680dd9b6f59b6fa40f2d904db3bb2806.ppt
- Количество слайдов: 79

1

Blood Components & Plasma derivatives Ahmad Sh. Silmi Msc, FIBMS Clinical Hematologist & Immunologist IUG 2

Introduction Whole Blood (WB) • Collected directly from donors into blood transfusion bag containing anticoagulant • 500 ml transfusion bag is used (contains 63 ml of anticoagulant + 450 ml blood) 3

Anticoagulants in blood units 1) Acid-Citrate-Dextrose (ACD) 2) Citrate-Phosphate- Dextrose (CPD) 3) Citrate, Phosphate, Dextrose, Adenine (CPDA-1) 4
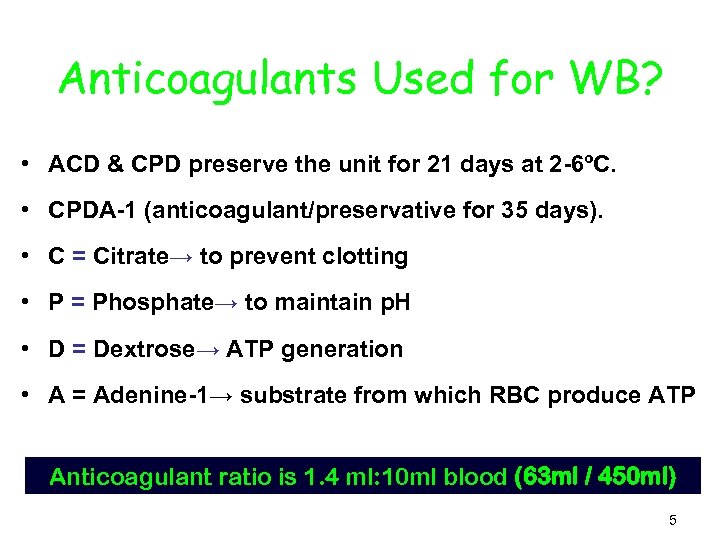
Anticoagulants Used for WB? • ACD & CPD preserve the unit for 21 days at 2 -6ºC. • CPDA-1 (anticoagulant/preservative for 35 days). • C = Citrate→ to prevent clotting • P = Phosphate→ to maintain p. H • D = Dextrose→ ATP generation • A = Adenine-1→ substrate from which RBC produce ATP Anticoagulant ratio is 1. 4 ml: 10 ml blood (63 ml / 450 ml) 5

Additive solutions • (SAGM) → Saline-Adenine-Glucose- Manitol • Purpose of additive solution, to improve RBCs storage viability till 42 days @ 2 -6ºC * Added only to PRBCs 6
Blood Components • Human blood consists of plasma, in which cells are suspended • The plasma also contains other specialised substances, which are important for blood clot formation (e. g. clotting factors) • Whole blood can be separated at the blood bank into various components 7
BLOOD COMPONENTS • Blood separated into different parts: 1. 2. 3. 4. 5. 6. 7. • Packed red cells Platelets Fresh frozen plasma Cryoprecipitate Granulocytes Factor IX conc. Factor VIII conc. There are more than 20 different products available 8
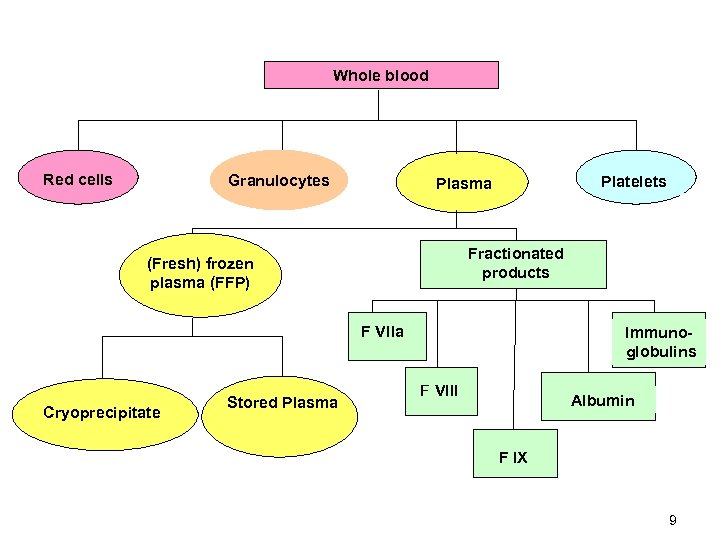
Whole blood Red cells Granulocytes Platelets Plasma Fractionated products (Fresh) frozen plasma (FFP) F Vlla Cryoprecipitate Stored Plasma Immunoglobulins F Vlll Albumin F l. X 9

