66fc3235e580aea2b2ab50c752cdb28c.ppt
- Количество слайдов: 39

ﺍﻟﻤﻤﺎﺭﺳﺎﺕ ﺍﻟﺠﻴﺪﺓ ﺍﻟﻤﺘﻌﺪﺩﺓ ﻓﻲ ﺍﻟﺼﻨﺎﻋﺎﺕ ﺍﻟﺪﻭﺍﺋﻴﺔ Gx. P ﺗﻘﺪﻳﻢ: ﺩ. ﺣﺒﻴﺐ ﻋﺒﻮﺩ ﻣﺪﻳﺮ ﻣﺨﺎﺑﺮﺍﻟﺮﻗﺎﺑﺔﻭﺍﻟﺒﺤﻮﺙﺍﻟﺪﻭﺍﺋﻴﺔ ﻃﺮﻃﻮﺱ 7 /01/ 6102

Gx. P G = Good x (variable replaced with Manufacturing, Clinical, Laboratory, Storage, Distribution and Review, Pharmacy) P = Practice
Gx. P • “Gx. P” is a collective term for the Good Practice quality guidelines and regulations used in many fields, encompassing such internationallyrecognized standards as GMP, GCP, GLP, GSP, GDP and GRP.

List of Gx. P’s in Pharmaceuticals 1. 2. 3. 4. 5. 6. GMP – (Good manufacturing Practice) GCP – (Good Clinical Practice) GLP – (Good Laboratory Practice) GSP – (Good Storage Practice) GDP – (Good Distribution practice) GRP – (Good Review Practice)

Gx. P guidelines are designed to ensure that products are safe, meet their intended use and, in regulated industries such as drugs, food, medical devices and cosmetics, adhere to quality processes during manufacturing, control, storage and distribution.

Gx. P

Who Governs
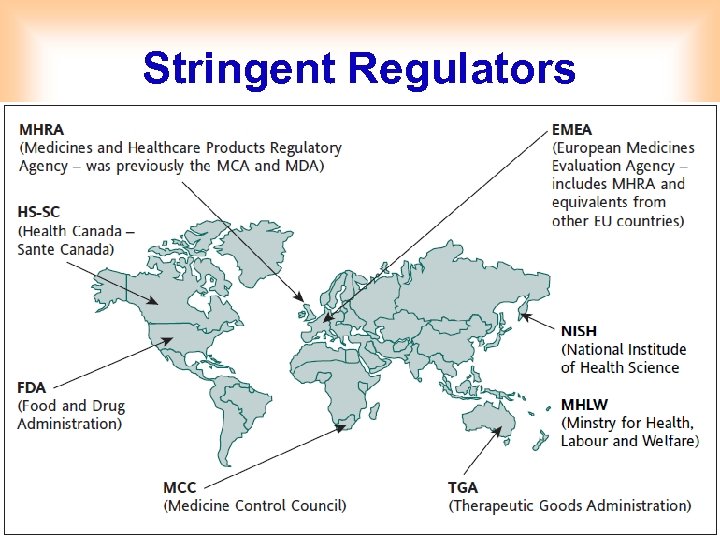
Stringent Regulators
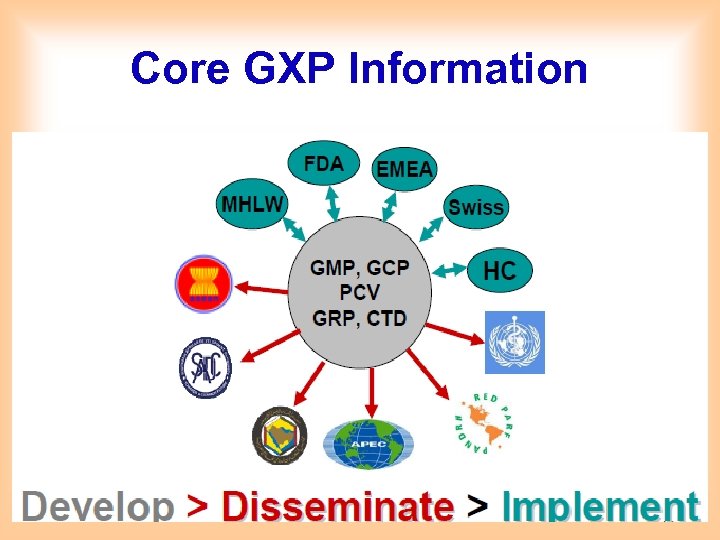
Core GXP Information

Regional Harmonization Initiatives


GMP guidelines • GMP as per Schedule “M” www. cdsco. nic. in • GMP as per WHO www. who. int • GMP as per MCA now known as MHRA www. mca. gov. uk • GMP as per TGA www. tga. gov. au • GMP as per US FDA www. fda. gov • GMP as per ICH guidelines www. ich. org

GMP • • GMP in solid dosage forms GMP in semisolid dosage forms GMP in Liquid orals GMP in Parenterals Production GMP in Ayurvedic medicines GMP in Bio technological products GMP in Nutraceuticals and cosmeceuticals GMP in Homeopathic medicines

List of important documents in Gx. P • • • Policies SOP Specifications MFR (Master Formula Record) BMR Manuals Master plans/ files Validation protocols Forms and Formats Records

10 attributes of a good document 1. Accurate 2. Clear 3. Complete 4. Consistent 5. Indelible 6. Legible 7. Timely 8. Direct 9. Authentic 10. Authorized

Preamble • The Pharmaceutical Industry is constantly being challenged to comply with rigorous regulatory requirements. • Satisfying regulatory agencies that a company’s processes are being operated at a level of control that will ensure that their products will meet predetermined safety, efficacy and quality specifications. • Compliance is evolving from an isolated departmental initiative to an enterprise level risk management challenge. • However, compliance is not a one-time event and organizations are redesigning their compliance programs to make them repeatable processes that could be sustained.

