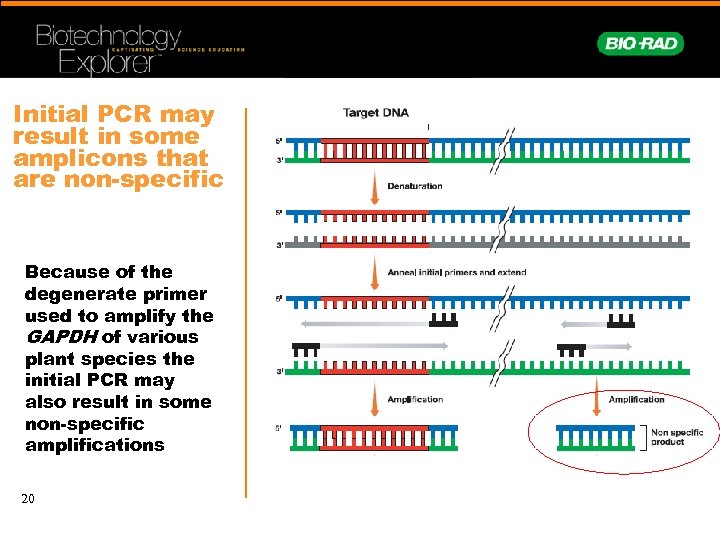 Initial PCR may result in some amplicons that are non-specific Because of the degenerate primer used to amplify the GAPDH of various plant species the initial PCR may also result in some non-specific amplifications 20
Initial PCR may result in some amplicons that are non-specific Because of the degenerate primer used to amplify the GAPDH of various plant species the initial PCR may also result in some non-specific amplifications 20
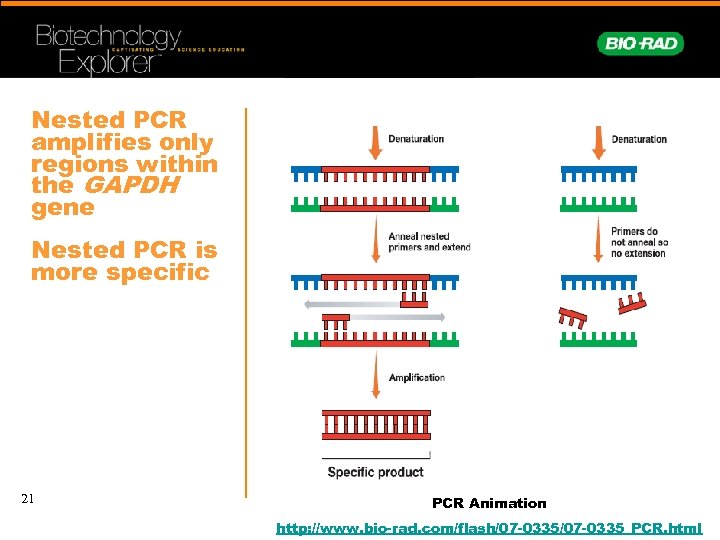 Nested PCR amplifies only regions within the GAPDH gene Nested PCR is more specific 21 PCR Animation http: //www. bio-rad. com/flash/07 -0335_PCR. html
Nested PCR amplifies only regions within the GAPDH gene Nested PCR is more specific 21 PCR Animation http: //www. bio-rad. com/flash/07 -0335_PCR. html
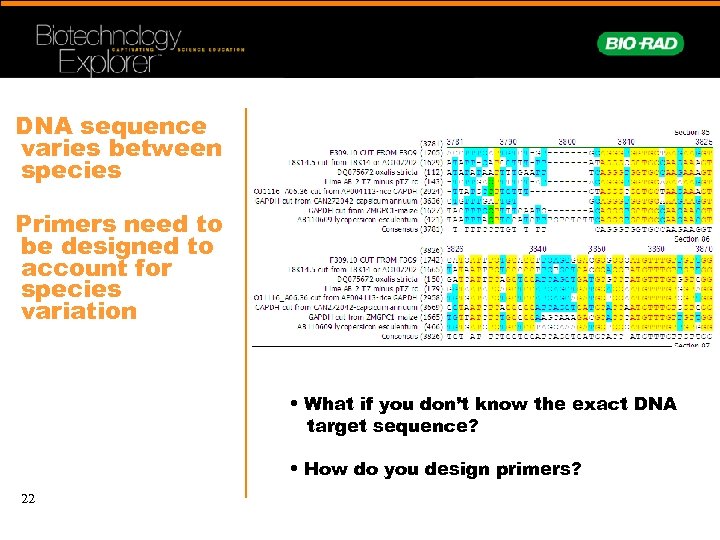 DNA sequence varies between species Primers need to be designed to account for species variation • What if you don’t know the exact DNA target sequence? • How do you design primers? 22
DNA sequence varies between species Primers need to be designed to account for species variation • What if you don’t know the exact DNA target sequence? • How do you design primers? 22
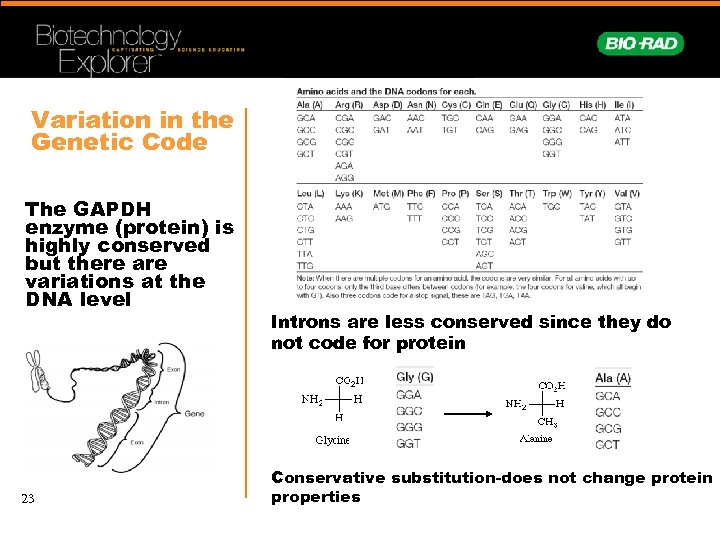 Variation in the Genetic Code The GAPDH enzyme (protein) is highly conserved but there are variations at the DNA level 23 Introns are less conserved since they do not code for protein Conservative substitution-does not change protein properties
Variation in the Genetic Code The GAPDH enzyme (protein) is highly conserved but there are variations at the DNA level 23 Introns are less conserved since they do not code for protein Conservative substitution-does not change protein properties
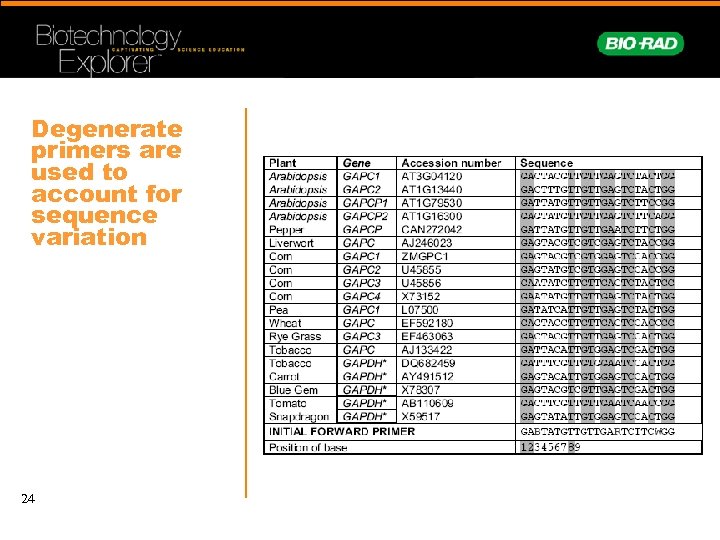 Degenerate primers are used to account for sequence variation 24
Degenerate primers are used to account for sequence variation 24
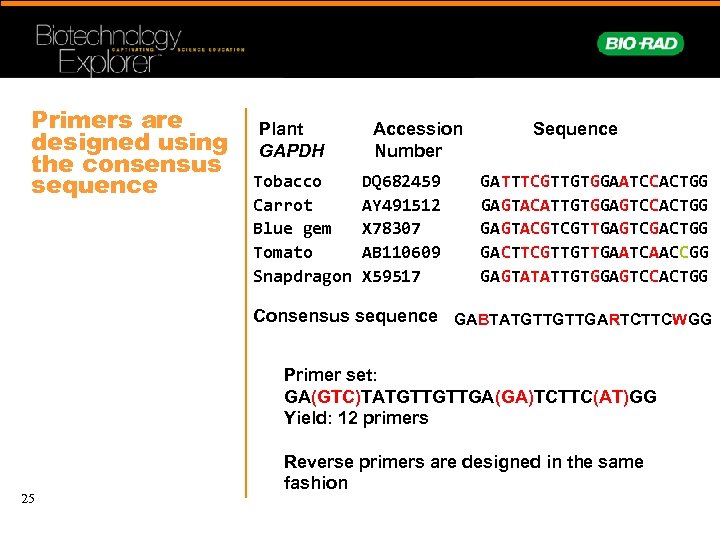 Primers are designed using the consensus sequence Plant GAPDH Tobacco Carrot Blue gem Tomato Snapdragon Accession Number DQ 682459 AY 491512 X 78307 AB 110609 X 59517 Sequence GATTTCGTTGTGGAATCCACTGG GAGTACATTGTGGAGTCCACTGG GAGTACGTCGTTGAGTCGACTGG GACTTCGTTGTTGAATCAACCGG GAGTATATTGTGGAGTCCACTGG Consensus sequence GABTATGTTGTTGARTCTTCWGG Primer set: GA(GTC)TATGTTGTTGA(GA)TCTTC(AT)GG Yield: 12 primers 25 Reverse primers are designed in the same fashion
Primers are designed using the consensus sequence Plant GAPDH Tobacco Carrot Blue gem Tomato Snapdragon Accession Number DQ 682459 AY 491512 X 78307 AB 110609 X 59517 Sequence GATTTCGTTGTGGAATCCACTGG GAGTACATTGTGGAGTCCACTGG GAGTACGTCGTTGAGTCGACTGG GACTTCGTTGTTGAATCAACCGG GAGTATATTGTGGAGTCCACTGG Consensus sequence GABTATGTTGTTGARTCTTCWGG Primer set: GA(GTC)TATGTTGTTGA(GA)TCTTC(AT)GG Yield: 12 primers 25 Reverse primers are designed in the same fashion
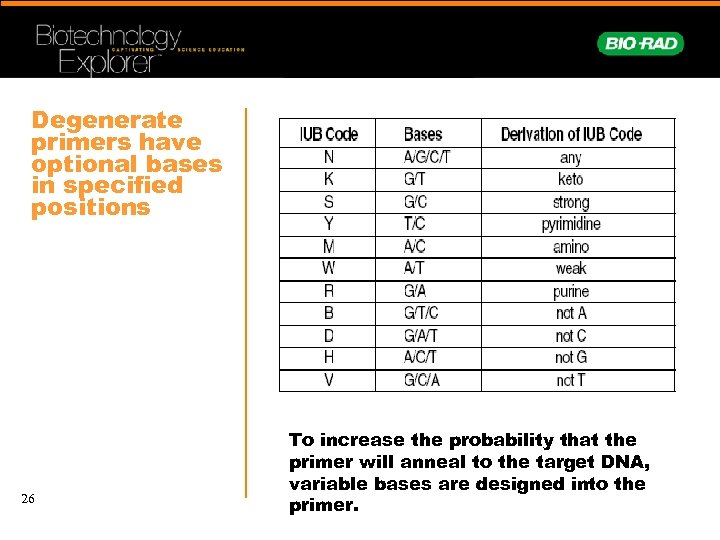 Degenerate primers have optional bases in specified positions 26 To increase the probability that the primer will anneal to the target DNA, variable bases are designed into the primer.
Degenerate primers have optional bases in specified positions 26 To increase the probability that the primer will anneal to the target DNA, variable bases are designed into the primer.
