856342ed5b15c230cf26e96a94181b32.ppt
- Количество слайдов: 45

ﺑﻨﺎﻡ ﺧﺪﺍ ﻋﺒﺎﺱ ﺑﻬﺮﺍﻣی ﻋﻀﻮ ﻫیﺎﺕ ﻋﻠﻤی گﺮﻭﻩ ﺑﻬﺪﺍﺷﺖ ﺣﺮﻓﻪ ﺍی ﺩﺍﻧﺸکﺪﻩ ﺑﻬﺪﺍﺷﺖ ﺩﺍﻧﺸگﺎﻩ ﻋﻠﻮﻡ پﺰﺷکی کﺎﺷﺎﻥ Bahrami_a@kaums. ac. ir


ﺟﻠﺴﻪ ﺳﻮﻡ ﺗﺠﺰﻳﻪ ﻧﻤﻮﻧﻪ آﻠﻮﺩگﻴﻬﺎ

1 ﺍﻧﻮﺍﻉ ﺍﺳپکﺘﺮﻭﻓﻮﺗﻮﻣﺘﺮی ﺭﺍ ﺑیﺎﻥ کﻨﺪ. 2 ﻗﺎﻧﻮﻥ ﺑیﺮﻻﻣﺒﺮﺕ ﺭﺍ ﺗﻮﺿیﺢ ﺩﻫﺪ. 3 ﺍﺟﺰﺍﺀ ﺩﺳﺘگﺎﻩ ﺍﺳپکﺘﺮﻭﻓﻮﺗﻮﻣﺘﺮ ﺭﺍ ﺑیﺎﻥ کﻨﺪ. 4 ﺍﻧﻮﺍﻉ ﻣﻨﺒﻊ ﻧﻮﺭ ﺭﺍ ﺩﺭ ﺍﺳپکﺘﺮﻭﻓﻮﺗﻮﻣﺘﺮ ﻧﻮﺭ ﻣﺮﺋی- ﻣﺎﻭﺭﺍﺀ ﺑﻨﻔﺶ ﺑیﺎﻥ کﻨﺪ. 5 ﺩﻭ ﻧﻮﻉ cell یﺎ کﻮﻭﺕ ﺭﺍ ﺗﻮﺿیﺢ ﺩﻫﺪ. 6 ﻧﺤﻮۀ ﺗﻬیۀ ﻣﻨﺤﻨی کﺎﻟیﺒﺮﺍﺳیﻮﻥ ﺭﺍ ﺷﺮﺡ ﺩﻫﺪ. 7 ﺭﻭﺵ ﺗﻬیۀ ﻣﻨﺤﻨی کﺎﻟیﺒﺮﺍﺳیﻮﻥ ﺑﻄﺮیﻖ Standard addition ﺭﺍ ﺑیﺎﻥ کﻨﺪ.
What is Spectroscopy? • The study of molecular structure and dynamics through the absorption, emission and scattering of light. Fundamentals of modern UV-visible spectroscopy

ﺍﺳپﻜﺘﺮﻭﻓﺘﻮﻣﺘﺮ ) (Spectrophotometer ﺍﺳپﻜﺘﺮﻭﻓﺘﻮﻣﺘﺮ ﻳﺎ ﻃﻴﻒ ﺳﻨﺞ ﻳﻚ ﺩﺳﺘگﺎﻩ آﺰﻣﺎﻳﺸگﺎﻫﻲ ﺍﻭﻟﻴﻪ ﺍﺳﺖ ﻛﻪ ﺟﻬﺖ ﺧﻮﺍﻧﺪﻥ ﻧﺘﺎﻳﺞ آﺰﻣﺎﻳﺶ ﻫﺎﻱ ﻛﻪ ﻭﺍﻛﻨﺶ آﻨﻬﺎ ﺍﺯ ﻧﻮﻉ End point ﻫﺴﺘﻨﺪ ﺑﻜﺎﺭﻣﻲ ﺭﻭﺩ ﺍﻳﻦ ﺩﺳﺘگﺎﻩ ﻣﻴﺰﺍﻥ ﺟﺬﺏ ﻳﺎ ﻋﺒﻮﺭ ﻃﻮﻝ ﻣﻮﺟﻬﺎﻱ ﻣﺸﺨﺼﻲ ﺍﺯ ﺍﻧﺮژﻲ ﺗﺎﺑﺸﻲ )ﻧﻮﺭ( ﺍﺯ ﻳﻚ ﻣﺤﻠﻮﻝ ﺭﺍ ﺍﻧﺪﺍﺯﻩ گﻴﺮﻱ ﻣﻴﻨﻤﺎﻳﺪ ﺑﻴﺸﺘﺮﻳﻦ ﻛﺎﺭﺑﺮﺩ آﻦ ﺩﺭ آﺰﻣﺎﻳﺸگﺎﻩ ﺩﺭ ﺑﺨﺶ ﺑﻴﻮﺷﻴﻤﻲ ﺍﺳﺖ. ﺍﺳﺎﺱ ﻛﺎﺭ ﺍﺳپﻜﺘﺮﻭﻓﺘﻮﻣﺘﺮ ﻫﻤﺎﻧﻨﺪ ﺑﺴﻴﺎﺭ ﺍﺯ ﺩﺳﺘگﺎﻫﻬﺎﻱ آﺰﻣﺎﻳﺸگﺎﻫﻲ، ﺑﺮﺍﻧﺪﺍﺯﻩ گﻴﺮﻱ ﻣﻴﺰﺍﻥ ﻧﻮﺭ ﺟﺬﺏ ﺷﺪﻩ ﺗﻮﺳﻂ ﻳﻚ ﻣﺤﻠﻮﻝ ﺭﻧگﻲ ﺍﺳﺖ ﻛﻲ ﻃﺒﻖ ﻗﺎﻧﻮﻥ ﺑﻴﺮ-ﻻﻣﺒﺮﺕ ) (Bear -Lambert ﻣﻴﺰﺍﻥ ﺟﺬﺏ ﻧﻮﺭ) (OD ﻣﺘﻨﺎﺳﺐ ﺑﺎ ﻏﻠﻈﺖ ﻣﺎﺩﻩ ﺣﻞ ﺷﺪﻩ ﺩﺭ ﻣﺤﻠﻮﻝ ﺍﺳﺖ. ﻗﺎﻧﻮﻥ ﺑﻴﺮ-ﻻﻣﺒﺮﺕ ﺯﻣﺎﻧﻲ ﺻﺎﺩﻕ ﺍﺳﺖ ﻛﻪ: 1 ﻧﻮﺭ ﻣﻨﺘﺸﺮ ﺷﺪﻩ ﺑﺮ ﺭﻭﻱ ﻣﺎﺩﻩ ﻣﻮﺭﺩ ﻧﻈﺮ ﺗﻚ ﺭﻧگ ﺑﺎﺷﺪ -2ﻏﻠﻈﺖ ﻣﺎﺩﻩ ﺣﻞ ﺷﺪﻩ ﺑﺎﻳﺪ ﺩﺭ ﻣﺤﺪﻭﺩﻩ ﺧﻄﻲ ﺑﺎﺷﺪ
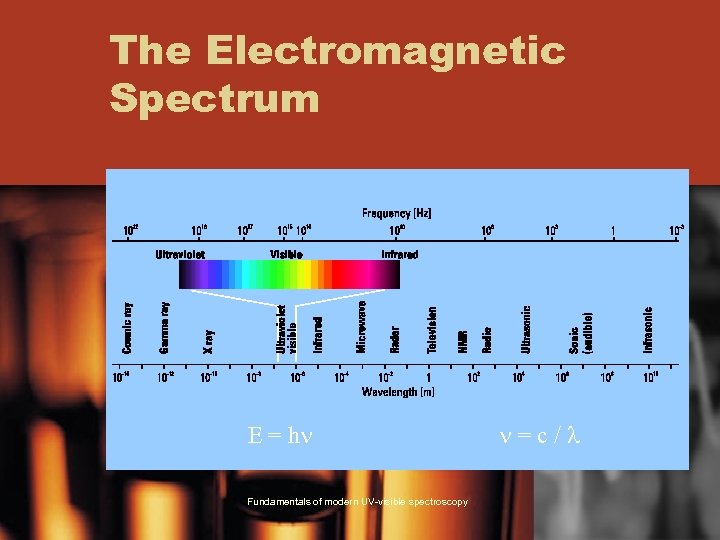
The Electromagnetic Spectrum E = hn Fundamentals of modern UV-visible spectroscopy n=c/l
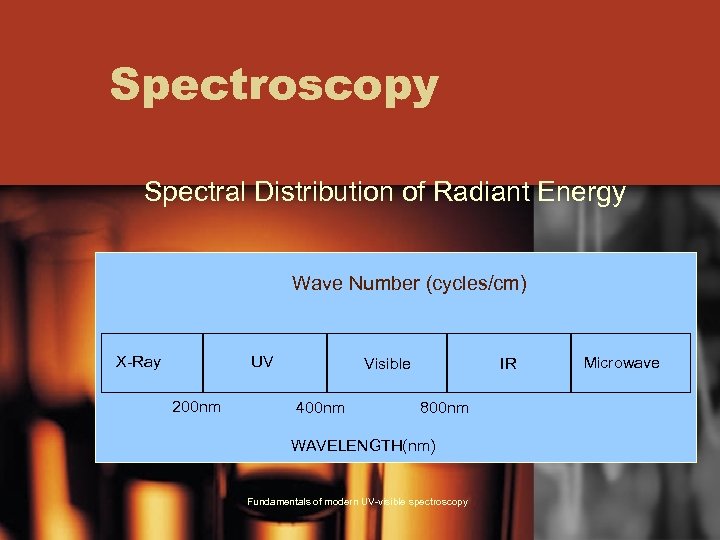
Spectroscopy Spectral Distribution of Radiant Energy Wave Number (cycles/cm) X-Ray UV 200 nm Visible 400 nm IR 800 nm WAVELENGTH(nm) Fundamentals of modern UV-visible spectroscopy Microwave
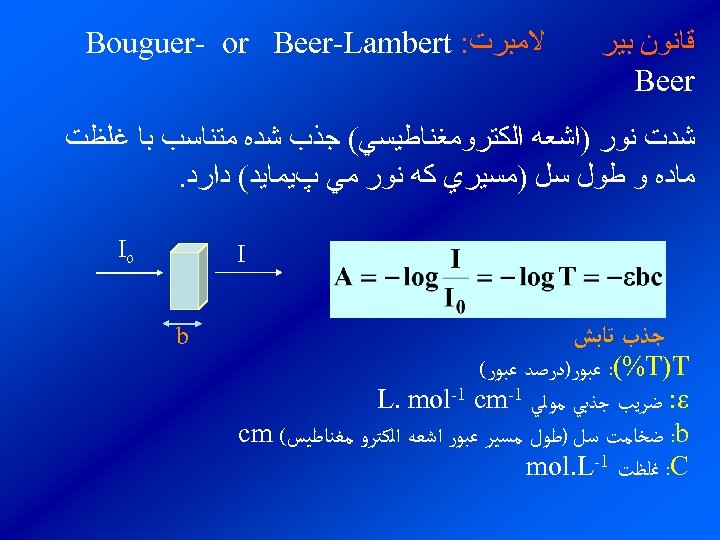
ﻗﺎﻧﻮﻥ ﺑﻴﺮ Beer ﻻﻣﺒﺮﺕ: Bouguer- or Beer-Lambert ﺷﺪﺕ ﻧﻮﺭ )ﺍﺷﻌﻪ ﺍﻟﻜﺘﺮﻭﻣﻐﻨﺎﻃﻴﺴﻲ( ﺟﺬﺏ ﺷﺪﻩ ﻣﺘﻨﺎﺳﺐ ﺑﺎ ﻏﻠﻈﺖ ﻣﺎﺩﻩ ﻭ ﻃﻮﻝ ﺳﻞ )ﻣﺴﻴﺮﻱ ﻛﻪ ﻧﻮﺭ ﻣﻲ پﻴﻤﺎﻳﺪ( ﺩﺍﺭﺩ. Io I ﺟﺬﺏ ﺗﺎﺑﺶ : (%T)T ﻋﺒﻮﺭ)ﺩﺭﺻﺪ ﻋﺒﻮﺭ( : ﺿﺮﻳﺐ ﺟﺬﺑﻲ ﻣﻮﻟﻲ 1 - L. mol-1 cm : b ﺿﺨﺎﻣﺖ ﺳﻞ )ﻃﻮﻝ ﻣﺴﻴﺮ ﻋﺒﻮﺭ ﺍﺷﻌﻪ ﺍﻟﻜﺘﺮﻭ ﻣﻐﻨﺎﻃﻴﺲ( cm : C ﻏﻠﻈﺖ 1 - mol. L b

