a170e2b53b02e227787bb14d5d849203.ppt
- Количество слайдов: 150

ﺍﻛﺘﺸﺎﻑ ﺍﻟﺪﻭﺍﺀ ﻭﺻﻮﻻ ﻟﻼﺧﺘﺒﺎﺭﺍﺕ ﺍﻟﺴﺮﻳﺮﻱ ﻭآﻠﻴﺔ ﺍﻟﺤﺼﻮﻝ ﻋﻠﻰ ﺗﺮﺧﻴﺺ ﻣﻨﻈﻤﺔ ﺍﻟﺪﻭﺍﺀ ﻭﺍﻟﻐﺬﺍﺀ ﺇﻋﺪﺍﺩ ﺣﺴــﻦ ﺑﻦ ﻋﺒﺪ ﺍﻟﻘﺎﺩﺭ ﺣﺴــﻦ ﺍﻟﺒــــــﺎﺭ ﻗﺴﻢ ﺍﻟﻜﻴﻤﻴﺎﺀ – ﻛﻠﻴﺔ ﺍﻟﻌﻠﻮﻡ ﺟﺎﻣﻌﺔ ﺍﻟﻤﻠﻚ ﻋﺒﺪ ﺍﻟﻌﺰﻳﺰ 8002 ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

ﺍﻟﻤﺤﺎﺿﺮﺓ 1 ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

ﺗﺼﻤﻴﻢ ﺗﺮﻛﻴﺒﺔ ﺟﺮﻋﺔ ﺍﻟﺪﻭﺍﺀ 1( ﻃﺮﻳﻘﺔ ﻭﻣﺴﺎﺭ ﺗﻌﺎﻃﻲ ﺍﻟﻤﺮﻳﺾ ﺍﻟﺪﻭﺍﺀ 2( ﺣﺮﻛﻴﺔ ﺻﻴﺪﻻﻧﻴﺔ 3( ﺩﻳﻨﻤﻜﺔ ﺻﻴﺪﻻﻧﻴﺔ 4( ﺻﻴﺪﻟﺔ ﻭﺳﻤﻴﺔ ﻭﺳﻼﻣﺔ ﺍﻟﺪﻭﺍﺀ ﺍﻟﻤﺤﺘﻤﻠﺔ 5( ﻣﻌﺮﻓﺔ ﺧﻮﺍﺹ ﺍﻟﺪﻭﺍﺀ ﺍﻟﻜﻴﻤﻴﺎﺋﻴﺔ ﻭﺍﻟﻔﻴﺰﻳﺎﺋﻴﺔ ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