 Multiple oligos comprise the forward primer GAGTATGTTGTTGA(GA)TCTTC(AT)GG Position 3 GATTATGTTGTTGA(GA)TCTTC(AT)GG GACTATGTTGTTGA(GA)TCTTC(AT)GG has 3 bases GA(GTC)TATGTTGTTGAGTCTTC(AT)GG Position 15 GA(GTC)TATGTTGTTGAATCTTC(AT)GG has 2 bases GA(GTC)TATGTTGTTGA(GA)TCTTCAGG Position 21 GA(GTC)TATGTTGTTGA(GA)TCTTCTGG has 2 bases 3 x 2 = 12 different oligonucleotides comprising the forward primer 27
Multiple oligos comprise the forward primer GAGTATGTTGTTGA(GA)TCTTC(AT)GG Position 3 GATTATGTTGTTGA(GA)TCTTC(AT)GG GACTATGTTGTTGA(GA)TCTTC(AT)GG has 3 bases GA(GTC)TATGTTGTTGAGTCTTC(AT)GG Position 15 GA(GTC)TATGTTGTTGAATCTTC(AT)GG has 2 bases GA(GTC)TATGTTGTTGA(GA)TCTTCAGG Position 21 GA(GTC)TATGTTGTTGA(GA)TCTTCTGG has 2 bases 3 x 2 = 12 different oligonucleotides comprising the forward primer 27
 DNA Isolation and Amplification To identify differences in GAPDH code we must isolate plant DNA and amplify the gene of interest using PCR first with degenerate primers (primers that account for variation in the DNA code) A second PCR reaction (Nested PCR) is necessary to amplify the region which contains one of the GAPDH gene sequences (second set of primers are nested inside the initial PCR product sequence) Biotechnology Explorer PCR primers are color-coded 28
DNA Isolation and Amplification To identify differences in GAPDH code we must isolate plant DNA and amplify the gene of interest using PCR first with degenerate primers (primers that account for variation in the DNA code) A second PCR reaction (Nested PCR) is necessary to amplify the region which contains one of the GAPDH gene sequences (second set of primers are nested inside the initial PCR product sequence) Biotechnology Explorer PCR primers are color-coded 28
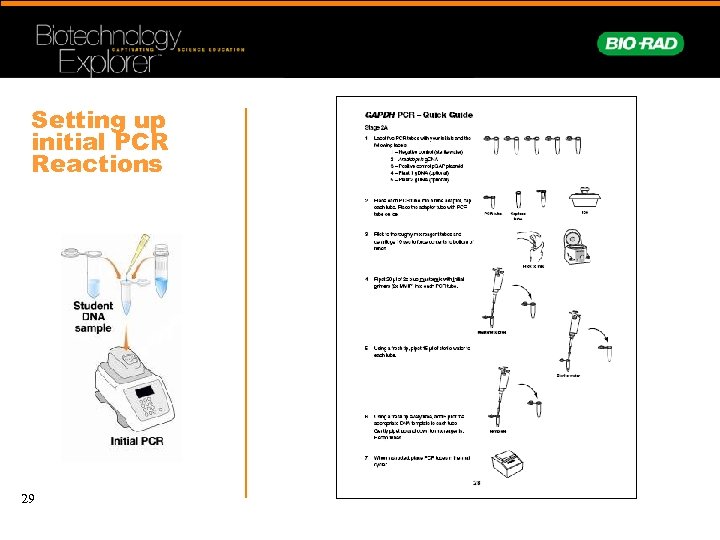 Setting up initial PCR Reactions 29
Setting up initial PCR Reactions 29
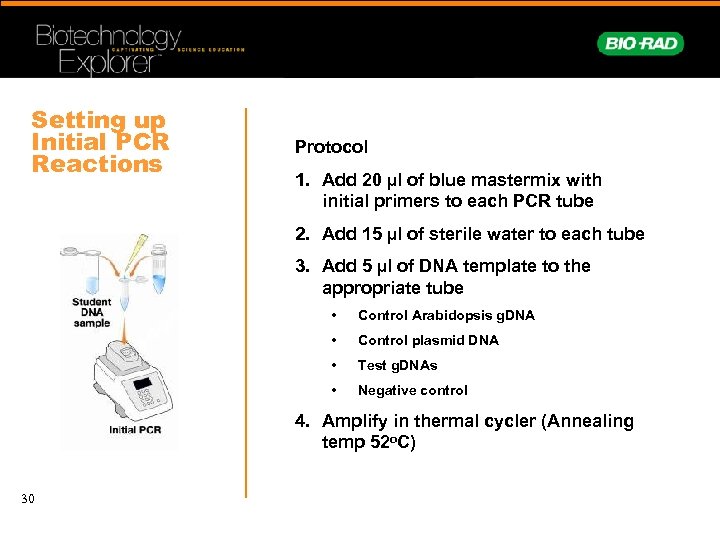 Setting up Initial PCR Reactions Protocol 1. Add 20 µl of blue mastermix with initial primers to each PCR tube 2. Add 15 µl of sterile water to each tube 3. Add 5 µl of DNA template to the appropriate tube • Control Arabidopsis g. DNA • Control plasmid DNA • Test g. DNAs • Negative control 4. Amplify in thermal cycler (Annealing temp 52 o. C) 30
Setting up Initial PCR Reactions Protocol 1. Add 20 µl of blue mastermix with initial primers to each PCR tube 2. Add 15 µl of sterile water to each tube 3. Add 5 µl of DNA template to the appropriate tube • Control Arabidopsis g. DNA • Control plasmid DNA • Test g. DNAs • Negative control 4. Amplify in thermal cycler (Annealing temp 52 o. C) 30
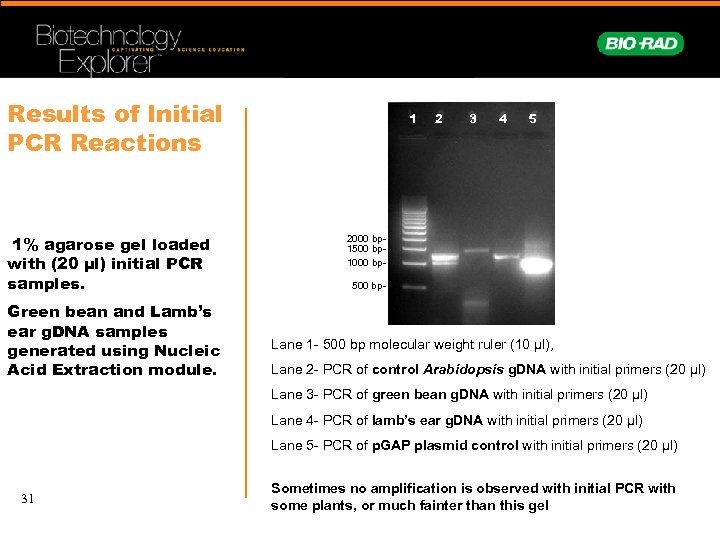 Results of Initial PCR Reactions 1% agarose gel loaded with (20 µl) initial PCR samples. Green bean and Lamb’s ear g. DNA samples generated using Nucleic Acid Extraction module. 1 2 3 4 5 2000 bp 1500 bp 1000 bp 500 bp- Lane 1 - 500 bp molecular weight ruler (10 µl), Lane 2 - PCR of control Arabidopsis g. DNA with initial primers (20 µl) Lane 3 - PCR of green bean g. DNA with initial primers (20 µl) Lane 4 - PCR of lamb’s ear g. DNA with initial primers (20 µl) Lane 5 - PCR of p. GAP plasmid control with initial primers (20 µl) 31 Sometimes no amplification is observed with initial PCR with some plants, or much fainter than this gel