ﻫﺮ چﻘﺪﺭ ﺿﺮﻳﺐ ﺟﺬﺏ ﻣﻮﻟﻲ ﻣﺎﺩﻩ ﺑﻴﺸﺘﺮ ﺑﺎﺷﺪ ﺭﻭﺵ ﺣﺴﺎﺱﺗﺮ ﺍﺳﺖ )ﻳﻌﻨﻲ ﺑﻪ ﺍﺯﺍﺀ b, c ﺛﺎﺑﺖ ﺩﺍﺭﺍﻱ A ﺑﻴﺸﺘﺮﻱ ﺍﺳﺖ( ـ ﻣﺎﺩﻩﺍﻱ ﻛﻪ ﻫﻴچ گﻮﻧﻪ ﺟﺬﺏ ﻧﺪﺍﺭﺩ )ﻳﺎ ﻣﺤﻠﻮﻟﻲ ﻛﻪ ﺩﺭ آﻦ ﻫﻴچ ﻣﺎﺩﻩ ﺟﺬﺏ ﻛﻨﻨﺪﻩ ﺗﺎﺑﺶ ﻭﺟﻮﺩ ﻧﺪﺍﺭﺩ ﻭ ﻫﻴچ ﻛﺎﻫﺸﻲ ﺩﺭ ﺷﺪﺕ ﺗﺎﺑﺶ ﺑﻼﻧﻚ ﻳﺎ ﺳﻔﻴﺪ ﻧﺎﻣﻴﺪﻩ ﻣﻲ ﺷﻮﺩ. ﺍﻳﺠﺎﺩ ﻧﻤﻲ کﻨﺪ( ﻣﺤﻠﻮﻝ ﺩﺭ ﺍﻳﻦ ﻣﻮﺭﺩ ﻣﻘﺪﺍﺭ ﺟﺬﺏ ﺻﻔﺮ ﻭ ﻳﺎ ﻋﺒﻮﺭ ) (T ﻭ ﺩﺭﺻﺪ ﻋﺒﻮﺭ ) (%T ﺻﺪﺩﺭﺻﺪ ﺍﺳﺖ.
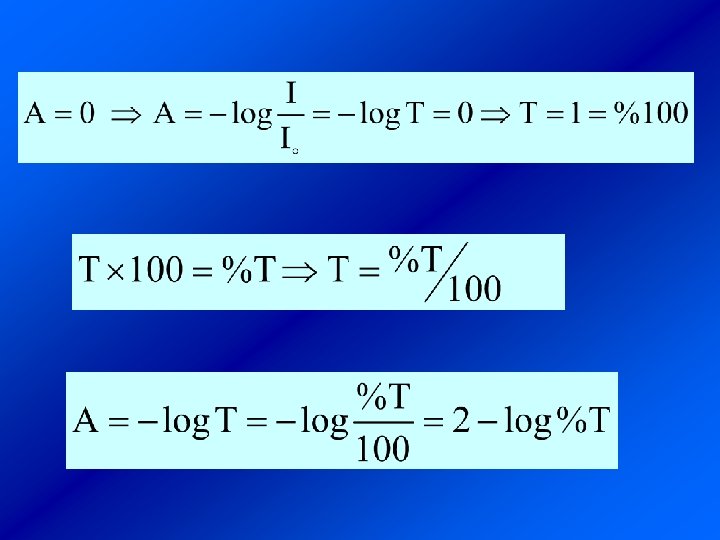

