7c3e3bfa8087b3920c3f3c9a9c733277.ppt
- Количество слайдов: 69
 1
1
 2
2
 3
3
 RADIATION Energy • in the form of particles • or electromagnetic waves • emitted from the nuclei of unstable atoms 4
RADIATION Energy • in the form of particles • or electromagnetic waves • emitted from the nuclei of unstable atoms 4
 RADIATION § The term really includes all forms of electromagnetic radiation § Radio Waves, Infrared, Visible Light § Ultraviolet, X-rays, -rays § Commonly used today to describe particle radiation 5
RADIATION § The term really includes all forms of electromagnetic radiation § Radio Waves, Infrared, Visible Light § Ultraviolet, X-rays, -rays § Commonly used today to describe particle radiation 5
 NUCLEAR REACTIONS PRODUCE RADIATION § Protons and neutrons determine nuclear reactions § One must understand atomic structure to understand radiation 6
NUCLEAR REACTIONS PRODUCE RADIATION § Protons and neutrons determine nuclear reactions § One must understand atomic structure to understand radiation 6
 NUCLEAR PARTICLES Protons and Neutrons are the two basic nuclear particles. Together they contain practically all the mass of an atom and are determinants of an atom’s nuclear characteristics. 7
NUCLEAR PARTICLES Protons and Neutrons are the two basic nuclear particles. Together they contain practically all the mass of an atom and are determinants of an atom’s nuclear characteristics. 7
 RADIOACTIVE DECAY • Radioactive decay refers to the spontaneous emission of radiation from the nucleus of an unstable atomic nucleus 8
RADIOACTIVE DECAY • Radioactive decay refers to the spontaneous emission of radiation from the nucleus of an unstable atomic nucleus 8
 DEFINITION OF RADIOACTIVE DECAY “Radioactive decay is the process of spontaneous emission of radiation in the form of particles or photons from the nuclei of unstable atoms” 9
DEFINITION OF RADIOACTIVE DECAY “Radioactive decay is the process of spontaneous emission of radiation in the form of particles or photons from the nuclei of unstable atoms” 9
 CHARACTERISTICS OF RADIOACTIVE DECAY • It is a natural process in our universe • It is spontaneous – we cannot predict when an atom will undergo decay 10