Results of Initial PCR Reactions 1% agarose gel loaded with (20 µl) initial PCR samples. Green bean and Lamb’s ear g. DNA samples generated using Nucleic Acid Extraction module. 1 2 3 4 5 2000 bp 1500 bp 1000 bp 500 bp- Lane 1 - 500 bp molecular weight ruler (10 µl), Lane 2 - PCR of control Arabidopsis g. DNA with initial primers (20 µl) Lane 3 - PCR of green bean g. DNA with initial primers (20 µl) Lane 4 - PCR of lamb’s ear g. DNA with initial primers (20 µl) Lane 5 - PCR of p. GAP plasmid control with initial primers (20 µl) 31 Sometimes no amplification is observed with initial PCR with some plants, or much fainter than this gel
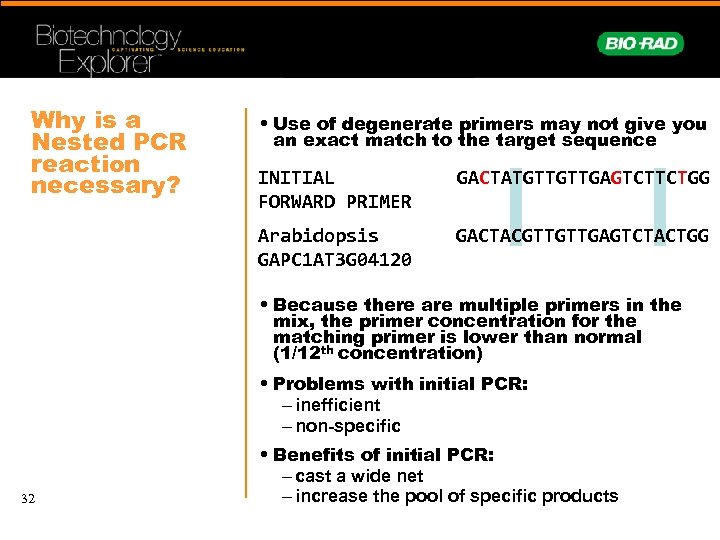 Why is a Nested PCR reaction necessary? • Use of degenerate primers may not give you an exact match to the target sequence INITIAL FORWARD PRIMER GACTATGTTGTTGAGTCTTCTGG Arabidopsis GAPC 1 AT 3 G 04120 GACTACGTTGTTGAGTCTACTGG • Because there are multiple primers in the mix, the primer concentration for the matching primer is lower than normal (1/12 th concentration) • Problems with initial PCR: – inefficient – non-specific 32 • Benefits of initial PCR: – cast a wide net – increase the pool of specific products
Why is a Nested PCR reaction necessary? • Use of degenerate primers may not give you an exact match to the target sequence INITIAL FORWARD PRIMER GACTATGTTGTTGAGTCTTCTGG Arabidopsis GAPC 1 AT 3 G 04120 GACTACGTTGTTGAGTCTACTGG • Because there are multiple primers in the mix, the primer concentration for the matching primer is lower than normal (1/12 th concentration) • Problems with initial PCR: – inefficient – non-specific 32 • Benefits of initial PCR: – cast a wide net – increase the pool of specific products
 Exonuclease 1 treatment is needed before the second round of PCR (nested PCR) is done • The primers that were not incorporated into PCR product in the first reaction must be removed so that they do not amplify target DNA in the second round of PCR. • Exonuclease I will be added to the PCR products • The enzyme must be inactivated before proceeding to the nested PCR 33
Exonuclease 1 treatment is needed before the second round of PCR (nested PCR) is done • The primers that were not incorporated into PCR product in the first reaction must be removed so that they do not amplify target DNA in the second round of PCR. • Exonuclease I will be added to the PCR products • The enzyme must be inactivated before proceeding to the nested PCR 33
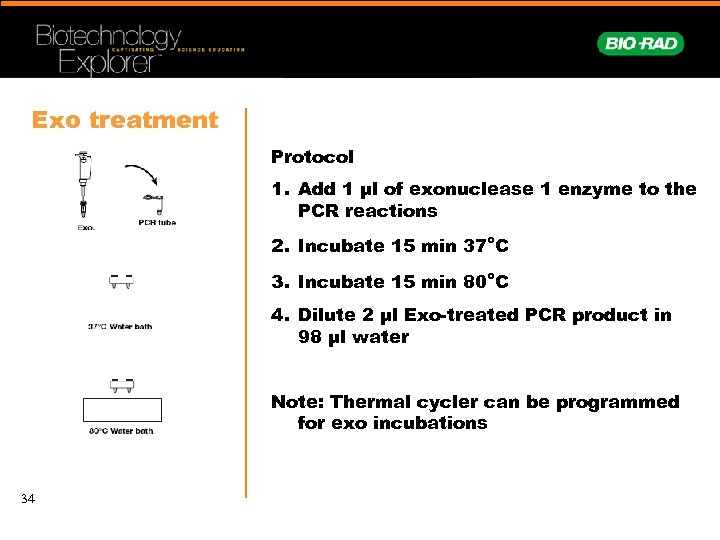 Exo treatment Protocol 1. Add 1 µl of exonuclease 1 enzyme to the PCR reactions 2. Incubate 15 min 37°C 3. Incubate 15 min 80°C 4. Dilute 2 µl Exo-treated PCR product in 98 µl water Note: Thermal cycler can be programmed for exo incubations 34
Exo treatment Protocol 1. Add 1 µl of exonuclease 1 enzyme to the PCR reactions 2. Incubate 15 min 37°C 3. Incubate 15 min 80°C 4. Dilute 2 µl Exo-treated PCR product in 98 µl water Note: Thermal cycler can be programmed for exo incubations 34
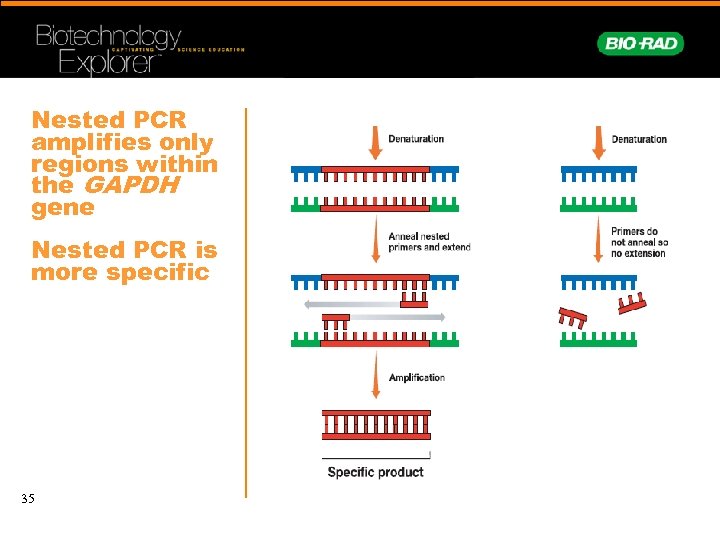 Nested PCR amplifies only regions within the GAPDH gene Nested PCR is more specific 35
Nested PCR amplifies only regions within the GAPDH gene Nested PCR is more specific 35
 Setting up Nested PCR Reactions Protocol 1. Mix 20 µl of diluted exo-treated template DNA with 20 µl of yellow mastermix with nested primers 2. For controls, mix 20 µl of control p. GAP plasmid and 20 µl of water with 20 µl of yellow mastermix with nested primers 3. Amplify in thermal cycler (Annealing temp 46 o. C) 36