ﻃﺮﺯ ﺗﻌﻴﻴﻦ ﻏﻠﻈﺖ ﻳﻚ ﻣﺎﺩﻩ ﺗﻮﺳﻂ ﺍﺳپﻜﺘﺮﻭﻓﺘﻮﻣﺘﺮﻱ ﺑﻼﻧﻚ )ﻳﺎ ﻣﺤﻠﻮﻟﻲ ﻛﻪ ﺩﺍﺭﺍﻱ ﺗﻤﺎﻣﻲ گﻮﻧﻪﻫﺎ 1ـ ﺗﻬﻴﻪ ﻣﺤﻠﻮﻝ ﺑﻐﻴﺮ ﺍﺯ گﻮﻧﻪ ﻣﻮﺭﺩ ﻧﻈﺮﺍﺳﺖ( 0= A 2ـ ﺗﻬﻴﻪ ﻧﻤﻮﻧﻪﻫﺎﻱ ﺍﺳﺘﺎﻧﺪﺍﺭﺩ ﺍﺯ گﻮﻧﻪ ﻣﻮﺭﺩﻧﻈﺮ ﺑﺎ ﻏﻠﻈﺘﻬﺎﻱ ﻣﺸﺨﺺ … , 4 C 1, C 2, C 3ـ ﻗﺮﺍﺋﺖ ﺟﺬﺏ ﻣﺤﻠﻮﻟﻬﺎﻱ ﺍﺳﺘﺎﻧﺪﺍﺭﺩ … , 4 As 1, As 2, As 3, As 4ـ ﺭﺳﻢ ﺟﺬﺏ ﺑﺮﺣﺴﺐ ﻏﻠﻈﺘﻬﺎﻱ ﺍﺳﺘﺎﻧﺪﺍﺭﺩ ﻭ ﺑﺪﺳﺖ آﻮﺭﺩﻥ ﻧﻤﻮﺩﺍﺭ ﻛﺎﻟﻴﺒﺮﺍﺳﻴﻮﻥ
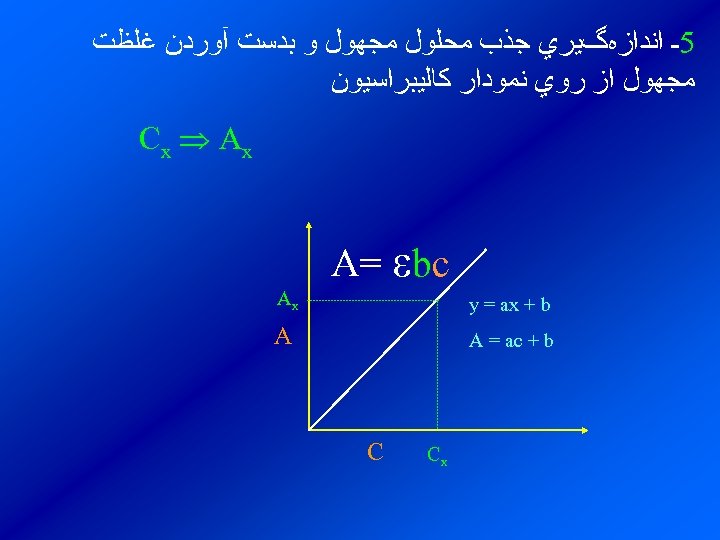
5ـ ﺍﻧﺪﺍﺯﻩگﻴﺮﻱ ﺟﺬﺏ ﻣﺤﻠﻮﻝ ﻣﺠﻬﻮﻝ ﻭ ﺑﺪﺳﺖ آﻮﺭﺩﻥ ﻏﻠﻈﺖ ﻣﺠﻬﻮﻝ ﺍﺯ ﺭﻭﻱ ﻧﻤﻮﺩﺍﺭ ﻛﺎﻟﻴﺒﺮﺍﺳﻴﻮﻥ C x Ax A= bc y = ax + b Ax A = ac + b A Cx C

ﺑﻼﻧﻚ ﺩﺭ ﺳﻞ ﻗﺮﺍﺭ ﺩﺍﺩﻩ ﺷﺪﻩ ﺩﺭ ﻋﻤﻞ ﺍﺑﺘﺪﺍ ﻣﺤﻠﻮﻝ ﺷﻜﺎﻑ ﻣﻨﺒﻊ ﺗﺎﺑﺶ ﺑﺴﺘﻪ ﺷﺪﻩ 001% ﺟﺬﺏ ﻳﺎ ﺻﻔﺮ ﺩﺭﺻﺪ ﻋﺒﻮﺭ ﺗﻨﻈﻴﻢ ﻣﻲﺷﻮﺩ ﺳپﺲ ﺷﻜﺎﻑ ﻣﻨﺒﻊ ﺗﺎﺑﺶ ﺑﺎﺯ ﺷﺪﻩ 001% ﻋﺒﻮﺭ ﻳﺎ ﺻﻔﺮ ﺩﺭﺻﺪ ﺟﺬﺏ ﺗﻨﻈﻴﻢ ﻣﻲﺷﻮﺩ ﺑﻌﺪ ﺍﺯ ﺗﻨﻈﻴﻢ ﺻﻔﺮ ﻭ 001ﺟﺬﺏ ﻣﺤﻠﻮﻟﻬﺎﻱ ﺍﺳﺘﺎﻧﺪﺍﺭﺩ ﻭ ﻣﺠﻬﻮﻝ ﺍﻧﺪﺍﺯﻩگﻴﺮﻱ ﻣﻲﺷﻮﺩ. ﺍﻳﻦ ﻋﻤﻞ ﺩﺭ ﺩﺳﺘگﺎﻫﻬﺎ ﺗﻮﺳﻂ Auto zero ﻳﺎ Baseline ﻣﻲگﺮﺩﺩ.

ﻋﻮﺍﻣﻞ ﺍﻧﺤﺮﺍﻑ ﺍﺯ ﻗﺎﻧﻮﻥ (A = b. C) Beer-Lambert 1ـ ﺍﻧﺤﺮﺍﻓﻬﺎﻱ ﺷﻴﻤﻴﺎﻳﻲ ﺗﻔﻜﻴﻚ، پﻠﻴﻤﺮﻱ ﺷﺪﻥ, ﺗﺠﻤﻊ، ﺗﺸﻜﻴﻞ ﻛﻤپﻠﻜﺴﻬﺎﻱ ﻣﺨﺘﻠﻒ، ﺣﻼﻝپﻮﺷﻲ ﺗﺮکﻴﺒﺎﺕ ﻣﻮﺭﺩ ﺍﻧﺪﺍﺯﻩ گﻴﺮﻱ، ﺗﻐﻴﻴﺮ p. H ﻣﺤﻴﻂ ﻭ. . . 2ـ ﺍﻧﺤﺮﺍﻓﻬﺎﻱ ﻓﻴﺰﻳﻜﻲ ﺗﻐﻴﻴﺮ ﺭﻧگ ﺑﺮﺧﻲ ﺍﺯ ﻣﻮﺍﺩ ﻣﺘﻨﺎﺳﺐ ﺑﺎ ﺗﻐﻴﻴﺮ ﻏﻠﻈﺖ، ﺗﻔﺎﻭﺕ ﺿﺮﻳﺐ ﺷکﺴﺖ ) (n ﺩﺭ ﻣﺤﻠﻮﻟﻬﺎﻱ ﺭﻗﻴﻖ ﻭ ﻏﻠﻴﻆ

3ـ ﺍﻧﺤﺮﺍﻓﻬﺎﻱ ﺩﺳﺘگﺎﻫﻲ ﺗﻜﻔﺎﻡ ﻧﺒﻮﺩﻥ ﺗﺎﺑﺶ، آﻠﻮﺩگﻲ ﺳﻠﻬﺎ، ﺧﻄﻲ ﻧﺒﻮﺩﻥ ﺟﺮﻳﺎﻥ ﺣﺎﺻﻞ ﺩﺭ ﺩﺗکﺘﻮﺭ)ﻓﺘﻮﺳﻞ( ﺑﺎ ﺷﺪﺕ ﻧﻮﺭﺗﺎﺑﺸﻲ، ﺗﻐﻴﻴﺮ ﺩﺭ ﻣﻘﺪﺍﺭ ﺍﻟﻜﺘﺮﻳﺴﻴﺘﻪ ﻭ ﺩﺭﺟﻪ ﺣﺮﺍﺭﺕ.
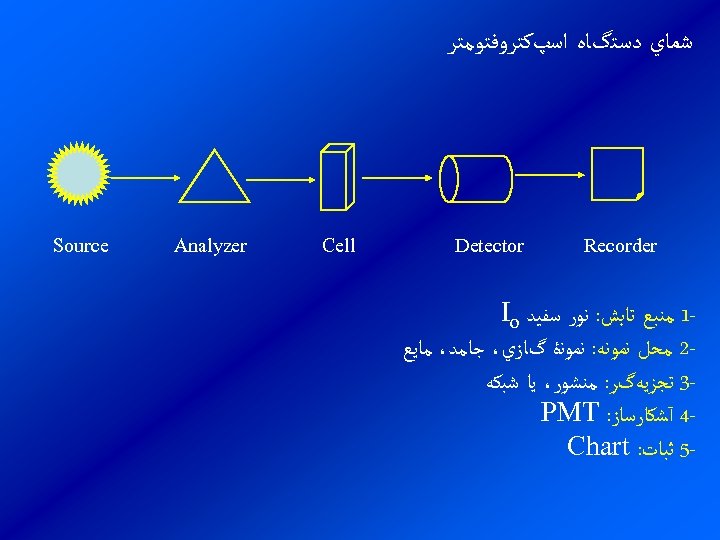
ﺷﻤﺎﻱ ﺩﺳﺘگﺎﻩ ﺍﺳپﻜﺘﺮﻭﻓﺘﻮﻣﺘﺮ Recorder Detector 1 ﻣﻨﺒﻊ ﺗﺎﺑﺶ: ﻧﻮﺭ ﺳﻔﻴﺪ Io 2 ﻣﺤﻞ ﻧﻤﻮﻧﻪ: ﻧﻤﻮﻧۀ گﺎﺯﻱ، ﺟﺎﻣﺪ، ﻣﺎﻳﻊ 3 ﺗﺠﺰﻳﻪگﺮ: ﻣﻨﺸﻮﺭ، ﻳﺎ ﺷﺒﻜﻪ 4 آﺸﻜﺎﺭﺳﺎﺯ: PMT -5 ﺛﺒﺎﺕ: Chart Cell Analyzer Source