Blood Components • Refers to a product separated from a single unit of whole blood • The term plasma derivative indicates a blood product separated from a large volume of pooled plasma by a process called fractionation 10

Blood Components • Separating WB into components of blood is necessary to avoid wasting of units. 11

Blood Components Separation Goals • Decrease harmful effects of blood transfusion. • Giving patients specific component needed. • Allow a longer survival for components. • More than one patient will use the unit. 12
Centrifugation Types? There are two types of centrifugation: - • Light spin; (2000 rpm at 20ºC for 11 min) • Heavy spin; (3500 rpm at 20ºC for 11 min) 13
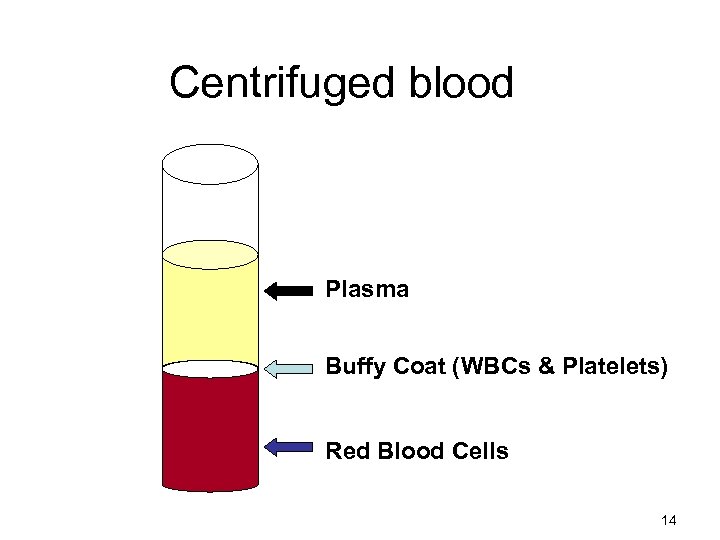
Centrifuged blood Plasma Buffy Coat (WBCs & Platelets) Red Blood Cells 14
Blood Components • Blood components – Oxygen carrying components – Red cell concentrates (RCC) – Leukocyte poor blood – Frozen-thawed red cells – Platelet products – Platelet rich plasma (PRP) – Platelet concentrates (PC) – Plasma products – – Fresh frozen plasma (FFP) Frozen plasma (FP) Cryoprecipitate Stored plasma • Plasma Derivatives – Coagulation Factor concentrates • Factor VIII concentrates • Factor IX complex concentrates & others – Oncotic agents • Albumin • Plasma protein fraction (PPF) – Immune serum Globulin • • Hepatitis B Ig (HBIG) Varicella-zoster Ig (VZIG) Rh Ig (Rh. IG) Tetanus Ig (TIG) 15