Setting up Nested PCR Reactions Protocol 1. Mix 20 µl of diluted exo-treated template DNA with 20 µl of yellow mastermix with nested primers 2. For controls, mix 20 µl of control p. GAP plasmid and 20 µl of water with 20 µl of yellow mastermix with nested primers 3. Amplify in thermal cycler (Annealing temp 46 o. C) 36
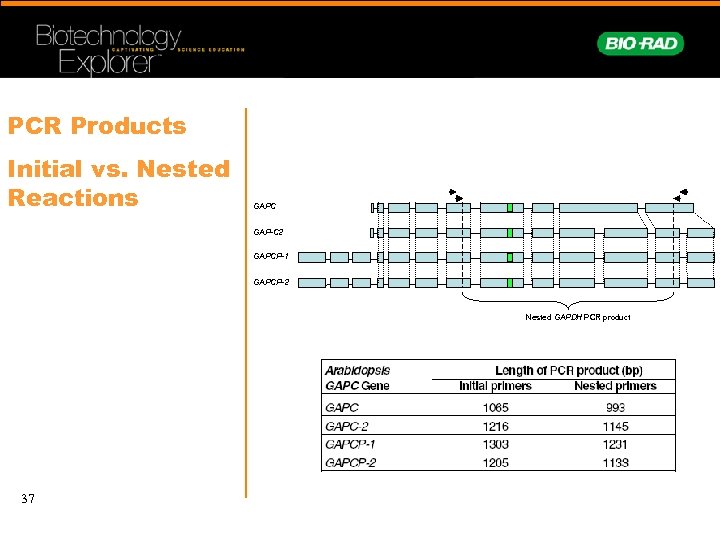 PCR Products Initial vs. Nested Reactions GAPC GAP-C 2 GAPCP-1 GAPCP-2 Nested GAPDH PCR product 37
PCR Products Initial vs. Nested Reactions GAPC GAP-C 2 GAPCP-1 GAPCP-2 Nested GAPDH PCR product 37
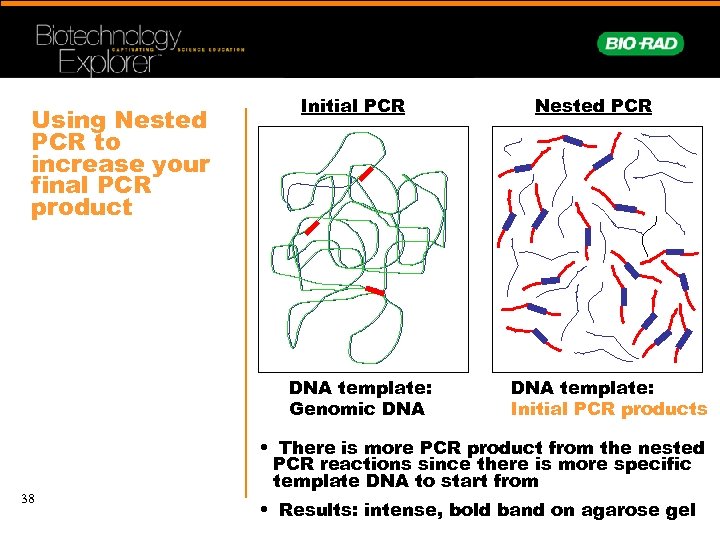 Using Nested PCR to increase your final PCR product Initial PCR DNA template: Genomic DNA 38 Nested PCR DNA template: Initial PCR products • There is more PCR product from the nested PCR reactions since there is more specific template DNA to start from • Results: intense, bold band on agarose gel
Using Nested PCR to increase your final PCR product Initial PCR DNA template: Genomic DNA 38 Nested PCR DNA template: Initial PCR products • There is more PCR product from the nested PCR reactions since there is more specific template DNA to start from • Results: intense, bold band on agarose gel
 Chapter 3 3: Electrophoresis 39
Chapter 3 3: Electrophoresis 39
 40
40
 Electrophoresis Quick Guide 41
Electrophoresis Quick Guide 41
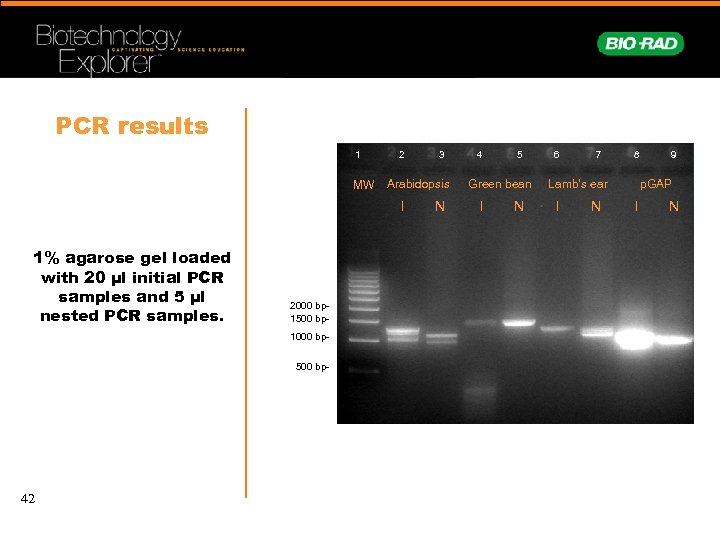 PCR results 1 MW 2 Arabidopsis I 1% agarose gel loaded with 20 µl initial PCR samples and 5 µl nested PCR samples. 2000 bp 1500 bp 1000 bp 500 bp- 42 3 N 4 5 Green bean I N 6 7 8 Lamb’s ear I N 9 p. GAP I N
PCR results 1 MW 2 Arabidopsis I 1% agarose gel loaded with 20 µl initial PCR samples and 5 µl nested PCR samples. 2000 bp 1500 bp 1000 bp 500 bp- 42 3 N 4 5 Green bean I N 6 7 8 Lamb’s ear I N 9 p. GAP I N
 Laboratory Overview Genomic DNA Extraction DNA Precipitation DNA Quantitation Bioinformatics Sequence Data Editing Contig Assembly Intron-Exon Prediction GAPDH PCR Nested PCR Degenerate primers Exonuclease Sequencing Automated sequencing Gel Electrophoresis DNA Gel Interpretation Band Identification Standard Curve Use Plasmid Miniprep Restriction Enzyme Digestion Gel Electrophoresis PCR Purification Size Exclusion Chromatography Microbial Culturing Antibiotic Selection Sterile Technique 43 Cloning Direct PCR cloning Transformation Ligation
Laboratory Overview Genomic DNA Extraction DNA Precipitation DNA Quantitation Bioinformatics Sequence Data Editing Contig Assembly Intron-Exon Prediction GAPDH PCR Nested PCR Degenerate primers Exonuclease Sequencing Automated sequencing Gel Electrophoresis DNA Gel Interpretation Band Identification Standard Curve Use Plasmid Miniprep Restriction Enzyme Digestion Gel Electrophoresis PCR Purification Size Exclusion Chromatography Microbial Culturing Antibiotic Selection Sterile Technique 43 Cloning Direct PCR cloning Transformation Ligation
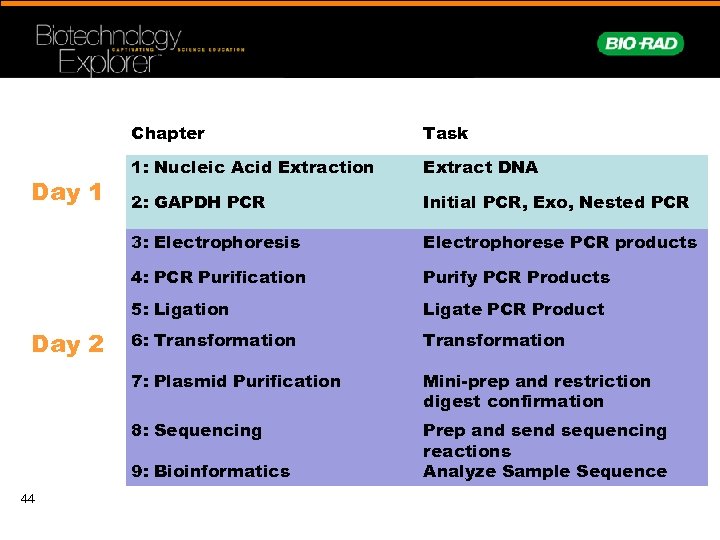 Chapter Extract DNA 2: GAPDH PCR Initial PCR, Exo, Nested PCR Electrophorese PCR products 4: PCR Purification Purify PCR Products 5: Ligation Ligate PCR Product 6: Transformation 7: Plasmid Purification Mini-prep and restriction digest confirmation 8: Sequencing Day 2 1: Nucleic Acid Extraction 3: Electrophoresis Day 1 Task Prep and sequencing reactions Analyze Sample Sequence 9: Bioinformatics 44
Chapter Extract DNA 2: GAPDH PCR Initial PCR, Exo, Nested PCR Electrophorese PCR products 4: PCR Purification Purify PCR Products 5: Ligation Ligate PCR Product 6: Transformation 7: Plasmid Purification Mini-prep and restriction digest confirmation 8: Sequencing Day 2 1: Nucleic Acid Extraction 3: Electrophoresis Day 1 Task Prep and sequencing reactions Analyze Sample Sequence 9: Bioinformatics 44