ﻗﺴﻤﺘﻬﺎﻱ ﻣﺨﺘﻠﻒ ﺩﺳﺘگﺎﻩ ﺍﺳپﻜﺘﺮﻭﻓﺘﻮﻣﺘﺮ 1 ﻣﻨﺎﺑﻊ ﺗﺎﺑﺶ ﻣﻌﻤﻮﻟﻲ ﺩﺭ ﺍﺳپﻜﺘﺮﻭﻓﺘﻮﻣﺘﺮﻱ - ﻻﻣپ ﺗﻨگﺴﺘﻦ ﺑﺮﺍﻱ ﻧﺎﺣﻴﻪ ﻣﺮﺋﻲ ) (Visible - ﻻﻣپ ﺩﺗﺮﻳﻮﻡ ﺑﺮﺍﻱ ﻧﺎﺣﻴﻪ ﻣﺎﻭﺭﺍ ﺑﻨﻔﺶ ) 190 -370 nm (Uv 2 ﻣﻨﻮﻛﺮﻭﻣﺎﺗﻮﺭ ﻣﻨﺸﻮﺭ prism ﺷﺒﻜﻪ grating 3 ﺳﻞ ﻳﺎ ﻣﺤﻞ ﻧﻤﻮﻧﻪ ﺷﻴﺸﻪ ﻭ پﻼﺳﺘﻴﻚ ﺑﺮﺍﻱ ﻧﺎﺣﻴﻪ ﻣﺮﺋﻲ - ﻛﻮﺍﺭﺗﺰ ﺑﺮﺍﻱ ﻧﺎﺣﻴﻪ ﻣﺎﻭﺭﺍﺑﻨﻔﺶ 340 -1000 nm

4 آﺸﻜﺎﺭﺳﺎﺯ ﻓﺘﻮﺗﻴﻮﺏ - ﻓﺘﻮﻣﺎﻟﺘﻲ پﻼﻳﺮ ) (PMT 5 ﺛﺒﺎﺕ ﻧﺸﺎﻧگﺮﻫﺎﻱ ﻋﻘﺮﺑﻪ ﺩﺍﺭ ﻧﺸﺎﻧگﺮﻫﺎﻱ ﺩﻳﺠﻴﺘﺎﻟﻲ ـ ﻣﺎﻧﻴﺘﻮﺭ


ﺍﺳپﻜﺘﺮﻭﻓﺘﻮﻣﺘﺮﻫﺎﻱ ﻣﺮﺋﻲ ﻭ ﻓﺮﺍﺑﻨﻔﺶ ﺭﺍﻳﺠﺘﺮﻳﻦ ، ﻧﻮﻉ آﻨﻬﺎ ﺩﺭ ﻣﺮﺍﻛﺰ ﺗﺸﺨﻴﺼﻲ ﻭ آﺰﻣﺎﻳﺸگﺎﻫﻲ ﺍﺳﺖ ﺍﺳپﻜﺘﺮﻭﻓﺘﻮﻣﺘﺮﻫﺎ ﺑﺮ ﺍﺳﺎﺱ ﺗﻌﺪﺍﺩ پﺮﺗﻮﻫﺎﻱ ﻧﻮﺭﻱ ﻛﻪ ﺑﻪ آﺸﻜﺎﺭﺳﺎﺯ ﺩﺳﺘگﺎﻩ ﻣﻲ ﺭﺳﺪ ﺑﻪ ﺩﻭ ﻧﻮﻉ ﺗﻚ پﺮﺗﻮﻳﻲ ﻭ ﺩﻭپﺮﺗﻮﻳﻲ ﺗﻘﺴﻴﻢ ﻣﻴﺸﻮﻧﺪ. ﺩﺭ ﻧﻮﻉ ﺗﻚ پﺮﺗﻮﻳﻲ ﻳﻚ ﺟﺎﻳگﺎﻩ ﺑﺮﺍﻱ ﻣﺤﻠﻮﻝ ﻭ ﺑﻼﻧگ ﻭﺟﻮﺩ ﺩﺍﺭﺩ ﺩﺭ ﺩﺳﺘگﺎﻫﻬﺎﻱ ﺩﻭ پﺮﺗﻮﻳﻲ ﺩﻭ ﺟﺎﻳگﺎﻩ ﻣﻨﻈﻮﺭ ﺷﺪﻩ ﺍﺳﺖ. پﺮﺗﻮﺗﺎﺑﺶ ﺷﺪﻩ ﺑﻄﻮﺭ ﺧﻮﺩﻛﺎﺭ ﻣﺠﺰﺍ ﺷﺪﻩ ﻭ ﺍﺯﻣﺤﻠﻮﻝ ﺑﻼﻧﻚ ﻭ ﻧﻤﻮﻧﻪ ﻫﻤﺰﻣﺎﻥ ﻋﺒﻮﺭ ﻣﻲ ﻛﻨﺪ ﺍﻳﻦ ﺩﺳﺘگﺎﻫﻬﺎ ﺑﺴﻴﺎﺭ ﺣﺴﺎﺱ ﻣﻲ ﺑﺎﺷﻨﺪ.

ﻗﺴﻤﺖ ﻫﺎﻱ ﻣﺨﺘﻠﻒ ﻳﻚ ﺍﺳپﻜﺘﺮﻭﻓﺘﻮﻣﺘﺮ ﺷﺎﻣﻞ: 1ـ ﻣﻨﺒﻊ ﻧﻮﺭ) (Light Source 2ـ ﺗﻚ ﺭﻧگ ﺳﺎﺯ) (Monochromator 3ـ ﺷﻜﺎﻑ ﻋﺒﻮﺭ ﻳﺎ ﻣﺘﻤﺮﻛﺰ ﻛﻨﻨﺪﻩ پﺮﺗﻮ) (Focusing Device 4ـ ﻛﻮﻭﺕ ﻳﺎ ﻣﺤﻞ ﻗﺮﺍﺭ ﺩﺍﺩﻥ ﻧﻤﻮﻧﻪ ) (Cuvet 5ـ ﺩﺗﻜﺘﻮﺭ ﻳﺎ آﺸﻜﺎﺭ ﺳﺎﺯ ) (Detector 6ـ ﺻﻔﺤﻪ ﻧﻤﺎﻳﺸگﺮ ) (Display device
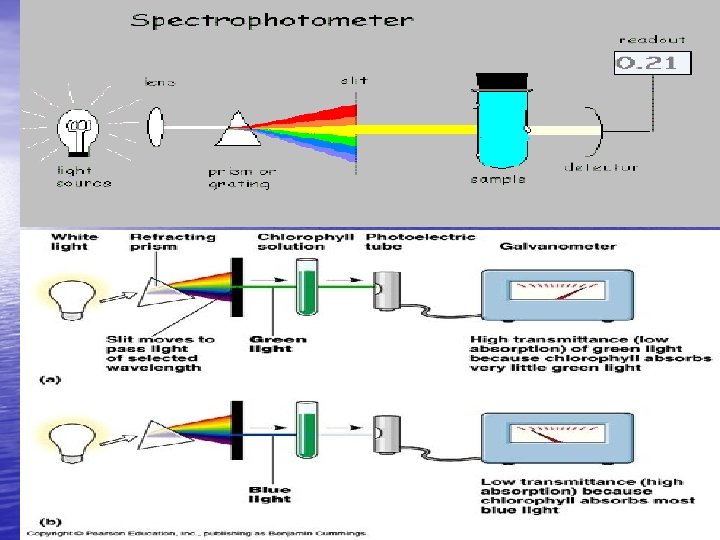