A- Blood components that carry oxygen • Increase the oxygen carrying capacity of the blood by increasing the circulating red blood cell mass. • Carry oxygen and nourishment to the tissues and take away carbon dioxide. 16
1 - PRBCs How to make (PRBCs)? § RBCs have higher specific gravity than plasma, it moves to lower portion of the bag by centrifugation § WB (Light spin) Two products: 1) PRBCs 2) Platelet Rich Plasma (PRP) 17
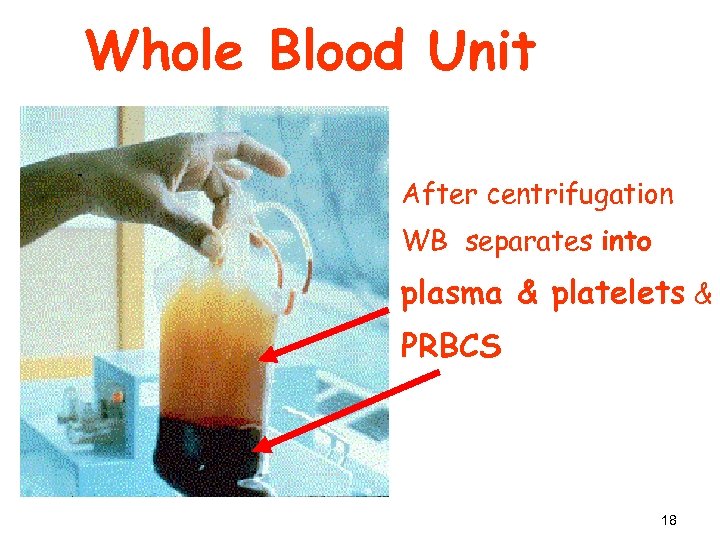
Whole Blood Unit After centrifugation WB separates into plasma & platelets & PRBCS 18
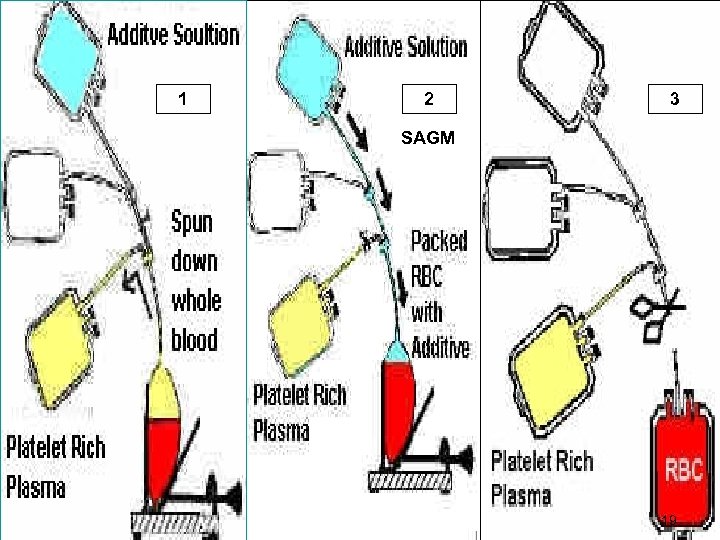
1 2 3 SAGM 19
1 - Red blood cell concentrates • Prepared by removing approx. 200 ml of plasma from whole blood after centrifugation • RBCs plus 100 ml of residual plasma • In CPD-A can be stored for 35 days at 4 o. C 20
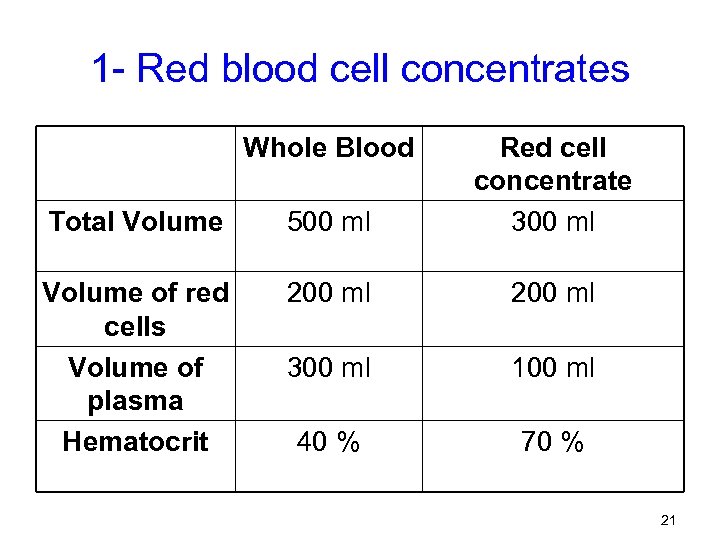
1 - Red blood cell concentrates Whole Blood Total Volume 500 ml Red cell concentrate 300 ml Volume of red cells Volume of plasma Hematocrit 200 ml 300 ml 100 ml 40 % 70 % 21