CHARACTERISTICS OF RADIOACTIVE DECAY • It is a natural process in our universe • It is spontaneous – we cannot predict when an atom will undergo decay 10
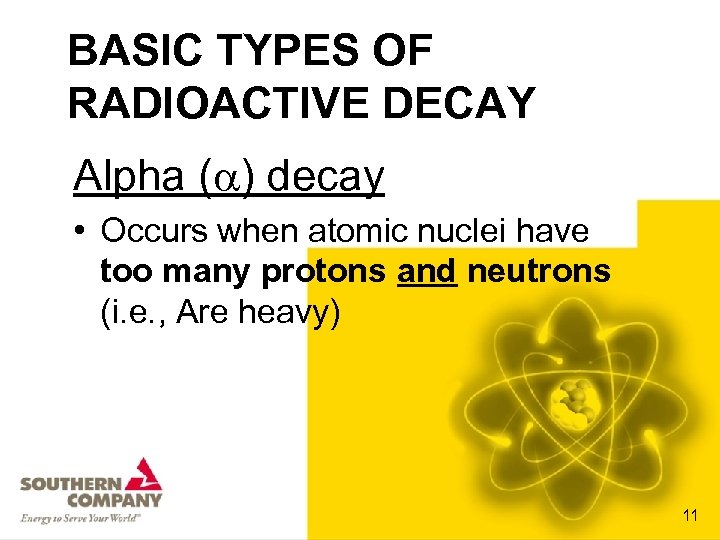 BASIC TYPES OF RADIOACTIVE DECAY Alpha ( ) decay • Occurs when atomic nuclei have too many protons and neutrons (i. e. , Are heavy) 11
BASIC TYPES OF RADIOACTIVE DECAY Alpha ( ) decay • Occurs when atomic nuclei have too many protons and neutrons (i. e. , Are heavy) 11
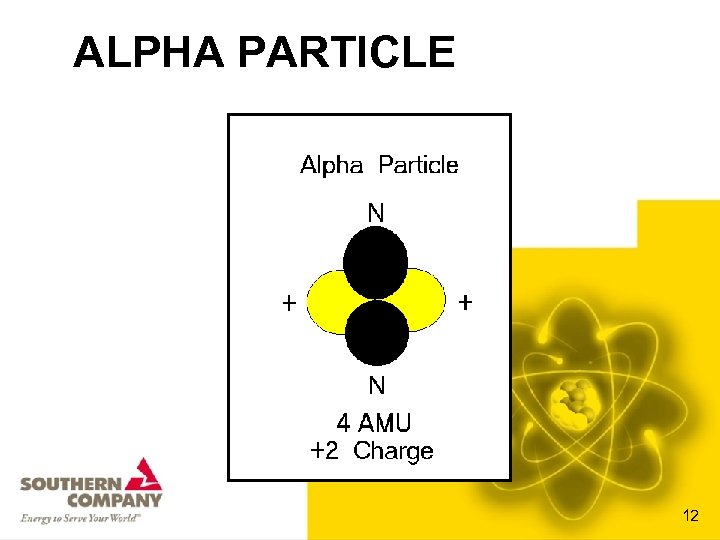 ALPHA PARTICLE 12
ALPHA PARTICLE 12
 CHARACTERISTICS OF ALPHA PARTICLES • • Consist of 2 protons and 2 neutrons Mass of an alpha particle is 4 amu Charge = +2 The isotope’s Atomic Mass goes down four; • The Atomic Number goes down two • Are highly ionizing • Have low penetrating abilities (only cm in air and mm in water) 13
CHARACTERISTICS OF ALPHA PARTICLES • • Consist of 2 protons and 2 neutrons Mass of an alpha particle is 4 amu Charge = +2 The isotope’s Atomic Mass goes down four; • The Atomic Number goes down two • Are highly ionizing • Have low penetrating abilities (only cm in air and mm in water) 13
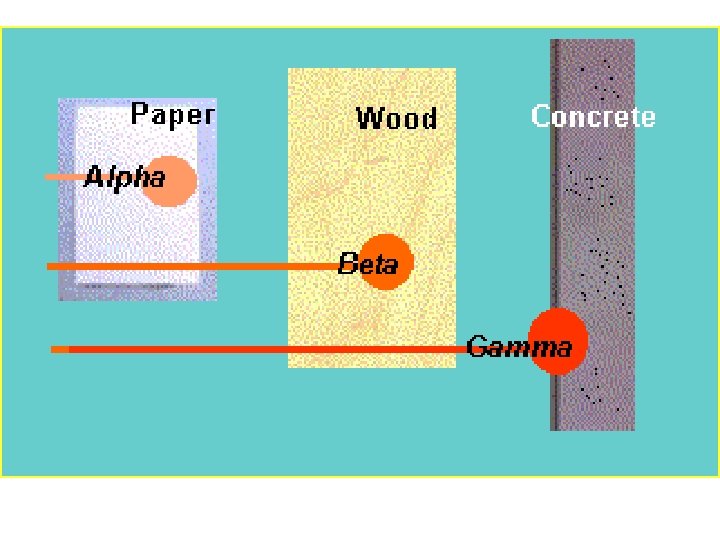
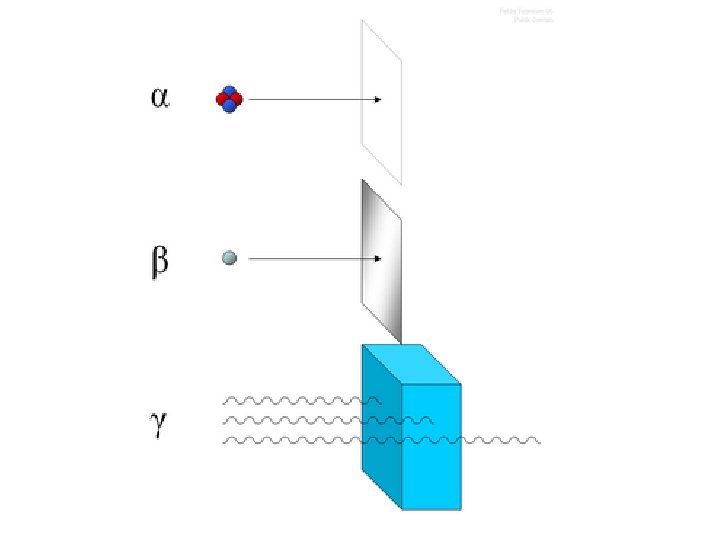
 MORE ABOUT ALPHA PARTICLES • Easily shielded; common types of shielding are paper, cardboard, air, clothing; will not penetrate skin • Health hazard when taken internally • Not commonly used in medicine • Common sources = smoke detectors (Am-241) and lantern mantles (thorium nitrate) 14
MORE ABOUT ALPHA PARTICLES • Easily shielded; common types of shielding are paper, cardboard, air, clothing; will not penetrate skin • Health hazard when taken internally • Not commonly used in medicine • Common sources = smoke detectors (Am-241) and lantern mantles (thorium nitrate) 14
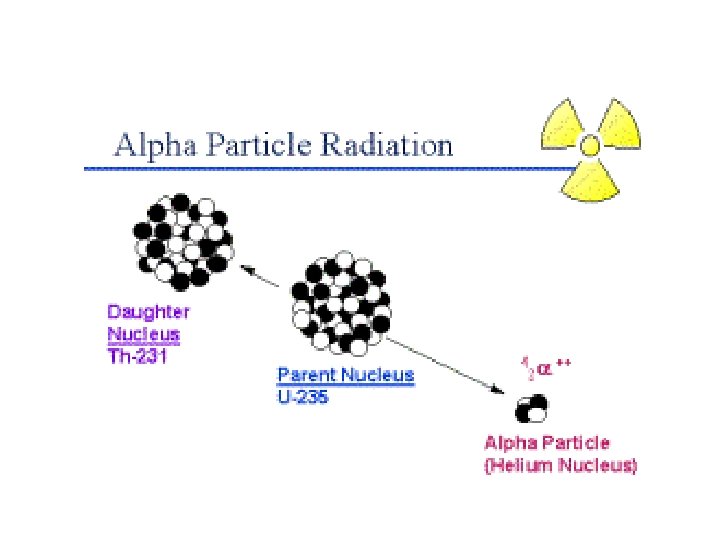
 ALPHA PARTICLE DECAY • Changes both the mass and identity of the nucleus of the parent radionuclide • This means that the decay results in the formation of a new element as the daughter product 15
ALPHA PARTICLE DECAY • Changes both the mass and identity of the nucleus of the parent radionuclide • This means that the decay results in the formation of a new element as the daughter product 15