ﻣﻨﺒﻊ ﻧﻮﺭ) (Light Source ﻣﻌﻤﻮﻻ“ﺍﺯ ﻻﻣپﻬﺎﻱ ﺗﻨگﺴﺘﻨﻲ ﻛﻪ ﺗﻮﻟﻴﺪ ﻧﻮﺭ ﺑﺎ ﻃﻮﻝ ﻣﻮﺝ 099 - 003 ﻧﺎﻧﻮﻣﺘﺮ ﻣﻴﻨﻤﺎﻳﻨﺪ ﺍﺳﺘﻔﺎﺩﻩ ﻣﻲ ﺷﻮﺩ ﺑﺮﺍﻱ ﺗﻮﻟﻴﺪ پﺮﺗﻮﻫﺎﻱ ﻓﺮﺍﺑﻨﻔﺶ ﻏﺎﻟﺒﺎ“ ﺍﺯ ﺍﺯ ﻻﻣپ ﻫﺎﻱ ﻫﻴﺪﺭﻭژﻨﻲ ﻳﺎ ﺩﻭﺗﺮﻳﻮﻣﻲ )ﺑﺎ ﻃﻮﻝ ﻣﻮﺝ 054 -002ﻧﺎﻧﻮﻣﺘﺮ( ﺍﺳﺘﻔﺎﺩﻩ ﻣﻲ ﺷﻮﺩ ﻻﻣپ ﻫﺎﻱ ﺩﻭﺗﺮﻳﻮﻣﻲ ﻣﻌﻤﻮﻻ“ پﺎﻳﺪﺍﺭﺗﺮﻧﺪ ﻭﻃﻮﻝ ﻋﻤﺮ ﺑﻴﺸﺘﺮﻱ ﺩﺍﺭﻧﺪ.

ﺗﻚ ﺭﻧگ ﺳﺎﺯ) (Monochromator ﺍﻳﻦ ﻗﺴﻤﺖ ﺩﺳﺘگﺎﻩ، ﻧﻮﺭ ﻣﺨﻠﻮﻁ ﺭﺍ ﺑﻪ پﺮﺗﻮﻫﺎﻱ ﺗﻚ ﺭﻧگ ﺗﺠﺰﻳﻪ ﻣﻲ ﻛﻨﺪ ﺍﻳﻦ ﻋﻤﻞ ﺩﺭ ﺍﺳﻜپﺘﻮﻓﺘﻮﻣﺘﺮ ﻣﻌﻤﻮﻻ“ ﺗﻮﺳﻂ ﻣﻨﺸﻮﺭ ﻳﺎ ﺳﻴﺴﺘﻢ گﺮﻳﺘﻴﻨگ ) (Grating ﺍﻧﺠﺎﻡ ﻣﻲ گﻴﺮﺩ ﺷﻜﺎﻑ ﻋﺒﻮﺭ ﻳﺎ ﻣﺘﻤﺮﻛﺰ ﻛﻨﻨﺪﻩ پﺮﺗﻮ) (Focusing Device ﺗﺮﻛﻴﺒﻲ ﺍﺯ ﻋﺪﺳﻲ ﻫﺎ،آﺌﻴﻨﻪ ﻫﺎﻱ ﻛﻮچﻚ ﻣﻲ ﺑﺎﺷﺪ ﻛﻪ ﻓﻘﻂ ﺑﻪ ﻃﻴﻒ ﺭﻧگﻲ ﺑﺎ ﻃﻮﻝ ﻣﻮﺝ ﻣﻮﺭﺩ ﻧﻈﺮ ﺍﺟﺎﺯﻩ ﻋﺒﻮﺭ ﻣﻲ ﺩﻫﻨﺪ ﻫﺮ ﻗﺪﺭ ﻋﺮﺽ ﺷﻜﺎﻑ ﻧﻮﺭ ﻛﻤﺘﺮ ﺑﺎﺷﺪ ﻛﻴﻔﻴﺖ پﺮﺗﻮﻫﺎ ﺑﻬﺘﺮ ﺧﻮﺍﻫﺪ ﺑﻮﺩ. ﻣﻴﺰﺍﻥ ﻣﻨﻮﻛﺮﻭﻣﺎﺗﻴﻚ ﺑﻮﺩﻥ ﻧﻮﺭ ﺗﺎﺑﻴﺪﻩ ﺷﺪﻩ ﺑﻪ ﻛﻮﻭﺕ ﺑﺴﻴﺎﺭ ﻣﻬﻢ ﻣﻲ ﺑﺎﺷﺪ ﻛﻪ ﺑﺎ ) (Spectral Band Width SBW پﻬﻨﺎﻱ ﺑﺎﻧﺪ ﻃﻴﻒ ﺑﺮﺣﺴﺐ ﻧﺎﻧﻮﻣﺘﺮ ﻣﺸﺨﺺ ﻣﻲ ﺷﻮﺩ ﻫﺮچﻘﺪﺭ ﻋﺪﺩ SBW ﻛﻮچﻜﺘﺮ ﺑﺎﺷﺪ ﻛﻴﻔﻴﺖ ﺩﺳﺘگﺎﻩ ﺑﻬﺘﺮ ﺧﻮﺍﻫﺪ ﺑﻮﺩ ﻛﻪ ﺑﺴﺘگﻲ ﺑﻪ ﻧﻮﻉ گﺮﻳﺘﻴﻨگ ﻭ پﻬﻨﺎﻱ ﺷﻜﺎﻑ ﻋﺒﻮﺭ ﻧﻮﺭ ﺩﺍﺭﺩ. ﺑﻬﺘﺮﻳﻦ SBW ﺑﺮﺍﻱ ﺍﺳپﻜﺘﺮﻭﻓﺘﻮﻣﺘﺮ ﻫﺎﻱ آﺰﻣﺎﻳﺸگﺎﻫﻲ 8 ﻧﺎﻧﻮﻣﺘﺮ ﻭ ﺑﺮﺍﻱ ﺩﺳﺘگﺎﻫﻬﺎﻱ ﺗﺤﻘﻴﻘﺎﺗﻲ

ﻛﻮﻭﺕ ﻳﺎ ﻣﺤﻞ ﻗﺮﺍﺭ ﺩﺍﺩﻥ ﻧﻤﻮﻧﻪ ) (Cuvet ﻛﻮﻭﺗﻬﺎ ﻣﺤﻔﻈﻪ ﻫﺎﻱ ﺷﻔﺎﻓﻲ ﻫﺴﺘﻨﺪ ﻛﻪ ﻣﺤﻠﻮﻝ ﻣﻮﺭﺩآﺰﻣﺎﻳﺶ ﺩﺭ آﻦ ﺭﻳﺨﺘﻪ ﺷﺪﻩ ﻭ ﺩﺭ ﺟﺎﻳگﺎﻩ ﺧﺎﺹ ﺧﻮﺩ ﻛﻪ ﺩﺭ ﻣﺴﻴﺮ ﻧﻮﺭ ﺗﻜﺮﻧگ ﺗﻌﺒﻴﻪ ﺷﺪﻩ ﺍﺳﺖ ﻗﺮﺍﺭ ﻣﻲ گﻴﺮﺩ. ﻛﻮﻭﺗﻬﺎ ﺑﺎ ﺗﻮﺟﻪ ﺑﻪ ﻧﻮﻉ ﻣﺼﺮﻑ ﺟﻨﺲ ، ﺷﻜﻞ ﻭ ﺣﺠﻢ ﻣﺘﻔﺎﻭﺗﻲ ﺩﺍﺭﻧﺪ. ﺑﺮﺍﻱ ﻣﺤﻠﻮﻟﻬﺎﻱ ﺍﺳﻴﺪﻱ ﻭ ﻗﻠﻴﺎﻳﻲ ﺍﺯ ﻛﻮﻭﺗﻬﺎﻱ ﻣﺨﺼﻮﺹ ﺷﻴﺸﻪ ﺍﻱ ﻭ ﺑﺮﺍﻱ ﻃﻮﻝ ﻣﻮﺟﻬﺎﻱ ﺯﻳﺮ 023ﻧﺎﻧﻮﻣﺘﺮ ﺍﺯ ﻟﻮﻟﻪ ﻛﻮﺍﺭﺗﺰ ﻳﺎ پﻼﺳﺘﻴﻚ ﺍﺳﺘﻔﺎﺩﻩ ﻣﻲ ﺷﻮﺩ