1 - Red blood cell concentrates • High hematocrit → viscous → infuse slowly • Rate of infusion increased by adding saline • Other fluids should not used – Calcium containing fluids (eg. Ringer’s lactate) should not be added – May cause clotting – Glucose solutions – can cause clumping • Only saline can be added to blood 22

Expiration date! – Once the PRBC unit is “opened” it has a 24 hour expiration date! 24 hours 23
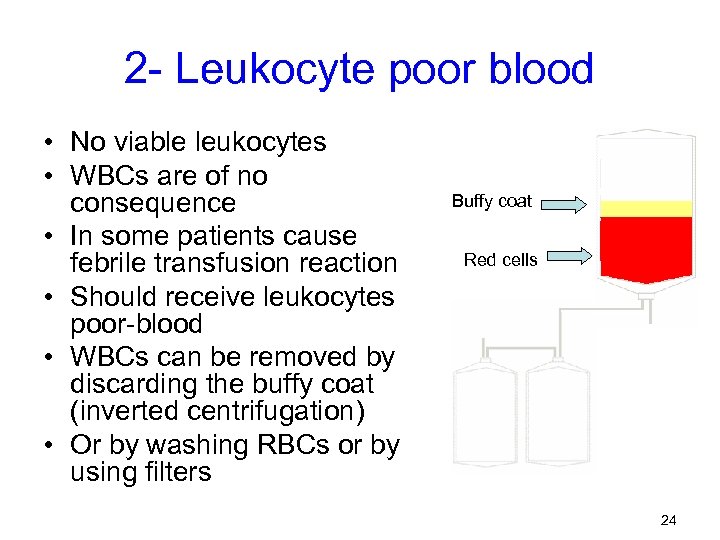
2 - Leukocyte poor blood • No viable leukocytes • WBCs are of no consequence • In some patients cause febrile transfusion reaction • Should receive leukocytes poor-blood • WBCs can be removed by discarding the buffy coat (inverted centrifugation) • Or by washing RBCs or by using filters Buffy coat Red cells 24

Leukocyte Reduction Filters (maintains closed system) http: //www. pall. com/39378_39479. asp Final unit must have less than 5 x 106 WBCs 25

3 - Frozen-thawed red cells • Red cells can be frozen with use of cryopreservation techniques • Permit storage for up to 10 years • Expensive procedure & recommended only in special circumstances – e. g. Individuals with rare blood types – For auto-transfusion 26

3 - Frozen-thawed red cells • The RBC's are first incubated in a 40% glycerol solution which acts as an "antifreeze" within the cells. • The units are then placed in special sterile containers in a deep freezer at less than -60 degrees C. • Cryopreserved units are thawed and washed free of glycerol prior to use as saline suspended RBC's. 27

3 - Frozen-thawed red cells • Deglycerolized RBCs – RBCs that have had the glycerin removed – Thawed at 37°C – A blood cell processor washes the cells with varying concentrations of saline – Considered “open”, expires in 24 hrs. 28

4 - Washed RBCs • Washed RBCs – Not effective in reducing WBCs – For patients (with anti-Ig. A) that may react with plasma proteins containing Ig. A – Reactions may be allergic, febrile, or anaphylactic 29
5 - Irradiated RBCs • Irradiated RBCs – Prevents T-cell proliferation that may cause transfusion-associated graft versus host disease (GVHD) – GVHD is fatal in 90% of those affected – Used for: • • Donor units from a blood relative HLA-matched donor unit Intrauterine transfusion Immunodeficiency Premature newborns Chemotherapy and irradiation Patients who received marrow or stem cells 30

6 - Synthetic oxygen carrying agents • Synthetic oxygen carrying agents – Perfluorochemical (e. g. Fluosol-DA ) • • Fluorinated hydrocarbons Readily dissolve oxygen Poor soluble in plasma Side effects: – Hypotension – DIC – Chemically modified hemoglobin • Free Hb has a very short half life • Chemically modified to: – increase intravascular survival – and to make it more effective in carrying oxygen 31
B- Platelets • Important in maintaining hemostasis • Help stop bleeding and form a platelet plug (primary hemostasis) • People who need platelets: – Cancer patients – Bone marrow recipients – Postoperative bleeding 32