Cost of Non – Compliance Regulators Act ! Non Compliance Observations Warning Letter (WL) Import Alerts Withheld Approvals Cancellation Of Government Contracts Product Recalls Seizures A Consent Decree Of Permanent Injunction Civil Money Penalties Suspension / Revocation Prosecution

Challenges • Rising Standards of Quality • Rising Regulatory requirements and reporting mandates • International Regulatory Requirement Harmonization Compliance is not one time requirement, it’s required Round the Clock

Warning Letters Issued • Total 26 warning letters issued in the year 2011

Regulators Speak through Regulations GLP GAMP Good Laboratory Practices 21 CFR Part 58 40 CFR Part 160 ( EPA ) Good Automated Manufacturing Practices GAMP 5(ISPE) Guide c. GMP Current Good Manufacturing Practices 21 CFR Part 211 ( Pharma ) 21 CFR Part 820 ( Med devices) 21 CFR Part 110 ( Food ) 21 CFR 600 – 680 Biologics GALP Good Automated Laboratory Practices EPA Directive 2185 ( 1995 Ed. ) ICH Guidelines Quality, Efficacy, Safety, CTD / e – CTD GCP Good Clinical Practices 21 CFR 312 Sub part Q 1 A(R 2) Q 1 B Q 1 C Q 1 D Q 1 E Q 7 Q 8(R 1) Q 9 Q 10 M 4(R 3)* Stability Testing Good Manufacturing Practice Guide for Active Pharmaceutical Ingredients Pharmaceutical Development Quality Risk Management Pharmaceutical Quality System Organization of the Common Technical Document for the Registration of Pharmaceuticals for Human Use

Regulators Speak through Regulations • GMP guidelines comprise strong recommendation on Quality Management, Personnel Production, Facility and Equipment, Documentation and Records, Product and inprocess Control, Packaging and Identification, Labeling, Storage and Distribution, Laboratory Controls, Validation, Complaint and Recalls, and Contract Manufacturers. • The WHO version of GMPs was prepared in 2004 (20 th World Assembly) from then, there are several amendments and extensions of the guidelines. • Schedule M (1987): Good Manufacturing Practices and requirements of Premises , Plant and Equipment for Pharmaceutical Products were regulated. • Schedule M (2001) : Stringent norms to match with Global requirements were devised.

Regulatory Guideline Updates In the year 2011, total 46 changes in the guidelines were published.

FDA Today

FDA Today

Pillars of Compliance Man Materials Equipments Environment Facility & Infrastructure Operations R&D QA QC RA

Compliance Approach

Do w ply m Co I Quality Assurance Systems Ho Analytical Control Validation & Calibration Equipment Control Process Control Material Control Supplier Management

Supplier Management Importance of Knowing the supply chain “Using a pharmaceutical ingredient without knowing the manufacturing location, where it’s been, and how it got to you is like using a toothbrush found lying in a public restroom. ”

Challenges in Assuring Supply Chain – Quality System Vulnerabilities § Often vendor audits restricted to the evaluation questionnaire and obtaining TSE/BSE certifications § Certificates of analysis (COAs) – Over reliance on COAs – Original manufacturer’s COA not always obtained – COA often altered to remove true identity of manufacturer – Reported test results may be unreliable or falsified § Supplier qualification programs, quality agreements, and lifecycle monitoring are often deficient § Distant manufacturing sites

GRP – (Good Review Practice)

GRP – (Good Review Practice) • A good review practice (GRP) is a documented best practice within CDER that discusses any aspect related to the process, format, content, and/or management of a product review. • GRPs are developed over time as superior practices based on CDER’s collective experience to provide consistency to the overall review process of new products.

GRP – (Good Review Practice) • GRPs improve efficiency, clarity, and transparency of the review process and review management. • GRPs are expected to be adopted by review staff as standard processes through supervisor mentoring, implementation teams, and formal training when necessary. • Developing GRPs is an attempt to identify, collect, enhance, implement, and adopt may of these best practices as documented and

GRPs Fundamental Values − Quality — Consistent implementation of GRPs by review staff will enhance the quality of reviews, the review process, and the resultant regulatory action. − Efficiency — GRPs will improve the efficiency of the review process through standardization. − Clarity — GRPs support clarity throughout the review process, including critical review and decision activities that must be completed before

GRPs Fundamental Values − Transparency — Developing and documenting GRPs ensures that our review processes are readily available in one location via the Internet (through CDER’s Web site) to sponsors and the public. − Consistency — By offering a consistent approach and only deviating from it when appropriate (after supervisory concurrence), GRPs help reviewers achieve consistency with their reviews and provide standard review processes across divisions and offices.

Pharmaceutical Quality System Change Control Planned Modification Out Of Trend Training Internal Quality Audit Event Investigation CAPA Market Complaint

Road to Compliance

Road to Compliance

When it comes to ensuring drug product quality and ultimately Consumer/Patient Safety. . . We need to think and act globally!

66fc3235e580aea2b2ab50c752cdb28c.ppt