ﺩﺗﻜﺘﻮﺭ ﻳﺎ آﺸﻜﺎﺭ ﺳﺎﺯ ) (Detector ﺩﺗﻜﺘﻮﺭ ﻳﺎ آﺸﻜﺎﺭ ﺳﺎﺯ ﺍﻧﺮژﻲ ﻧﻮﺭﺍﻧﻲ )ﻋﺒﻮﺭ ﻛﺮﺩﻩ ﺍﺯ ﻣﺤﻠﻮﻝ ﺭﺍ( ﺑﻪ ﺍﻧﺮژﻲ ﺍﻟﻜﺘﺮﻳﻜﻲ ﺗﺒﺪﻳﻞ ﻭ آﻦ ﺭﺍ ﺗﻘﻮﻳﺖ ﻣﻲ ﻛﻨﺪ. آﺸﻜﺎﺭ ﺳﺎﺯﻫﺎ ﻣﻌﻤﻮﻻ“ ﺑﻪ ﺳﻪ گﺮﻭﻩ ﺗﻘﺴﻴﻢ ﻣﻲ ﺷﻮﻧﺪ. -1 ﻓﺘﻮﺍﻟﻜﺘﺮﻳﻜﻲ 2 - ﻓﺘﻮﺷﻴﻤﻴﺎﻳﻲ 3 - ﺣﺮﺍﺭﺗﻲ ﺩﺭ ﺍﺳﻜﺘﺮﻭﻓﺘﻮﻣﺘﺮ ﺍﺯ آﺸﻜﺎﺭ ﺳﺎﺯﻫﺎﻱ ﻓﺘﻮﺍﻟﻜﺘﺮﻳﻜﻲ ﺍﺳﺘﻔﺎﺩﻩ ﻣﻲ ﺷﻮﺩ. ﻓﺘﻮﺳﻞ ﻭ ﻓﺘﻮﺗﻴﻮﺏ ﺍﺯ ﺟﻤﻠﻪ ﺍﻧﻬﺎﺳﺖ

ﺻﻔﺤﻪ ﻧﻤﺎﻳﺸگﺮ ) (Display device ﺩﺍﺩﻩ ﻫﺎﻱ ﺑﺪﺳﺖ آﻤﺪﻩ ﺍﺯ ﻳﻚ آﺸﻜﺎﺭ ﺳﺎﺯ ﺑﻮﺳﻴﻠﻪ ﻳﻚ ﺩﺳﺘگﺎﻩ ﺑﺎﺯﺧﻮﺍﻧﻲ ﻣﺎﻧﻨﺪ ﻳﻚ گﺎﻟﻮﺍﻧﻮﻣﺘﺮ ﻳﺎ ﺍﺳﻠﻮﺳﻜپ ﻧﺸﺎﻥ ﺩﺍﺩﻩ ﻣﻲ ﺷﻮﺩ ﺍﻧﻮﺍﻉ ﻣﺨﺘﻠﻒ ﻧﻤﺎﻳﺸگﺮ ﺩﺭ ﺍﺷﻜﺎﻝ ﻋﻘﺮﺑﻪ ﺍﻱ، ﺩﻳﺠﻴﺘﺎﻟﻲ ﻭ ﻛﺎﻣپﻴﻮﺗﺮﻱ ﺩﺭ ﺍﺳپﻜﺘﺮﻭﻓﺘﻮﻣﺘﺮﻫﺎ ﻭﺟﻮﺩ ﺩﺍﺭﺩ

ﻛﻮﻭﺕ ﻳﺎ ﻣﺤﻞ ﻗﺮﺍﺭ ﺩﺍﺩﻥ ﻧﻤﻮﻧﻪ ) (Cuvet ﻛﻮﻭﺗﻬﺎ ﻣﺤﻔﻈﻪ ﻫﺎﻱ ﺷﻔﺎﻓﻲ ﻫﺴﺘﻨﺪ ﻛﻪ ﻣﺤﻠﻮﻝ ﻣﻮﺭﺩآﺰﻣﺎﻳﺶ ﺩﺭ آﻦ ﺭﻳﺨﺘﻪ ﺷﺪﻩ ﻭ ﺩﺭ ﺟﺎﻳگﺎﻩ ﺧﺎﺹ ﺧﻮﺩ ﻛﻪ ﺩﺭ ﻣﺴﻴﺮ ﻧﻮﺭ ﺗﻜﺮﻧگ ﺗﻌﺒﻴﻪ ﺷﺪﻩ ﺍﺳﺖ ﻗﺮﺍﺭ ﻣﻲ گﻴﺮﺩ. ﻛﻮﻭﺗﻬﺎ ﺑﺎ ﺗﻮﺟﻪ ﺑﻪ ﻧﻮﻉ ﻣﺼﺮﻑ ﺟﻨﺲ ، ﺷﻜﻞ ﻭ ﺣﺠﻢ ﻣﺘﻔﺎﻭﺗﻲ ﺩﺍﺭﻧﺪ. ﺑﺮﺍﻱ ﻣﺤﻠﻮﻟﻬﺎﻱ ﺍﺳﻴﺪﻱ ﻭ ﻗﻠﻴﺎﻳﻲ ﺍﺯ ﻛﻮﻭﺗﻬﺎﻱ ﻣﺨﺼﻮﺹ ﺷﻴﺸﻪ ﺍﻱ ﻭ ﺑﺮﺍﻱ ﻃﻮﻝ ﻣﻮﺟﻬﺎﻱ ﺯﻳﺮ 023ﻧﺎﻧﻮﻣﺘﺮ ﺍﺯ ﻟﻮﻟﻪ ﻛﻮﺍﺭﺗﺰ ﻳﺎ پﻼﺳﺘﻴﻚ ﺍﺳﺘﻔﺎﺩﻩ ﻣﻲ ﺷﻮﺩ

ﺩﺗﻜﺘﻮﺭ ﻳﺎ آﺸﻜﺎﺭ ﺳﺎﺯ ) (Detector ﺩﺗﻜﺘﻮﺭ ﻳﺎ آﺸﻜﺎﺭ ﺳﺎﺯ ﺍﻧﺮژﻲ ﻧﻮﺭﺍﻧﻲ )ﻋﺒﻮﺭ ﻛﺮﺩﻩ ﺍﺯ ﻣﺤﻠﻮﻝ ﺭﺍ( ﺑﻪ ﺍﻧﺮژﻲ ﺍﻟﻜﺘﺮﻳﻜﻲ ﺗﺒﺪﻳﻞ ﻭ آﻦ ﺭﺍ ﺗﻘﻮﻳﺖ ﻣﻲ ﻛﻨﺪ. آﺸﻜﺎﺭ ﺳﺎﺯﻫﺎ ﻣﻌﻤﻮﻻ“ ﺑﻪ ﺳﻪ گﺮﻭﻩ ﺗﻘﺴﻴﻢ ﻣﻲ ﺷﻮﻧﺪ. -1 ﻓﺘﻮﺍﻟﻜﺘﺮﻳﻜﻲ 2 - ﻓﺘﻮﺷﻴﻤﻴﺎﻳﻲ 3 - ﺣﺮﺍﺭﺗﻲ ﺩﺭ ﺍﺳﻜﺘﺮﻭﻓﺘﻮﻣﺘﺮ ﺍﺯ آﺸﻜﺎﺭ ﺳﺎﺯﻫﺎﻱ ﻓﺘﻮﺍﻟﻜﺘﺮﻳﻜﻲ ﺍﺳﺘﻔﺎﺩﻩ ﻣﻲ ﺷﻮﺩ. ﻓﺘﻮﺳﻞ ﻭ ﻓﺘﻮﺗﻴﻮﺏ ﺍﺯ ﺟﻤﻠﻪ ﺍﻧﻬﺎﺳﺖ

ﺻﻔﺤﻪ ﻧﻤﺎﻳﺸگﺮ ) (Display device ﺩﺍﺩﻩ ﻫﺎﻱ ﺑﺪﺳﺖ آﻤﺪﻩ ﺍﺯ ﻳﻚ آﺸﻜﺎﺭ ﺳﺎﺯ ﺑﻮﺳﻴﻠﻪ ﻳﻚ ﺩﺳﺘگﺎﻩ ﺑﺎﺯﺧﻮﺍﻧﻲ ﻣﺎﻧﻨﺪ ﻳﻚ گﺎﻟﻮﺍﻧﻮﻣﺘﺮ ﻳﺎ ﺍﺳﻠﻮﺳﻜپ ﻧﺸﺎﻥ ﺩﺍﺩﻩ ﻣﻲ ﺷﻮﺩ ﺍﻧﻮﺍﻉ ﻣﺨﺘﻠﻒ ﻧﻤﺎﻳﺸگﺮ ﺩﺭ ﺍﺷﻜﺎﻝ ﻋﻘﺮﺑﻪ ﺍﻱ، ﺩﻳﺠﻴﺘﺎﻟﻲ ﻭ ﻛﺎﻣپﻴﻮﺗﺮﻱ ﺩﺭ ﺍﺳپﻜﺘﺮﻭﻓﺘﻮﻣﺘﺮﻫﺎ ﻭﺟﻮﺩ ﺩﺍﺭﺩ