How platelets are processed • REMEMBER!!! • Requires 2 spins: – Soft – separates RBCs and WBCs from plasma and platelets – Heavy • platelets in platelet rich plasma (PRP) will be forced to the bottom of a satellite bag • 40 -60 m. L of plasma is expelled into another satellite bag, while the remaining bag contains platelet concentrate 33

Preparation of platelet concentrate Plasma RBCs PRP Platelet concentrate 34

B- Platelet Products • Platelet Rich Plasma (PRP) – Gentle centrifugation of whole blood – Supernatant transferred to the 2 nd bag • Platelet Concentrates – Prepared from PRP by a 2 nd centrifugation – Removal of all but 50 ml of plasma – Contain approx. 6 X 1010 platelets – 60 – 80% Plts present in whole blood unit – Remain 5 days – Longer at 22 o. C with continuous agitation 35
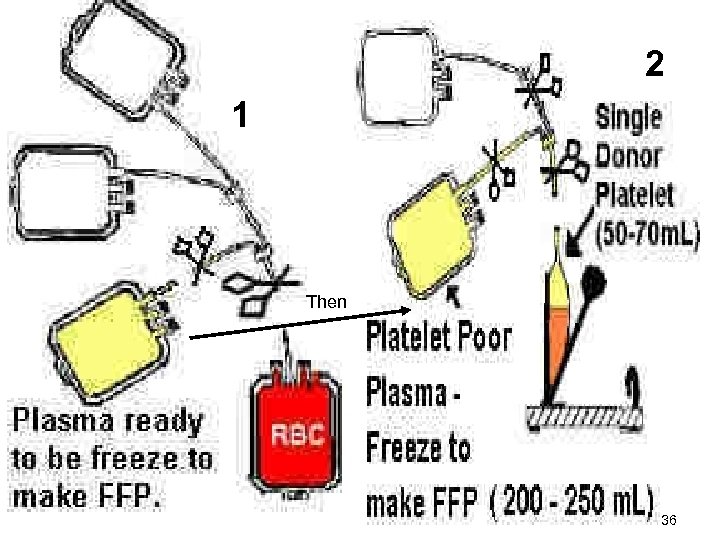
2 1 Then 36

Whole blood unit Centrifuge using LIGHT spin Express Platelets Rich Plasma (PRP) into satellite bag Take PRP and centrifuge again now using HEAVY spin Express PPP into satellite bag & freeze at -18ºC Final products : PRBCs, Platelets concentrate, FFP 37
B- Platelet Products • Contamination by WBCs & RBCs is usually small • But there is enough to induce alloimmunization • Plt concentrates from Rh +ve should not be administered to Rh –ve women • Storage at 22 o. C, therefore care to prevent contamination 38

C- Plasma Products • Plt poor plasma can be separated into a number of products – Fresh frozen plasma – Platelet concentrate – Frozen plasma – Cryoprecipitate – Stored plasma 39

1 - Fresh frozen plasma (FFP) • Prepared from whole blood within 6 hours of collection • Rapid freezing of plasma preserves the labile coagulation factors at maximum levels • Don't contain cellular elements • 200 ml volume 40

1 - Fresh frozen plasma (FFP) Freeze at -18ºC for 1 year from collection date. Or freeze at -70ºC for up to 7 yrs Cross match is not required, but of coarse should be ABO compatible. 41
Indications of FFP • Liver disease • Severe burns • Provides coagulation factors for – – – – Bleeding Abnormal clotting due to massive transfusion Patients on warfarin who are bleeding Treatment of TTP and HUS Factor deficiencies ATIII deficiency DIC when fibrinogen is <100 mg/d. L 42
43
2 - Platelets Concentrate (PC) How to prepare PC? Platelet Rich Plasma (PRP) centrifuged using (heavy spin), this will produce: 1) Fresh frozen plasma (FFP) 2) Platelets concentrate (PC) • PC are stored at room temperature on platelet agitator (prevent platelets clumping) • PC stored for 5 days at 20 -24°C. • Each unit should elevate the platelet count by 5000/µL 44
2 - Platelets concentrate • Indications: 1. To prevent bleeding due to thrombocytopenia or platelet dysfunction 2. To a patient undergoing an operation, if the platelet count is less than 20, 000/µL 45