 PCR Purification, Ligation, Transformation and Plasmid Miniprep Purification Plasmid Miniprep Restriction Enzyme Digestion Gel Electrophoresis PCR Purification Size Exclusion Chromatography Microbial Culturing Antibiotic Selection Sterile Technique Cloning Direct PCR cloning Transformation Ligation • Choose best PCR products for ligation • Transformation and selection • Plasmid Minipreparation 45
PCR Purification, Ligation, Transformation and Plasmid Miniprep Purification Plasmid Miniprep Restriction Enzyme Digestion Gel Electrophoresis PCR Purification Size Exclusion Chromatography Microbial Culturing Antibiotic Selection Sterile Technique Cloning Direct PCR cloning Transformation Ligation • Choose best PCR products for ligation • Transformation and selection • Plasmid Minipreparation 45
 Chapter 4 4: PCR Purification Additional tasks to perform prior to next stage: Starter cultures must be inoculated one day prior to the transformation with a starter colony from the HB 101 LB agar starter plate. Incubate cultures with shaking overnight at 37 o. C. 46
Chapter 4 4: PCR Purification Additional tasks to perform prior to next stage: Starter cultures must be inoculated one day prior to the transformation with a starter colony from the HB 101 LB agar starter plate. Incubate cultures with shaking overnight at 37 o. C. 46
 Optional: Electrophorese 5 µl of the purified sample along with 5 µl of the unpurified sample on an agarose gel. 47
Optional: Electrophorese 5 µl of the purified sample along with 5 µl of the unpurified sample on an agarose gel. 47
 Column Purification of PCR products SEC is used to purify large PCR products from the smaller primers, d. NTPs and enzymes. 48
Column Purification of PCR products SEC is used to purify large PCR products from the smaller primers, d. NTPs and enzymes. 48
 PCR Purification Quick Guide 49
PCR Purification Quick Guide 49
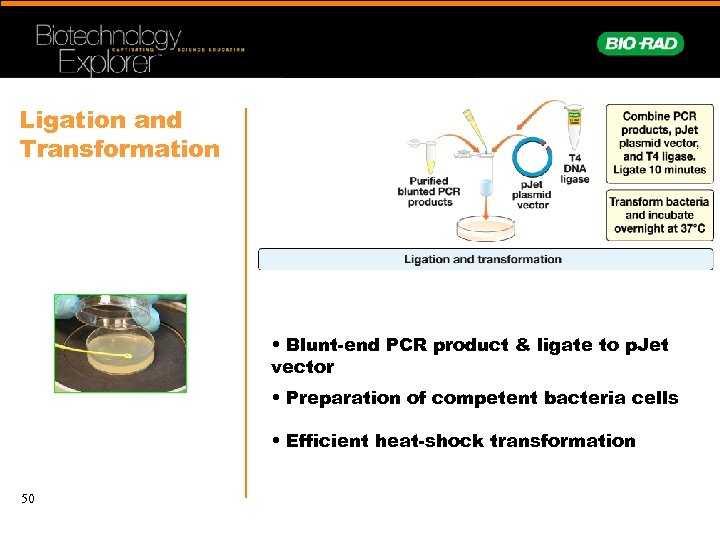 Ligation and Transformation • Blunt-end PCR product & ligate to p. Jet vector • Preparation of competent bacteria cells • Efficient heat-shock transformation 50
Ligation and Transformation • Blunt-end PCR product & ligate to p. Jet vector • Preparation of competent bacteria cells • Efficient heat-shock transformation 50
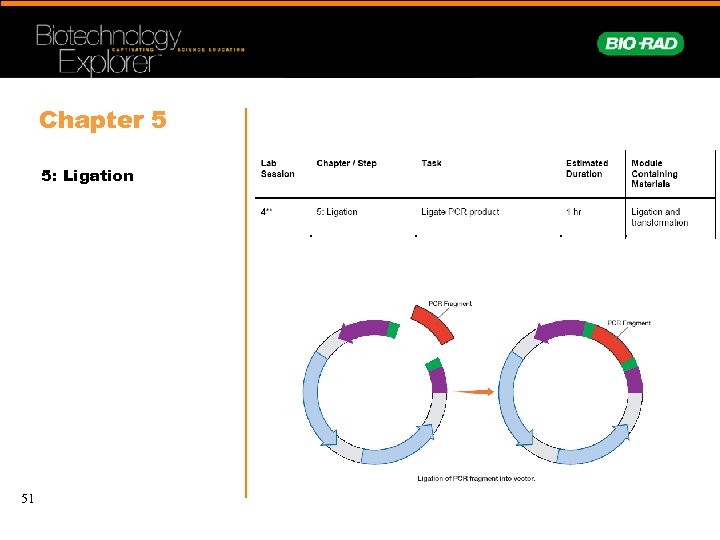 Chapter 5 5: Ligation 51
Chapter 5 5: Ligation 51
 52
52
 Ligation Sticky End Ligation: If the insert has sticky ends then the vector should be cut with the same enzyme to produce complementary ends Allows for directional cloning (c. DNA) Blunt End Ligation: All DNA ends are compatible, not necessary to cut vector and insert with the same restriction enzymes 53 Treating the PCR product with a proofreading DNA polymerase removes 3’-A added by Taq DNA polymerase in PCR, leaving blunt ends ready for ligation.
Ligation Sticky End Ligation: If the insert has sticky ends then the vector should be cut with the same enzyme to produce complementary ends Allows for directional cloning (c. DNA) Blunt End Ligation: All DNA ends are compatible, not necessary to cut vector and insert with the same restriction enzymes 53 Treating the PCR product with a proofreading DNA polymerase removes 3’-A added by Taq DNA polymerase in PCR, leaving blunt ends ready for ligation.