 QUESTIONS? ? 16
QUESTIONS? ? 16
 NEGATIVE BETA (ß-) DECAY Occurs when atoms have too many neutrons (i. e. , Are “neutron-rich”) and decay by emitting a negative beta particle (ß-) 17
NEGATIVE BETA (ß-) DECAY Occurs when atoms have too many neutrons (i. e. , Are “neutron-rich”) and decay by emitting a negative beta particle (ß-) 17
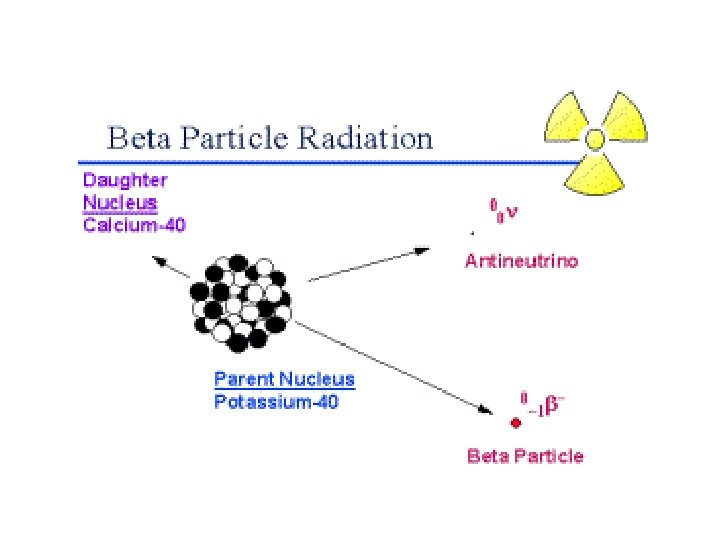
 WHAT ARE NEGATIVE BETA PARTICLES? During negative beta decay, neutrons are converted into protons and electrons. The protons remain in the nucleus but the new electrons are emitted as negative beta particles (ß-) or negatrons. You may wish to think of them as “nuclear electrons. ” 18
WHAT ARE NEGATIVE BETA PARTICLES? During negative beta decay, neutrons are converted into protons and electrons. The protons remain in the nucleus but the new electrons are emitted as negative beta particles (ß-) or negatrons. You may wish to think of them as “nuclear electrons. ” 18
 CHARACTERISTICS OF NEGATIVE BETA DECAY • Less ionizing than alphas due to decreased mass of negatrons • Changes the identity of the nucleus but not the mass • The Atomic Number is increased by one due to conversion of neutrons into protons 19
CHARACTERISTICS OF NEGATIVE BETA DECAY • Less ionizing than alphas due to decreased mass of negatrons • Changes the identity of the nucleus but not the mass • The Atomic Number is increased by one due to conversion of neutrons into protons 19
 CHARACTERISTICS OF NEGATIVE BETA PARTICLES (NEGATRONS) § Negatrons consist of nuclear electrons § The mass is the same as electrons § There is a charge of – 1 in negatrons § More penetrating than alpha particles; ~ 12 meters in air § They can penetrate skin– best shielding is wood, plastics, thick cardboard, etc. 20
CHARACTERISTICS OF NEGATIVE BETA PARTICLES (NEGATRONS) § Negatrons consist of nuclear electrons § The mass is the same as electrons § There is a charge of – 1 in negatrons § More penetrating than alpha particles; ~ 12 meters in air § They can penetrate skin– best shielding is wood, plastics, thick cardboard, etc. 20
 QUESTIONS? ? 24