ﻛﺎﺭ ﺑﺎ ﺍﺳپﻜﺘﺮﻭﻓﺘﻮﻣﺘﺮ 1 پﺲ ﺍﺗﺼﺎﻝ ﺑﻪ ﺑﺮﻕ ﺩﺳﺘگﺎﻩ ﺭﺍ ﺭﻭﺷﻦ ﻣﻲ ﻛﻨﻴﻢ ﺣﺪﻭﺩ 01 ﺩﻗﻴﻘﻪ ﺻﺒﺮ ﻣﻲ ﻛﻨﻴﻢ ﺗﺎ ﺑﻪ ﺍﺻﻄﻼﺡ ﺩﺳﺘگﺎﻩ گﺮﻡ ﺷﻮﺩ 2 ﻃﻮﻝ ﻣﻮﺝ ﻣﻮﺭﺩ ﻧﻈﺮ ﺭﺍ ﺍﻧﺘﺨﺎﺏ ﻣﻲ ﻛﻨﻴﻢ 3 ﺑﺎ ﺍﺳﺘﻔﺎﺩﻩ ﺑﻼﻧﻚ ﺩﺳﺘگﺎﻩ ﺭﺍ ﺻﻔﺮ ﻣﻴﻜﻨﻴﻢ 4ﺍﺳﺘﺎﻧﺪﺍﺭﺩآﺰﻣﺎﻳﺶ ﻭ ﻧﻤﻮﻧﻪ ﺑﻪ ﺗﺮﺗﻴﺐ ﺑﻪ ﻛﻮﻭﺕ ﻣﻨﺘﻘﻞ ﻧﻤﻮﺩﻩ ﻭ OD آﻦ ﺭﺍ ﻣﻲ ﺧﻮﺍﻧﻴﻢ 5 ﺑﺎ ﻳﻚ ﻣﺤﺎﺳﺒﻪ ﻏﻠﻈﺖ ﻧﻤﻮﻧﻪ ﺑﺪﺳﺖ ﻣﻲ آﻮﺭﻳﻢ A=ODt/ODs X S
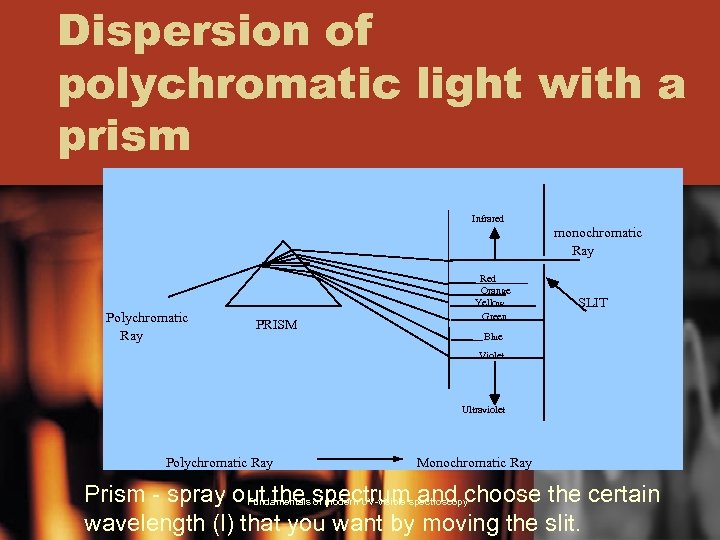
Dispersion of polychromatic light with a prism Infrared Polychromatic Ray PRISM Red Orange Yellow Green monochromatic Ray SLIT Blue Violet Ultraviolet Polychromatic Ray Monochromatic Ray Prism - spray out the spectrum and choose the certain Fundamentals of modern UV-visible spectroscopy wavelength (l) that you want by moving the slit.