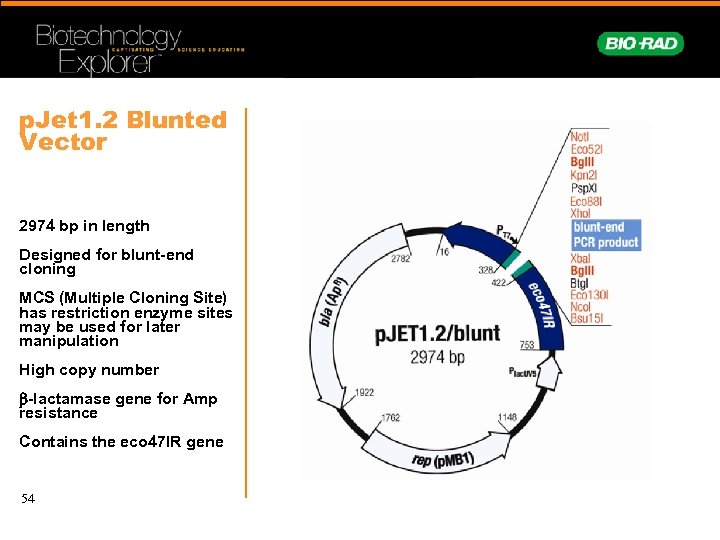 p. Jet 1. 2 Blunted Vector 2974 bp in length Designed for blunt-end cloning MCS (Multiple Cloning Site) has restriction enzyme sites may be used for later manipulation High copy number b-lactamase gene for Amp resistance Contains the eco 47 IR gene 54
p. Jet 1. 2 Blunted Vector 2974 bp in length Designed for blunt-end cloning MCS (Multiple Cloning Site) has restriction enzyme sites may be used for later manipulation High copy number b-lactamase gene for Amp resistance Contains the eco 47 IR gene 54
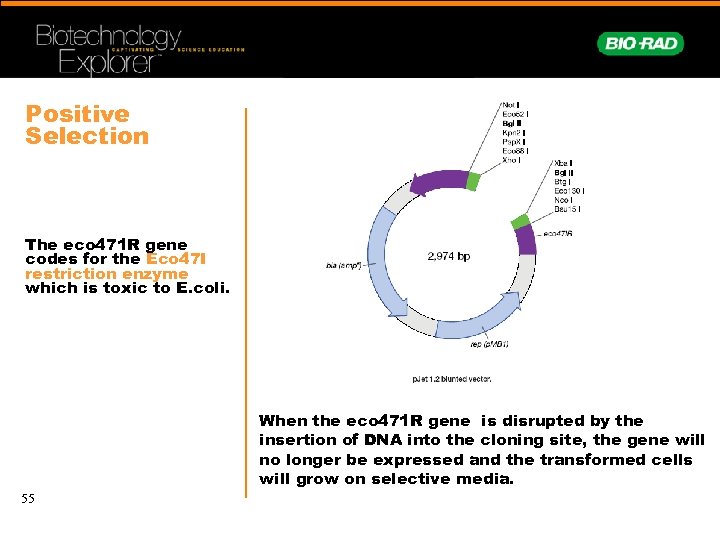 Positive Selection The eco 471 R gene codes for the Eco 47 I restriction enzyme which is toxic to E. coli. When the eco 471 R gene is disrupted by the insertion of DNA into the cloning site, the gene will no longer be expressed and the transformed cells will grow on selective media. 55
Positive Selection The eco 471 R gene codes for the Eco 47 I restriction enzyme which is toxic to E. coli. When the eco 471 R gene is disrupted by the insertion of DNA into the cloning site, the gene will no longer be expressed and the transformed cells will grow on selective media. 55
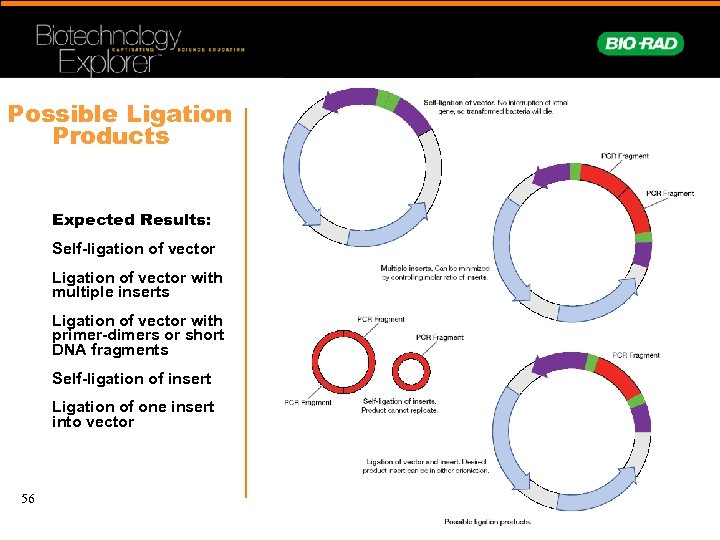 Possible Ligation Products Expected Results: Self-ligation of vector Ligation of vector with multiple inserts Ligation of vector with primer-dimers or short DNA fragments Self-ligation of insert Ligation of one insert into vector 56
Possible Ligation Products Expected Results: Self-ligation of vector Ligation of vector with multiple inserts Ligation of vector with primer-dimers or short DNA fragments Self-ligation of insert Ligation of one insert into vector 56
 Ligation Quick Guide 57
Ligation Quick Guide 57
 Chapter 6 6: Transformation 58
Chapter 6 6: Transformation 58
 59
59
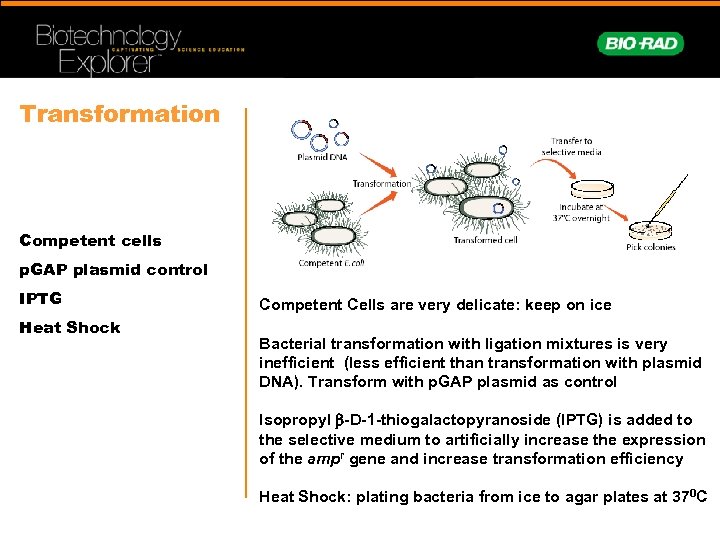 Transformation Competent cells p. GAP plasmid control IPTG Heat Shock Competent Cells are very delicate: keep on ice Bacterial transformation with ligation mixtures is very inefficient (less efficient than transformation with plasmid DNA). Transform with p. GAP plasmid as control Isopropyl b-D-1 -thiogalactopyranoside (IPTG) is added to the selective medium to artificially increase the expression of the ampr gene and increase transformation efficiency Heat Shock: plating bacteria from ice to agar plates at 370 C
Transformation Competent cells p. GAP plasmid control IPTG Heat Shock Competent Cells are very delicate: keep on ice Bacterial transformation with ligation mixtures is very inefficient (less efficient than transformation with plasmid DNA). Transform with p. GAP plasmid as control Isopropyl b-D-1 -thiogalactopyranoside (IPTG) is added to the selective medium to artificially increase the expression of the ampr gene and increase transformation efficiency Heat Shock: plating bacteria from ice to agar plates at 370 C
 Transformation Quick Guide 61
Transformation Quick Guide 61
 Chapter 7 7: Plasmid Purification 62
Chapter 7 7: Plasmid Purification 62
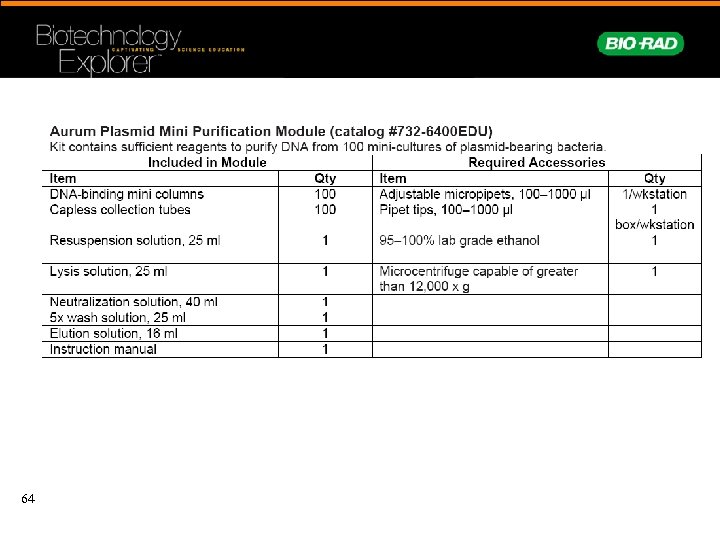
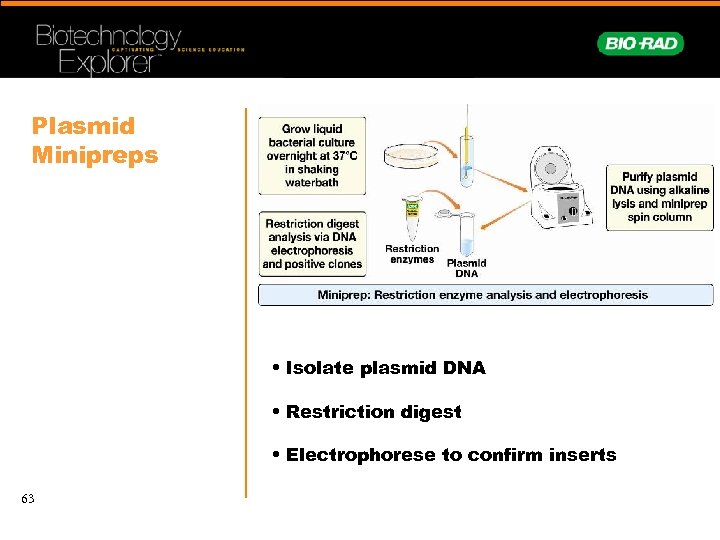 Plasmid Minipreps • Isolate plasmid DNA • Restriction digest • Electrophorese to confirm inserts 63
Plasmid Minipreps • Isolate plasmid DNA • Restriction digest • Electrophorese to confirm inserts 63
 64
64
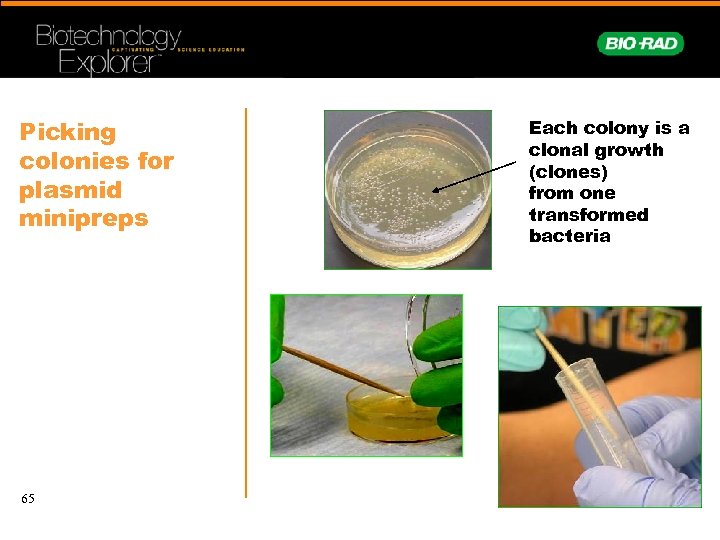 Picking colonies for plasmid minipreps 65 Each colony is a clonal growth (clones) from one transformed bacteria