QUESTIONS? ? 24
 GAMMA ( ) EMISSION Is a form of pure electromagnetic radiation emitted from nuclei that have excess energy. It is sometimes called gamma photon radiation. 25
GAMMA ( ) EMISSION Is a form of pure electromagnetic radiation emitted from nuclei that have excess energy. It is sometimes called gamma photon radiation. 25
 GAMMA RAYS Are photons emitted from unstable nuclei to rid themselves of excess energy. Gamma photons are subatomic packets of pure energy. They are higher in energy and more penetrating than the photons that make up visible light. 26
GAMMA RAYS Are photons emitted from unstable nuclei to rid themselves of excess energy. Gamma photons are subatomic packets of pure energy. They are higher in energy and more penetrating than the photons that make up visible light. 26
 PROPERTIES OF GAMMA ( ) RAYS § Charge is 0 (no charge) § Mass is 0 (no mass) § Low ionization § Penetration abilities can be extremely high; – penetrating power is dependent upon the energy of the emitted photons 28
PROPERTIES OF GAMMA ( ) RAYS § Charge is 0 (no charge) § Mass is 0 (no mass) § Low ionization § Penetration abilities can be extremely high; – penetrating power is dependent upon the energy of the emitted photons 28
 QUESTIONS? ? 29
QUESTIONS? ? 29
 What is a “packet” of light energy that behaves like a particle? 1. 2. 3. 4. Positron Negatron Megatron Photon
What is a “packet” of light energy that behaves like a particle? 1. 2. 3. 4. Positron Negatron Megatron Photon
 Which form of radiation penetrates the least? 1. 2. 3. 4. Alpha Decay Beta Decay Gamma Decay Delta Decay
Which form of radiation penetrates the least? 1. 2. 3. 4. Alpha Decay Beta Decay Gamma Decay Delta Decay
 Which radioactive particle increases the Parent Nucleus’s atomic number? 1. 2. 3. 4. Alpha Particle Beta Particle Gamma Particle Delta Particle
Which radioactive particle increases the Parent Nucleus’s atomic number? 1. 2. 3. 4. Alpha Particle Beta Particle Gamma Particle Delta Particle
 Which form of radiation penetrates the most? 1. 2. 3. 4. Alpha Decay Beta Decay Gamma Decay Delta Decay
Which form of radiation penetrates the most? 1. 2. 3. 4. Alpha Decay Beta Decay Gamma Decay Delta Decay