1 2 3 46

Platelet concentrate 47

3 - Frozen Plasma (FP) • Separated from whole blood within 24 hours of collection • Contains at least 50 % of original factor VIII & factor V frozen plasma • Adequate source for treatment of mild to moderate coagulation factor deficiencies • 200 ml volume • Storage at -30 o. C for up to 12 months 48
4 - Cryoprecipitate • Produced from freshly separated plasma by freezing at -70 o. C followed by thawing at 4 o. C • Flocculent precipitate is rich in factor VIII, fibrinogen and fibronectin • Once thawed, mixture is centrifuged to sediment the cryoprecipitate & all but 5 to 10 ml of supernatant plasma is removed • Contains 250 mg fibrinogen • 80 clotting units of factor VIII • Stored at -30 o. C for 12 months 49

4 - Cryoprecipitate • Increase of 2% of factor VIII level for each bag of cryoprecipitate infused • Supernatant plasma removed is called stored plasma – Must be used within 5 weeks if stored at 4 o. C – Lasts for 2 years at -30 o. C 50

Cryo. . Indications: Hemophilia A Von Willebrand disease (VWD) Congenital or acquired fibrinogen defects (i. e. , dysfibrinogenemia) 51

5 - Stored plasma • Plasma separated from whole blood after 24 hours of storage at 4 o. C • Can also be derived from cryoprecipitate production • Contain reduced levels of labile coagulation factors V VIII & fibrinogen • It is indicated for patients requiring volume expansion or protein replacement when labile clotting factors are not required • Plasma products do not require crossmatch prior to use but should be ABO compatible 52

Summary Blood Components Blood Component Centrifugation 1) PRBCs WB Light spin= 2) PC PRP heavy spin= 3) FFP 53 2000 rpm-20ºC -11 min. PRBCs + PRP 3500 rpm-20ºC -11 min. PC + FFP Storage Indication Temp Time 2 -6ºC +SAGM 42 d • Anemia • Newborn exchange transfusion R. T -18ºC -65ºC 3 -5 d • Bleeding • Operation if plt. Less than 20000/μl • Clotting factor 7 years deficiencies • Severe burns 1 year

Summary Blood Components Blood Comp Centrifugation Storage indication Temp Time 4) Cryo a. WB special heavy spin= 3500 rpm at 4ºC - -30ºC 1 year 11 min. RBC + Plasma b. Plasma store at -18 ºC then thaw at 4 ºC then heavy spin at 4ºC 54 • Hemophilia A • Von Willebrand disease

Plasma Derivatives 55

Plasma Derivatives • Certain plasma derivatives can be obtained by fractionating the fresh frozen plasma or stored plasma • Fractionation: § Allows the processing of large volumes of pooled plasma § Pooling of many units increases the risk of viral transmission to the recipient 56
Plasma protein fractionation • Plasma proteins are separated according to differences of each protein. • Fractionation involves changing the conditions of the pooled plasma (e. g. the temperature or the acidity) • Proteins that are normally dissolved in the plasma fluid become insoluble, forming large clumps, called precipitate. • The insoluble protein can be collected by centrifugation. 57
Plasma protein fractionation • One of the very effective ways for carrying out this process is the addition of alcohol to the plasma pool while simultaneously cooling the pool. • This process is sometimes called cold alcohol fractionation or ethanol fractionation. • This procedure is carried out in a series of steps so that a single pool of plasma yields several different protein products, such as albumin and immune globulin. 58
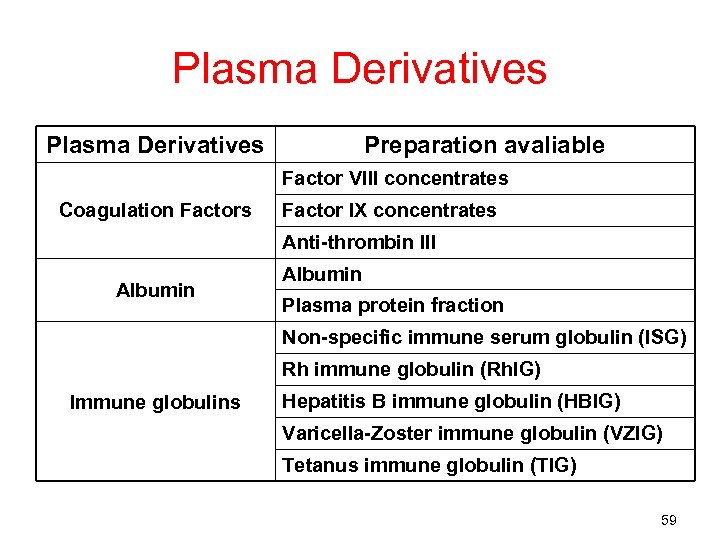
Plasma Derivatives Preparation avaliable Factor VIII concentrates Coagulation Factors Factor IX concentrates Anti-thrombin III Albumin Plasma protein fraction Non-specific immune serum globulin (ISG) Rh immune globulin (Rh. IG) Immune globulins Hepatitis B immune globulin (HBIG) Varicella-Zoster immune globulin (VZIG) Tetanus immune globulin (TIG) 59