Picking colonies for plasmid minipreps 65 Each colony is a clonal growth (clones) from one transformed bacteria
 Plasmid Purification and Restriction Digest Analysis Quick Guide 66
Plasmid Purification and Restriction Digest Analysis Quick Guide 66
 67 Optional: It is recommended that 5 µl of undigested DNA also be run next to your digested samples. Prepare these samples by combining 5 µl of miniprep DNA with 5 µl of sterile water.
67 Optional: It is recommended that 5 µl of undigested DNA also be run next to your digested samples. Prepare these samples by combining 5 µl of miniprep DNA with 5 µl of sterile water.
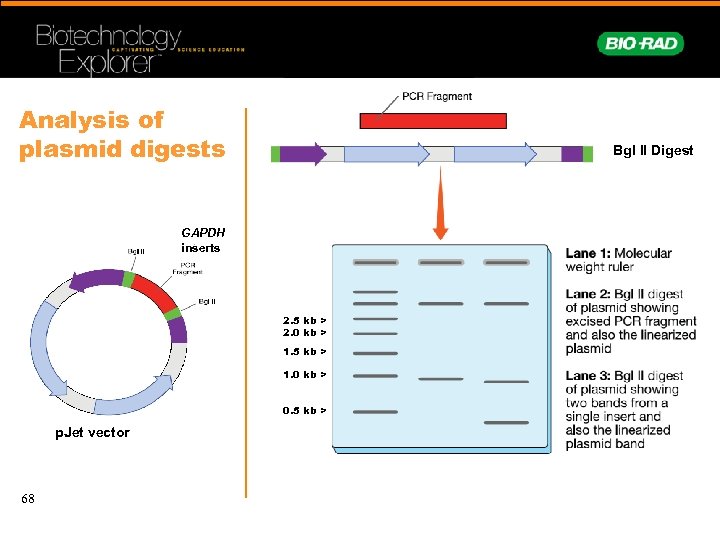 Analysis of plasmid digests Bgl II Digest GAPDH inserts 2. 5 kb > 2. 0 kb > 1. 5 kb > 1. 0 kb > 0. 5 kb > p. Jet vector 68
Analysis of plasmid digests Bgl II Digest GAPDH inserts 2. 5 kb > 2. 0 kb > 1. 5 kb > 1. 0 kb > 0. 5 kb > p. Jet vector 68
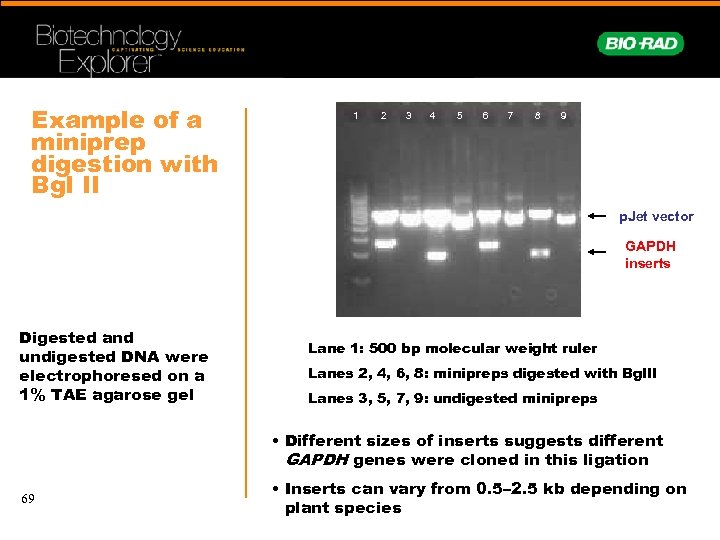 Example of a miniprep digestion with Bgl II 1 2 3 4 5 6 7 8 9 p. Jet vector GAPDH inserts Digested and undigested DNA were electrophoresed on a 1% TAE agarose gel Lane 1: 500 bp molecular weight ruler Lanes 2, 4, 6, 8: minipreps digested with Bgl. II Lanes 3, 5, 7, 9: undigested minipreps • Different sizes of inserts suggests different GAPDH genes were cloned in this ligation 69 • Inserts can vary from 0. 5– 2. 5 kb depending on plant species
Example of a miniprep digestion with Bgl II 1 2 3 4 5 6 7 8 9 p. Jet vector GAPDH inserts Digested and undigested DNA were electrophoresed on a 1% TAE agarose gel Lane 1: 500 bp molecular weight ruler Lanes 2, 4, 6, 8: minipreps digested with Bgl. II Lanes 3, 5, 7, 9: undigested minipreps • Different sizes of inserts suggests different GAPDH genes were cloned in this ligation 69 • Inserts can vary from 0. 5– 2. 5 kb depending on plant species
 Chapter 8 8: Sequencing 70
Chapter 8 8: Sequencing 70
 71
71
 Sequencing • Primers, control plasmid and barcoded 96 well plate are included. Actual sequencing service is not included with the purchase of the series. • Bio-Rad Laboratories has been working with Eurofins MWG/Operon which is a company that offers sequencing services around the world. We have been able to partner with Eurofins MWG/Operon so that they can offer a highly discounted rate. The benefit of being able to utilize the discounted services of Eurofins MWG/Operon is that their turnaround time is 1 -2 days and as an ISO 9001 company they adhere to specific quality standards. To contact Eurofins MWG/Operon please visit www. operon. com/bio-rad or call (800)6882248. • Alternate options for sequencing would include local universities and other professional sequencing services.
Sequencing • Primers, control plasmid and barcoded 96 well plate are included. Actual sequencing service is not included with the purchase of the series. • Bio-Rad Laboratories has been working with Eurofins MWG/Operon which is a company that offers sequencing services around the world. We have been able to partner with Eurofins MWG/Operon so that they can offer a highly discounted rate. The benefit of being able to utilize the discounted services of Eurofins MWG/Operon is that their turnaround time is 1 -2 days and as an ISO 9001 company they adhere to specific quality standards. To contact Eurofins MWG/Operon please visit www. operon. com/bio-rad or call (800)6882248. • Alternate options for sequencing would include local universities and other professional sequencing services.