 Which particle drops the Parent Nucleus’s atomic number by two? 1. 2. 3. 4. Alpha Particle Beta Particle Gamma Particle Delta Particle
Which particle drops the Parent Nucleus’s atomic number by two? 1. 2. 3. 4. Alpha Particle Beta Particle Gamma Particle Delta Particle
 Which particle resembles a Helium nucleus? 1. 2. 3. 4. Alpha Particle Beta Particle Gamma Particle Delta Particle
Which particle resembles a Helium nucleus? 1. 2. 3. 4. Alpha Particle Beta Particle Gamma Particle Delta Particle
 Which particle isn’t a particle but a photon? 1. 2. 3. 4. Alpha Particle Beta Particle Gamma Particle Delta Particle
Which particle isn’t a particle but a photon? 1. 2. 3. 4. Alpha Particle Beta Particle Gamma Particle Delta Particle
 5 • End of Part 1
5 • End of Part 1
 Turn to page 5 • the first answer you should have for box set #3 is:
Turn to page 5 • the first answer you should have for box set #3 is:
 Turn to page 6 • the last answer you should have for box set # 12 is:
Turn to page 6 • the last answer you should have for box set # 12 is:
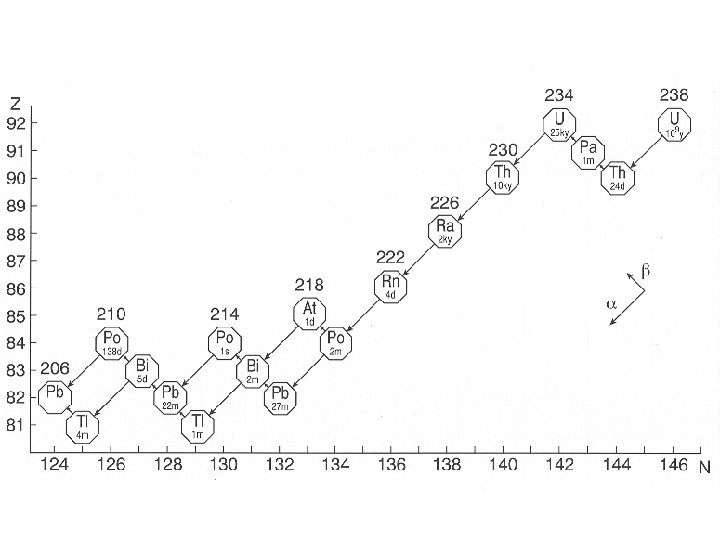
 Decay Systems • Each radioactive element will undergo various forms of radiation until it becomes stable • The particular elements that a “Parent Nucleus” changes into are always the same • This “path” is a Decay System
Decay Systems • Each radioactive element will undergo various forms of radiation until it becomes stable • The particular elements that a “Parent Nucleus” changes into are always the same • This “path” is a Decay System
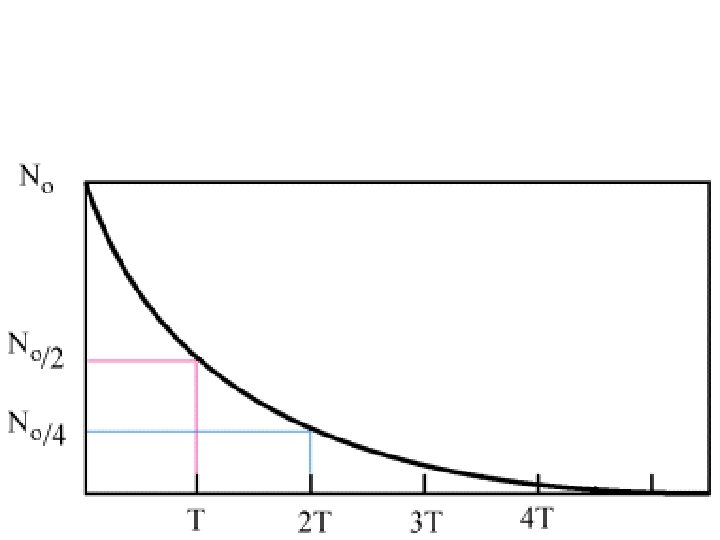
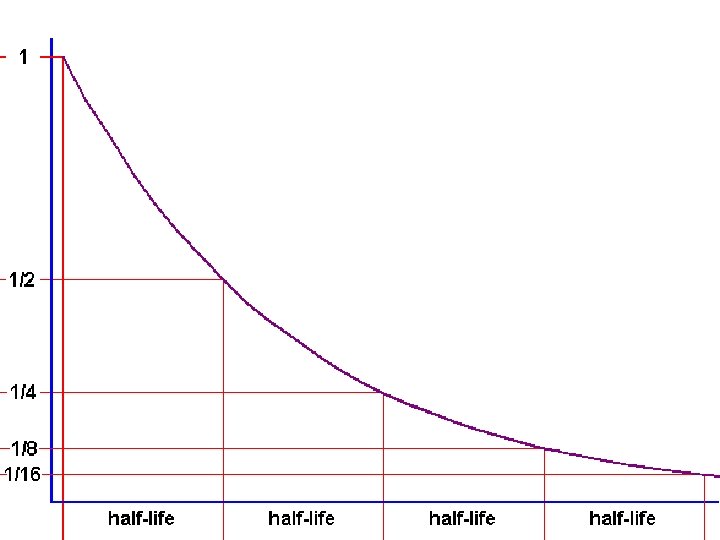
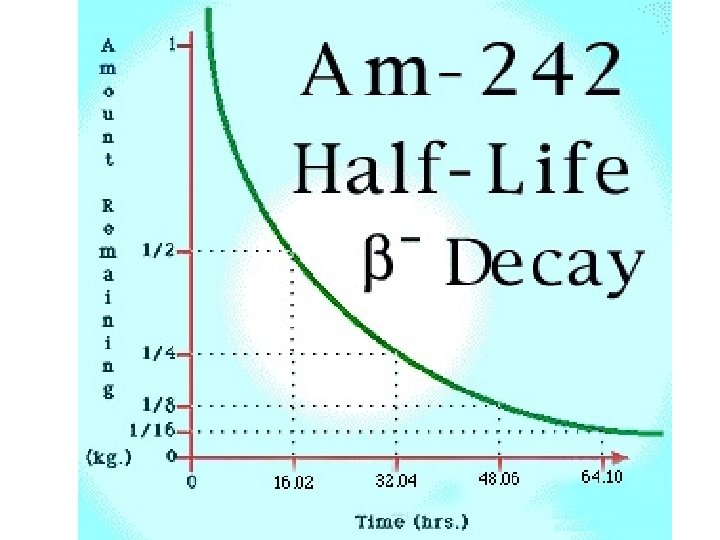
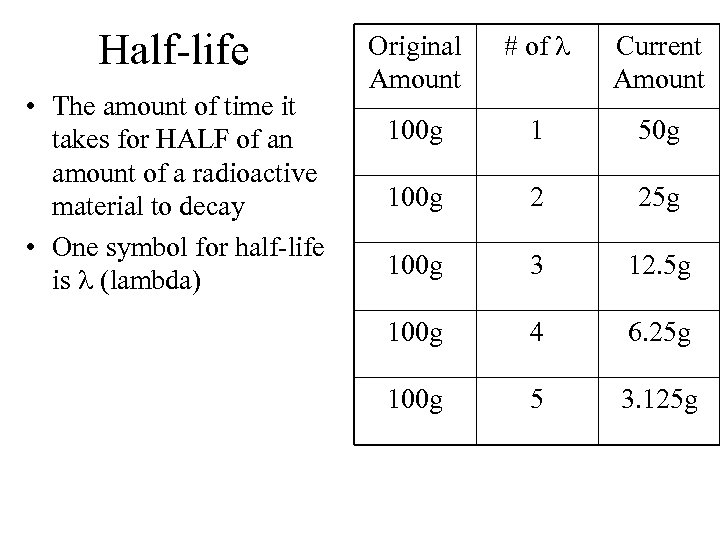 Half-life • The amount of time it takes for HALF of an amount of a radioactive material to decay • One symbol for half-life is (lambda) Original Amount # of Current Amount 100 g 1 50 g 100 g 2 25 g 100 g 3 12. 5 g 100 g 4 6. 25 g 100 g 5 3. 125 g
Half-life • The amount of time it takes for HALF of an amount of a radioactive material to decay • One symbol for half-life is (lambda) Original Amount # of Current Amount 100 g 1 50 g 100 g 2 25 g 100 g 3 12. 5 g 100 g 4 6. 25 g 100 g 5 3. 125 g