1 - Coagulation Factor Concentrates • Prepared in a freeze-dried form • Indicated for patients with congenital coagulation deficiencies – Risk of hepatitis is high • Should not used for mild acquired coagulation deficiencies – Should be treated with FP or FFP 60

Factor VIII Concentrate • Commercially prepared, lyophilized powder purified from human FFP • Contain also small amounts of fibrinogen & other proteins • Can contain blood group Abs • Treat patients with hemophilia A 61
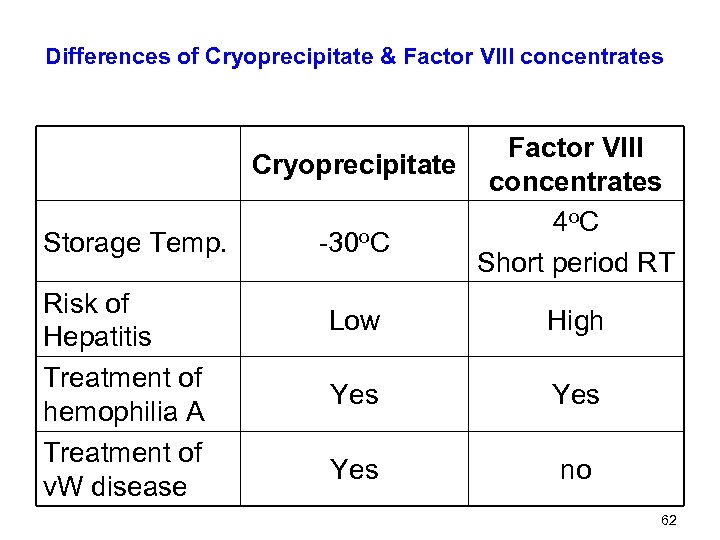
Differences of Cryoprecipitate & Factor VIII concentrates Factor VIII Cryoprecipitate concentrates 4 o. C Storage Temp. -30 o. C Short period RT Risk of Low High Hepatitis Treatment of Yes hemophilia A Treatment of Yes no v. W disease 62

Factor IX Concentrate • For the treatment Factor IX deficiency or Hemophilia B (Christmas Disease). • Have been used to treat patients with acquired inhibitors of factor VIII – Have factor VIII bypassing activity • Contains also factors II, VII & X in concentrated form • Vials containing 500 units of factor IX 63

Factor IX Concentrate & liver disease • It is contraindicated in patients with liver disease – Have low levels of circulating antithrombin III – Activation of clotting factors present in some factor IX concentrates, – cause DIC 64

Blood products & treatment of specific clotting factor deficiencies Deficiency Fibrinogen Factor VIII Blood product Indicated Cryoprecipitate Stored plasma Fresh frozen plasma Factor IX concentrate Stored plasma Factor VIII concentrate Cryoprecipitate Von Willebrand’s Disease Fresh frozen plasma Factor IX concentrate Factor X Stored plasma Factor XIII Stored plasma 65

2 - Oncotic Agents • Albumin: volume expansion • Other colloids are available for blood volume expansion – Dextran – Gelatin – Hydroxyethyl starch – Polyvinylpyrrolidone 66