Photomultiplier Tube Detector • High sensitivity at low light levels • Cathode material determines spectral sensitivity • Good signal/noise • Shock sensitive Anode Fundamentals of modern UV-visible spectroscopy
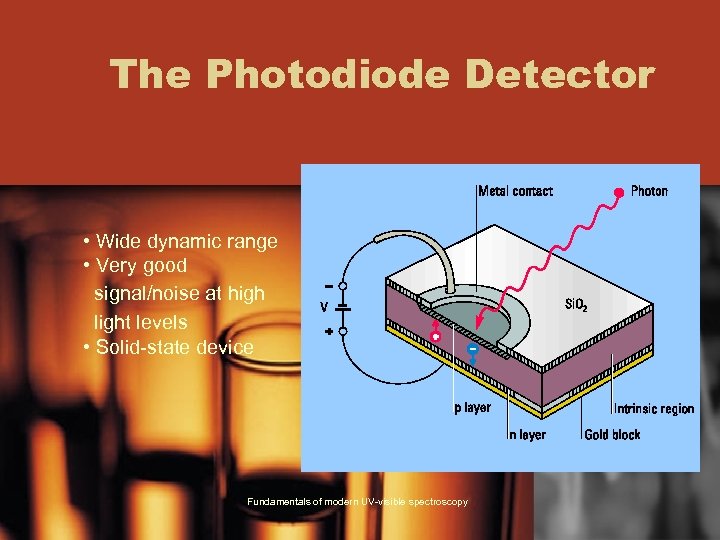
The Photodiode Detector • Wide dynamic range • Very good signal/noise at high light levels • Solid-state device Fundamentals of modern UV-visible spectroscopy
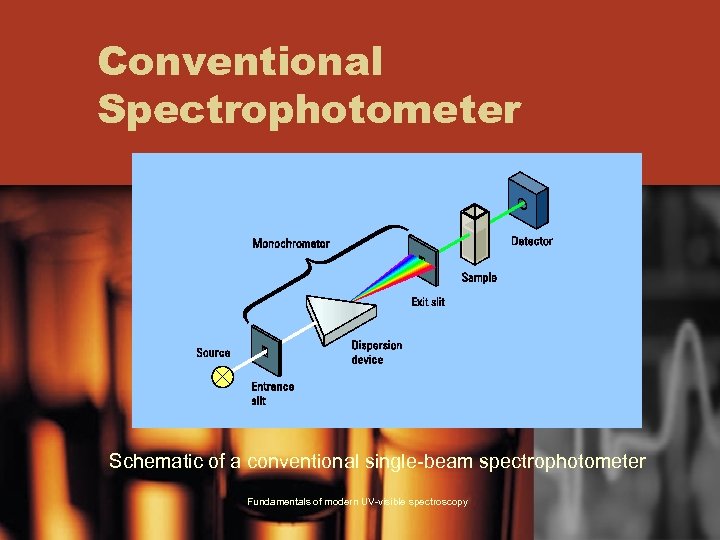
Conventional Spectrophotometer Schematic of a conventional single-beam spectrophotometer Fundamentals of modern UV-visible spectroscopy
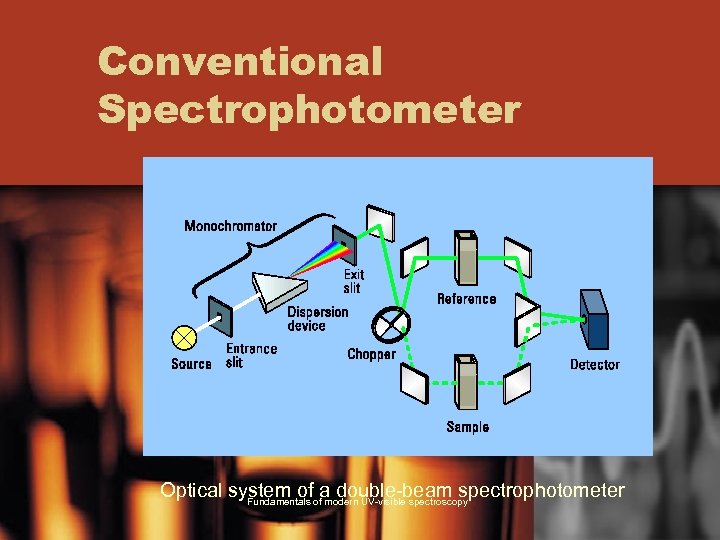
Conventional Spectrophotometer Optical system of a double-beam spectrophotometer Fundamentals of modern UV-visible spectroscopy
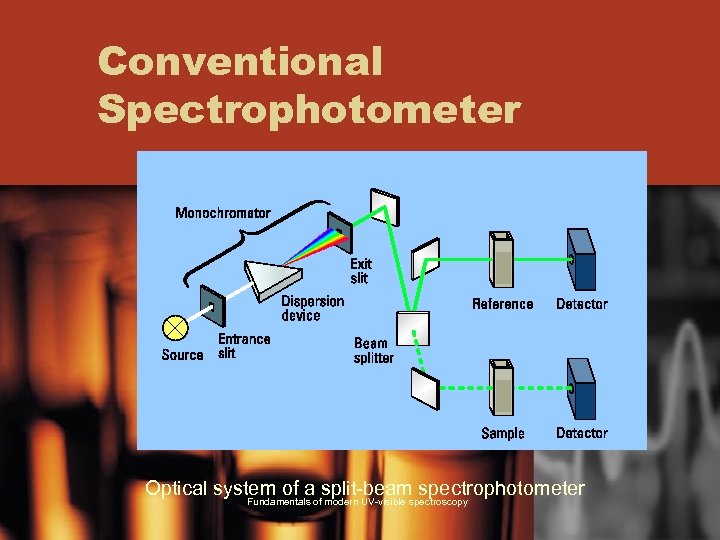
Conventional Spectrophotometer Optical system of a split-beam spectrophotometer Fundamentals of modern UV-visible spectroscopy
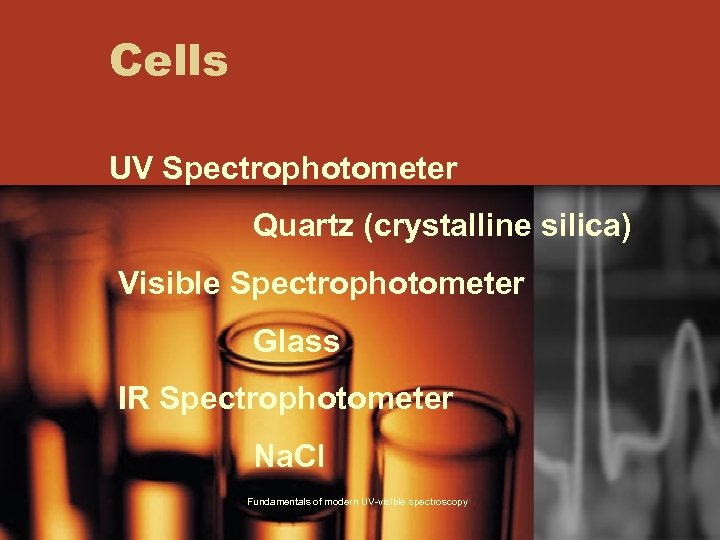
Cells UV Spectrophotometer Quartz (crystalline silica) Visible Spectrophotometer Glass IR Spectrophotometer Na. Cl Fundamentals of modern UV-visible spectroscopy
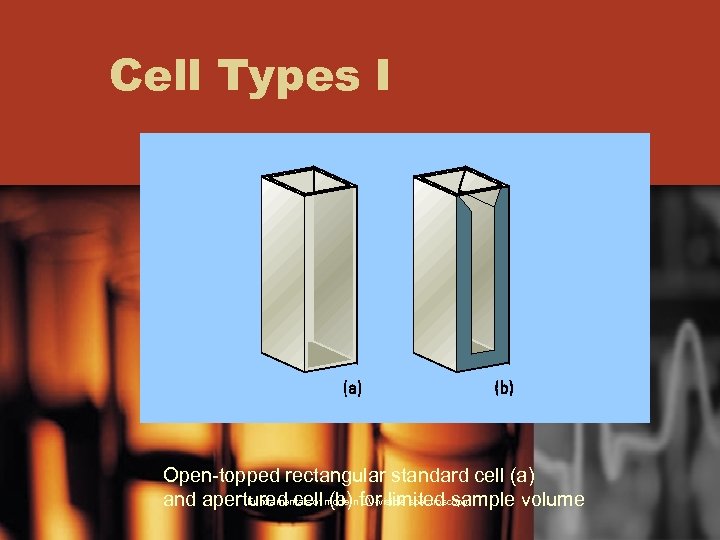
Cell Types I Open-topped rectangular standard cell (a) Fundamentals of modern UV-visible spectroscopy and apertured cell (b) for limited sample volume
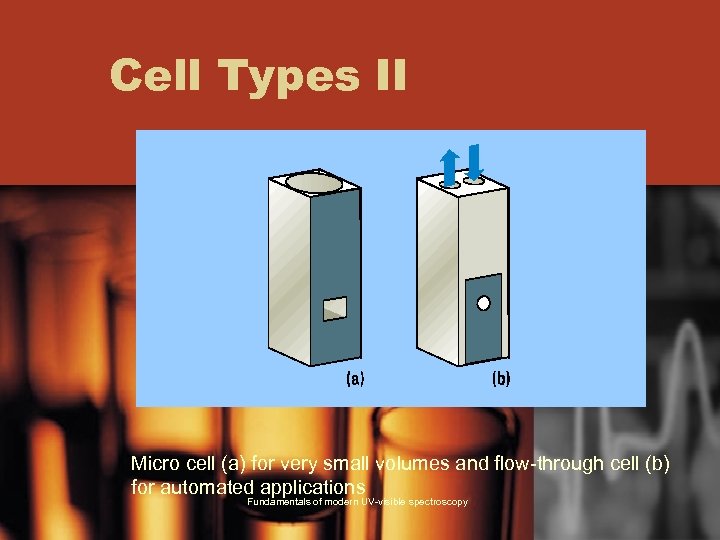
Cell Types II Micro cell (a) for very small volumes and flow-through cell (b) for automated applications Fundamentals of modern UV-visible spectroscopy
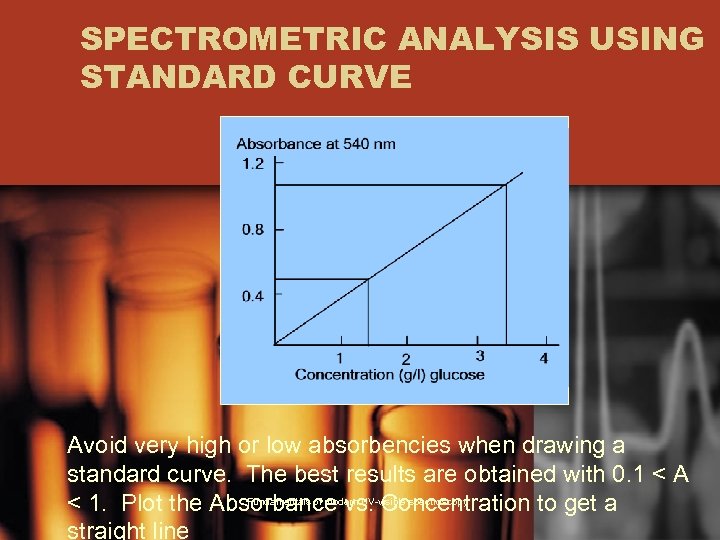
SPECTROMETRIC ANALYSIS USING STANDARD CURVE Avoid very high or low absorbencies when drawing a standard curve. The best results are obtained with 0. 1 < A Fundamentals of modern UV-visible spectroscopy < 1. Plot the Absorbance vs. Concentration to get a straight line

Relating Absorbance and Transmittance • Absorbance rises linearly with concentration. Absorbance is measured in units. • Transmittance decreases in a nonlinear fashion. • Transmittance is measured as a %. • Absorbance = log 10 – (100/% transmittance) Fundamentals of modern UV-visible spectroscopy
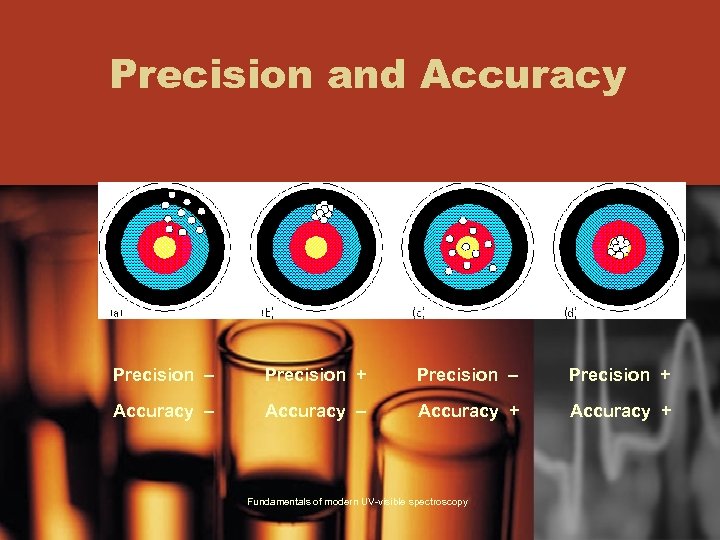
Precision and Accuracy Precision – Precision + Accuracy – Accuracy + Fundamentals of modern UV-visible spectroscopy

Thanks for your kind attention Fundamentals of modern UV-visible spectroscopy
856342ed5b15c230cf26e96a94181b32.ppt