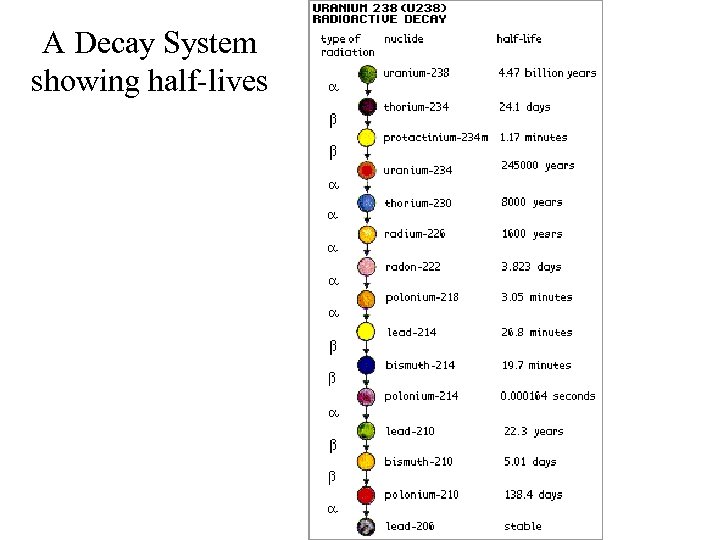 A Decay System showing half-lives
A Decay System showing half-lives
 Practice • 1. An isotope of cesium (cesium-137) has a half-life of 30 yrs. If 1. 0 mg of cesium-137 disintegrates over a period of 90 yrs. , how many mg of cesium-137 would remain? (1) (2) (3) 1. 0 mg 0. 5 mg 0. 25 mg 0. 125 mg 30 yrs 60 yrs 90 yrs
Practice • 1. An isotope of cesium (cesium-137) has a half-life of 30 yrs. If 1. 0 mg of cesium-137 disintegrates over a period of 90 yrs. , how many mg of cesium-137 would remain? (1) (2) (3) 1. 0 mg 0. 5 mg 0. 25 mg 0. 125 mg 30 yrs 60 yrs 90 yrs
 Practice • The half-life of Po-218 is three minutes. How much of a 2. 0 gram sample remains after 15 minutes? (2) (3) (4) (1) 2. 0 g 1. 0 g 0. 5 g 0. 25 g 0. 125 g 0. 0625 g 6 min 9 min 12 min 15 min 3 min
Practice • The half-life of Po-218 is three minutes. How much of a 2. 0 gram sample remains after 15 minutes? (2) (3) (4) (1) 2. 0 g 1. 0 g 0. 5 g 0. 25 g 0. 125 g 0. 0625 g 6 min 9 min 12 min 15 min 3 min
 Practice • 3. A 2. 5 gram sample of an isotope of strontium-90 was formed in a 1960 explosion of an atomic bomb at Johnson Island in the Pacific Test site. The half-life of strontium-90 is 28 yrs. In what year will only 0. 625 grams of this strontium-90 remain? 56 yrs to get to (2) (1) 2. 5 g 1. 25 g 0. 625 g 56 yrs 28 yrs 0. 625 grams… started in 1960… 1960 +56 = 2016
Practice • 3. A 2. 5 gram sample of an isotope of strontium-90 was formed in a 1960 explosion of an atomic bomb at Johnson Island in the Pacific Test site. The half-life of strontium-90 is 28 yrs. In what year will only 0. 625 grams of this strontium-90 remain? 56 yrs to get to (2) (1) 2. 5 g 1. 25 g 0. 625 g 56 yrs 28 yrs 0. 625 grams… started in 1960… 1960 +56 = 2016
 Practice • 5. Sodium-25 was to be used in an experiment, but it took 3. 0 minutes to get the sodium from the reactor to the laboratory. If 5. 0 mg of sodium-25 was removed from the reactor, how many mg of sodium-25 were placed in the reaction vessel 3. 0 minutes later if the half-life of sodium-25 is 60 seconds?