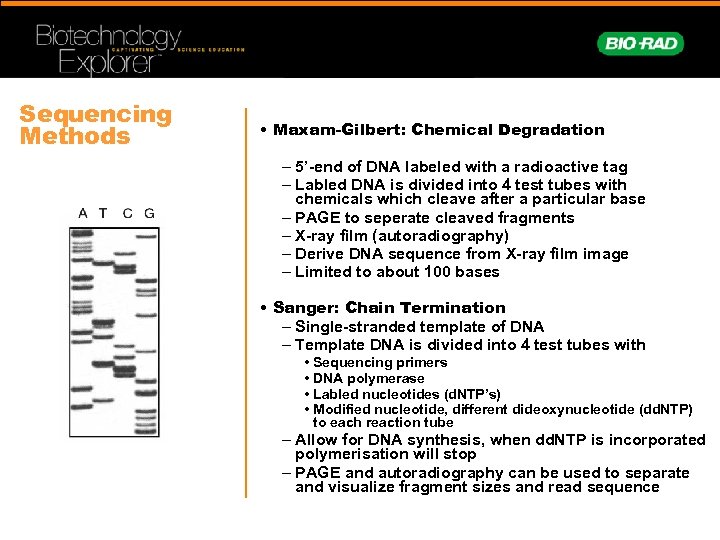 Sequencing Methods • Maxam-Gilbert: Chemical Degradation – 5’-end of DNA labeled with a radioactive tag – Labled DNA is divided into 4 test tubes with chemicals which cleave after a particular base – PAGE to seperate cleaved fragments – X-ray film (autoradiography) – Derive DNA sequence from X-ray film image – Limited to about 100 bases • Sanger: Chain Termination – Single-stranded template of DNA – Template DNA is divided into 4 test tubes with • Sequencing primers • DNA polymerase • Labled nucleotides (d. NTP’s) • Modified nucleotide, different dideoxynucleotide (dd. NTP) to each reaction tube – Allow for DNA synthesis, when dd. NTP is incorporated polymerisation will stop – PAGE and autoradiography can be used to separate and visualize fragment sizes and read sequence
Sequencing Methods • Maxam-Gilbert: Chemical Degradation – 5’-end of DNA labeled with a radioactive tag – Labled DNA is divided into 4 test tubes with chemicals which cleave after a particular base – PAGE to seperate cleaved fragments – X-ray film (autoradiography) – Derive DNA sequence from X-ray film image – Limited to about 100 bases • Sanger: Chain Termination – Single-stranded template of DNA – Template DNA is divided into 4 test tubes with • Sequencing primers • DNA polymerase • Labled nucleotides (d. NTP’s) • Modified nucleotide, different dideoxynucleotide (dd. NTP) to each reaction tube – Allow for DNA synthesis, when dd. NTP is incorporated polymerisation will stop – PAGE and autoradiography can be used to separate and visualize fragment sizes and read sequence
 Setting up Sequencing Reactions • Add sequencing primers to DNA • Load 96 -well plate • Send sealed plate off to chosen sequencing facility for sequencing 74
Setting up Sequencing Reactions • Add sequencing primers to DNA • Load 96 -well plate • Send sealed plate off to chosen sequencing facility for sequencing 74
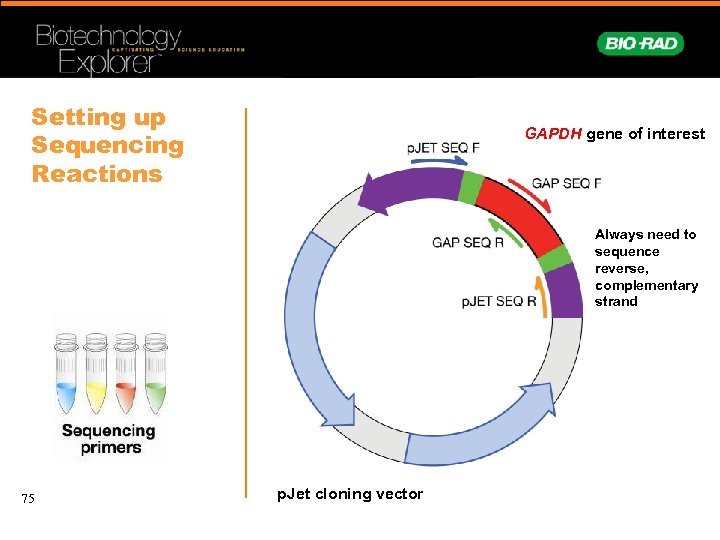 Setting up Sequencing Reactions GAPDH gene of interest Always need to sequence reverse, complementary strand 75 p. Jet cloning vector
Setting up Sequencing Reactions GAPDH gene of interest Always need to sequence reverse, complementary strand 75 p. Jet cloning vector
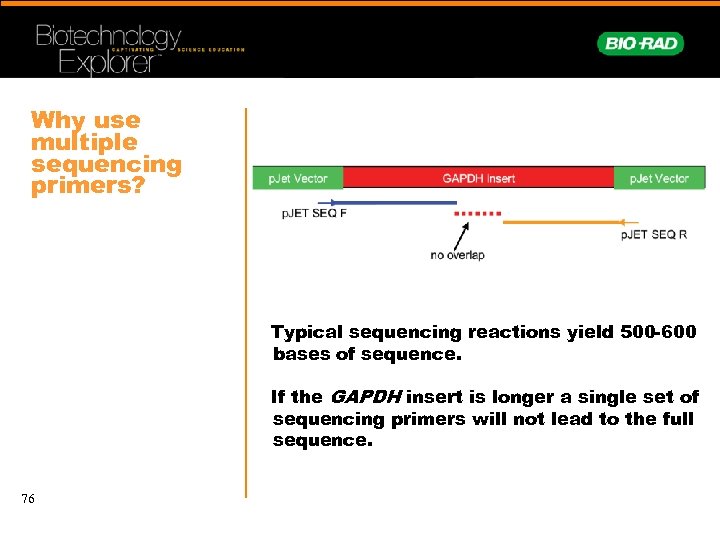 Why use multiple sequencing primers? Typical sequencing reactions yield 500 -600 bases of sequence. If the GAPDH insert is longer a single set of sequencing primers will not lead to the full sequence. 76
Why use multiple sequencing primers? Typical sequencing reactions yield 500 -600 bases of sequence. If the GAPDH insert is longer a single set of sequencing primers will not lead to the full sequence. 76
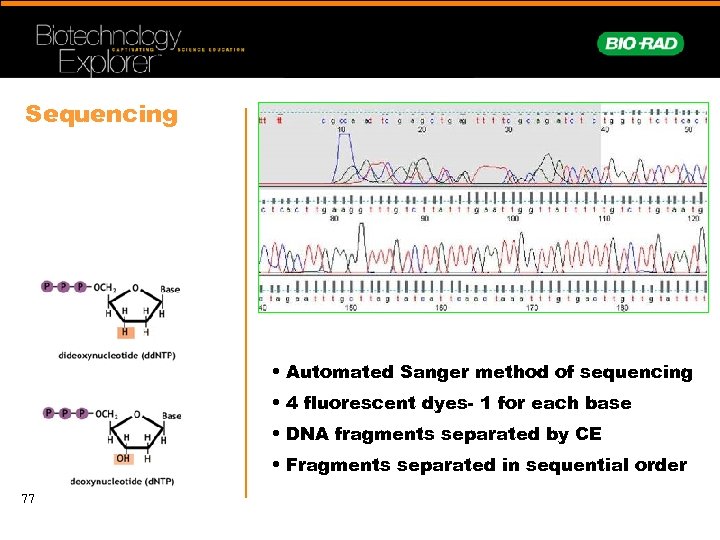 Sequencing • Automated Sanger method of sequencing • 4 fluorescent dyes- 1 for each base • DNA fragments separated by CE • Fragments separated in sequential order 77
Sequencing • Automated Sanger method of sequencing • 4 fluorescent dyes- 1 for each base • DNA fragments separated by CE • Fragments separated in sequential order 77
 Chapter 9 9: Bioinformatics 78
Chapter 9 9: Bioinformatics 78
 Bioinformatics • Two month subscription to genetic analysis software from Geospiza • Data is stored on i. Finch server • Data can be accessed 24/7 79
Bioinformatics • Two month subscription to genetic analysis software from Geospiza • Data is stored on i. Finch server • Data can be accessed 24/7 79