Albumin • Albumin is prepared by ethanol fractionation of pooled plasma • Available in 5% and 25% concentrations. • Have physiological sodium content • No risk of hepatitis, sterilized during preparation • No coagulation factors or blood group Abs 67

Albumin • Used for treatment of hypovolaemia and hypoalbuminaemia (result from abnormal synthesis, increased metabolism or loss) • It maintains capillary osmotic pressure • Carrier protein for drugs, hormones, enzymes & metabolites 68

Plasma protein Fraction • Partially purified albumin • Contains ≈ 85% albumin & 15% other plasma proteins 69

3 - Immune Globulins • Contains immune Ig. G antibodies, prepared from pools of plasma. • For disease prophylaxis, hepatitis A, measles, varicella and rubella. • For the treatment of hypogammaglobulinemia and agammaglobulinemia. 70

Immune Serum Globulin (ISG) • • • Primarily Ig. G Ab Prevention of some viral diseases Hypogammaglobulinemia Congenital immune deficiency Given by IM injection (aggregates of Ig. G) 71

Hepatitis B Immune Globulin (HBIG) • Contains Hepatitis B immune antibodies. • From plasma of donors with high titer of Ab to HBs. Ag • Provides passive immunization for HBV. • For treatment after exposure to HBs. Ag. • For the prevention of maternally transferred HBV (perinatal exposure). 72

Varicella-Zoster immune globulin (VZIG) • Derived from patients had recent Herpes Zoster infections • Herpes Zoster infections result in severe fatal infection in immunocompromised individuals • Passive administration of VZIG during 72 hours of exposure can prevent or attenuate infection 73

Rh Immune Globulin (Rh. IG) • Derived from Rh -ve individuals • Contains Ig. G antibodies to the D antigen on red blood cells. • Given during pregnancy and post-natally to Rh negative mothers to prevent the development of anti-D and hemolytic disease of the newborn (HDN) due to anti-D. • Given prophylacticaly following abortion, or invasive maternal procedures (e. g. , amniocentesis). 74

Tetanus Immune Globulin (TIG) • Prepared from individuals specifically immunized for tetanus toxoid • Available for individuals at risk following injury 75

• Changes in stored blood: – Certain percent of RBC are destroyed, in good anticoagulant only 10 -20 % of RBC are destroyed. – Blood become acidic. – Elevation in K concentration which is bad for heart. – Platelets will die within few hours after collection. So there will no Plts. – WBC are deteriorated also (no WBC in old blood). – Activity of coagulation factors will be grossly reduced (VIII, V). 76
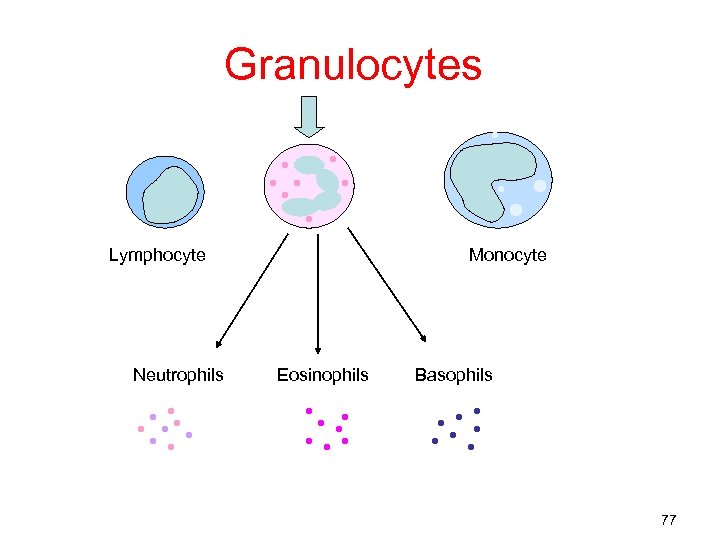
Granulocytes Lymphocyte Neutrophils Monocyte Eosinophils Basophils 77

Granulocytes • Neutrophils are the most numerous, involved in phagocytosis of bacteria/fungi • Although rare, it is useful for infants with bacteremia • Prepared by hemapheresis • ≥ 1. 0 x 1010 • Maintained at room temp for 24 hours 78

79
680dd9b6f59b6fa40f2d904db3bb2806.ppt