Practice • 5. Sodium-25 was to be used in an experiment, but it took 3. 0 minutes to get the sodium from the reactor to the laboratory. If 5. 0 mg of sodium-25 was removed from the reactor, how many mg of sodium-25 were placed in the reaction vessel 3. 0 minutes later if the half-life of sodium-25 is 60 seconds?
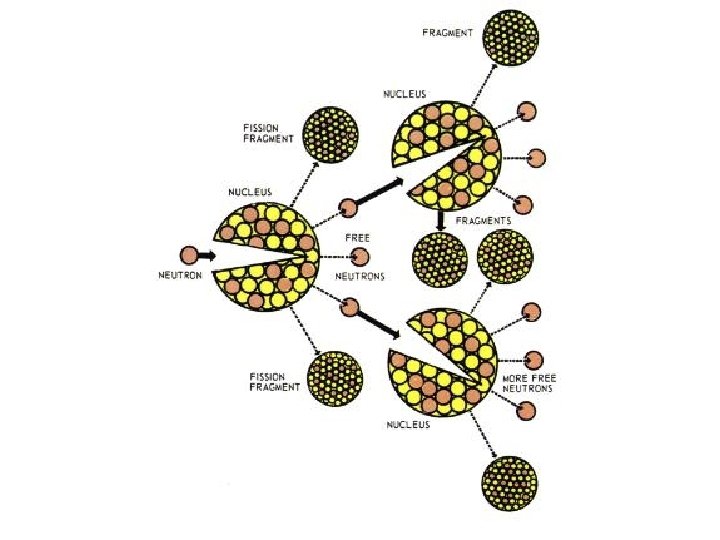
 Nuclear Processes • Fission – The splitting of a large, unstable nucleus into two or more stable nuclei – Scientists can cause fission by injecting a neutron at high speed – These are the uses associated with nuclear fission: • Power plants / reactors • Bombs • “Dirty bombs”
Nuclear Processes • Fission – The splitting of a large, unstable nucleus into two or more stable nuclei – Scientists can cause fission by injecting a neutron at high speed – These are the uses associated with nuclear fission: • Power plants / reactors • Bombs • “Dirty bombs”
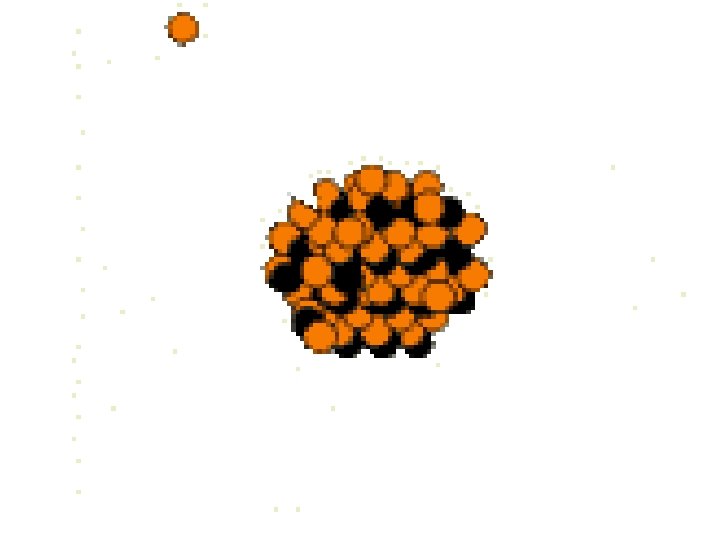
 Nuclear Processes • Fusion – The joining of two or more smaller nuclei into one larger, more stable nucleus – Scientists are working on fusion using high pressures, temperatures, and lasers – The only place that fusion occurs naturally is stars
Nuclear Processes • Fusion – The joining of two or more smaller nuclei into one larger, more stable nucleus – Scientists are working on fusion using high pressures, temperatures, and lasers – The only place that fusion occurs naturally is stars
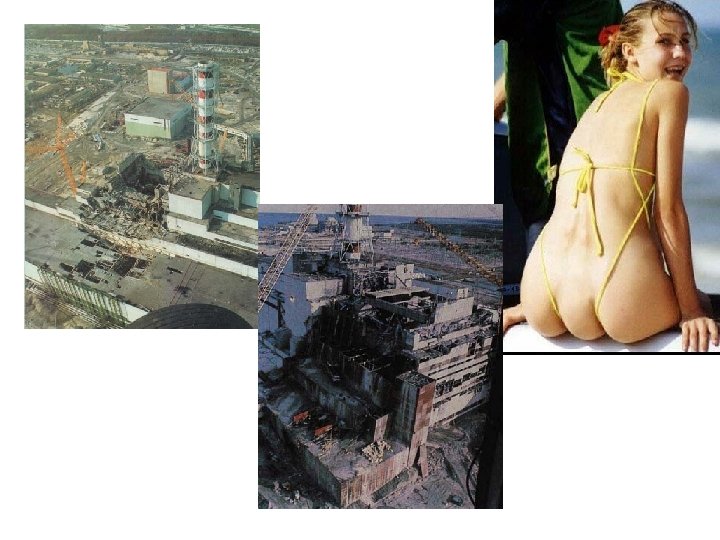
 Chernobyl Disaster • April 26, 1986 – flawed reactor design was operated by inadequately trained personnel and w/o regard to safety
Chernobyl Disaster • April 26, 1986 – flawed reactor design was operated by inadequately trained personnel and w/o regard to safety
 Chernobyl Disaster • Result – steam explosion and fire released at least 5% of the radioactive reactor core into the atmosphere & downwind • 28 people died within 4 months from radiation or thermal burns • 19 subsequently died and 9 deaths from thyroid cancer
Chernobyl Disaster • Result – steam explosion and fire released at least 5% of the radioactive reactor core into the atmosphere & downwind • 28 people died within 4 months from radiation or thermal burns • 19 subsequently died and 9 deaths from thyroid cancer
 TERMS TO REVIEW Radiation Alpha particle Negatron X-ray Gamma ray Half-Life Decay Systems Mother Nucleus Fission Alpha decay Radioactive decay Negative beta decay Photon Daughter Nuclei Fusion 30
TERMS TO REVIEW Radiation Alpha particle Negatron X-ray Gamma ray Half-Life Decay Systems Mother Nucleus Fission Alpha decay Radioactive decay Negative beta decay Photon Daughter Nuclei Fusion 30
 Half-life • 4. Thallium-201 has a half-life of 73 hours. If 4. 0 mg of thallium-201 disintegrates over a period of 6. 0 days and 2 hours, how many mg of thallium-201 will remain?
Half-life • 4. Thallium-201 has a half-life of 73 hours. If 4. 0 mg of thallium-201 disintegrates over a period of 6. 0 days and 2 hours, how many mg of thallium-201 will remain?
 Half-life • 6. The half-life of isotope X is 2. 0 years. How many years would it take for a 4. 0 mg sample of X to decay and have only 0. 50 mg of it remain?
Half-life • 6. The half-life of isotope X is 2. 0 years. How many years would it take for a 4. 0 mg sample of X to decay and have only 0. 50 mg of it remain?
 Half-life • 3. Actinium-226 has a half-life of 29 hours. If 100 mg of actinium-226 disintegrates over a period of 58 hours, how many mg of actinium 226 will remain?
Half-life • 3. Actinium-226 has a half-life of 29 hours. If 100 mg of actinium-226 disintegrates over a period of 58 hours, how many mg of actinium 226 will remain?
 Half-life • 7. Selenium-83 has a half-life of 25. 0 minutes. How many minutes would it take for a 10. 0 mg sample to decay and have only 1. 25 mg of it remain?
Half-life • 7. Selenium-83 has a half-life of 25. 0 minutes. How many minutes would it take for a 10. 0 mg sample to decay and have only 1. 25 mg of it remain?
 Half-life • 8. Element-106 has a half-life of 0. 90 seconds. If one million atoms of it were prepared, how many atoms would remain after 4. 5 seconds?
Half-life • 8. Element-106 has a half-life of 0. 90 seconds. If one million atoms of it were prepared, how many atoms would remain after 4. 5 seconds?
 Half-life • 9. The half-life of Po-218 is three minutes. How much of a 2. 0 gram sample remains after 15 minutes? Suppose you wanted to buy some of this isotope, and it required half an hour for it to reach you. How much should you order if you need to use 0. 10 gram of this material?
Half-life • 9. The half-life of Po-218 is three minutes. How much of a 2. 0 gram sample remains after 15 minutes? Suppose you wanted to buy some of this isotope, and it required half an hour for it to reach you. How much should you order if you need to use 0. 10 gram of this material?
 Half-life • 10. Three grams of Bismuth-218 decay to 0. 375 grams in one hour. What is the half-life of this isotope?
Half-life • 10. Three grams of Bismuth-218 decay to 0. 375 grams in one hour. What is the half-life of this isotope?
 Half-life • 11. The half-life of francium is 21 minutes. Starting with 4 x 1018 atoms of francium, how many atoms would disintegrate in 1 hour and 45 minutes? What fraction of the original sample remains?
Half-life • 11. The half-life of francium is 21 minutes. Starting with 4 x 1018 atoms of francium, how many atoms would disintegrate in 1 hour and 45 minutes? What fraction of the original sample remains?
 Half-life • 12. The half-life of a radioactive element is 30 seconds. In what period of time would the activity of the sample be reduced to onesixteenth of the original activity?
Half-life • 12. The half-life of a radioactive element is 30 seconds. In what period of time would the activity of the sample be reduced to onesixteenth of the original activity?