ﺑﺮﻧﺎﻣﺞ ﺍﻟﻤﺮﺣﻠﺔ ﺍﻟﺜﺎﻧﻮﻳﺔ ﻣﺎ ﻗﺒﻞ ﺍﻟﻄﺒﻴﺔ ﻳﺠﺐ ﺃﻦ ﻳﻜﻮﻥ ﺧﺎﺿﻊ ﻟﻨﻈﺎﻡ ﺗﻤﻴﺰ ﺍﻟﻤﻌﻤﻞ ﺍﻟﺠﻴﺪ GLP ﻭﺗﻜﻮﻥ ﺍﻟﻨﺘﺎﺋﺞ ﻣﺘﻤﺎﺷﻴﺔ ﻣﻊ ﺃﻨﻈﻤﺔ ﻣﻨﻈﻤﺔ ﺍﻟـ DFA ﻳﺠﺐ ﺃﻦ ﺗﻜﻮﻥ ﺍﻟﻤﺴﻴﺮﺓ ﺍﻟﺒﺤﺜﻴﺔ ﻭﺍﻟﺘﻄﺒﻴﻘﻴﺔ ﻟﻠﺪﻭﺍﺀ ﻣﺘﻮﺍﺋﻤﺔ ﻣﻊ ﻗﻮﺍﻋﺪ ﺍﻟـ . GLP " ﺩﺭﺍﺳﺎﺕ ﺍﻟﻤﺮﺣﻠﺔ ﺍﻟﺜﺎﻧﻮﻳﺔ ﻣﺎ ﻗﺒﻞ ﺍﻟﻄﺒﻴﺔ" )1( ﺍﻟﺼﻴﺪﻻﻧﻴﺔ ﺍﻻﺣﻴﺎﺋﻴﺔ Biopharmaceutics ﺍﻟﺬﻭﺑﺎﻧﻴﺔ – ﺗﻜﻮﻳﻦ ﻣﻠﺢ ﺍﻟﻤﺮﻛﺐ – –p. H –p. Ka ﺷﻜﻞ ﻭﺣﺠﻢ ﺍﻟﺨﺎﺭﺟﻲ ﻟﺒﻠﻮﺭﺍﺕ ﺍﻟﺪﻭﺍﺀ – ﺗﻌﺪﺩﻳﺔ ﺃﺸﻜﺎﻝ ﻭﺃﺤﺠﺎﻡ ﺑﻠﻮﺭﺍﺕ ﺍﻟﺪﻭﺍﺀ – ﺛﺒﺎﺗﻴﺔ ﺍﻟﺪﻭﺍﺀ )ﺟﻬﺔ ﺍﻟﻀﻮﺀ ﺍﻟﺤﺮﺍﺭﺓ ﺍﻷﻜﺴﺠﻴﻦ( – ﻧﻘﺎﻭﺓ ﺍﻟﺪﻭﺍﺀ – ﺗﺼﻨﻴﻌﻪ ﺑﻜﻤﻴﺎﺕ ﻛﺒﻴﺮﺓ. ﻭﺗﺸﻤﻞ ﺍﻟﻌﻨﺎﺻﺮ ﺍﻟﺘﺎﻟﻲ: - a ﻛﻴﻤﻴﺎﺀ ﺍﻟﻔﻴﺰﻳﺎﺀ Physicochemistry ﺍﻟﻤﻜﻮﻧﺎﺕ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ ﺍﻟﻨﺸﻄﺔ API ﺍﻟﺘﺮﻛﻴﺐ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ ﺍﻟﻤﺒﺪﺋﻴﺔ per-formulation Delivery system - b ﺩﺭﺍﺳﺔ ﺻﻴﺪﻻﻧﻴﺔ ﺃﻤﺎﻧﺔ Safety Pharmacology - c ﺍﻟﺤﺮﻛﻴﺔ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ (ADME) PK - d ﺍﻟﺪﻳﻨﻤﻜﺔ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ PD )2( ﺍﻟﺴﻤﻴﺔ )ﺍﻻﻣﺘﺼﺎﺹ – ﺍﻟﺘﻮﺯﻳﻊ – ﺍﻟﺘﻔﺎﻋﻼﺕ ﺍﻟﺤﻴﻮﻳﺔ – ﺍﻟﺤﺬﻑ – ﺍﻟﺴﻤﻴﺔ ﺑﺎﻟﺠﺴﻢ( )3( ﺍﻟﺘﺤﻜﻢ ﺍﻟﺘﺼﻨﻴﻌﻲ ﺍﻟﻜﻴﻤﻴﺎﺋﻲ : CMC ﻳﺘﻢ ﺗﻨﻔﻴﺬﻩ ﺧﻼﻝ ﻣﺮﺣﻠﺔ ﻣﺎ ﻗﺒﻞ ﺍﻟﻄﺒﻴﺔ ﻳﻌﺘﻤﺪ ﻋﻠﻰ ﺍﻟﻤﻜﻮﻧﺎﺕ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ ﺍﻟﻨﺸﻄﺔ API ﻭﻫﻲ ﺗﺨﺘﺺ ﺑﺎﻟﺪﻭﺍﺀ ﻧﻔﺴﻪ ﻋﻠﻰ ﺍﻟﻨﺤﻮ ﺍﻟﺘﺎﻟﻲ: )1( ﻗﺎﺑﻠﻴﺔ ﺗﺼﻨﻴﻌﻪ )2( ﺗﺼﻨﻴﻌﻪ ﺑﻜﻤﻴﺎﺕ ﺍﻗﺘﺼﺎﺩﻳﺔ )3( ﻳﺨﻀﻊ ﻷﻨﻈﻤﺔ (4) GMP ﻧﺒﺬﺓ ﻋﻦ ﺍﻟﺸﻮﺍﺋﺐ )5( ﺑﻘﺎﻳﺎ ﺍﻟﻤﺬﻳﺒﺎﺕ )6( ﺍﻟﺘﺤﻜﻢ ﻓﻲ ﻃﺮﻳﻘﺔ ﺍﻹﻧﺘﺎﺝ )7ﻡ 8002 ﺳﺘﻤﺒﺮﺍﻟﺪﻭﺍﺀ )8( ﺗﺤﻮﺭ ﺍﻟﺪﻭﺍﺀ ﺍﻟﺘﺸﻜﻴﻠﻲ )9( ﺍﻟﻜﻴﻤﻴﺎﺀ ﺍﻟﻔﺮﺍﻏﻴﺔ ( ﺻﻼﺣﻴﺔ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ )01( ﻣﺴﺎﺋﻞ ﺑﺮﺍﺀﺓ ﺍﻷﺨﺘﺮﺍﻉ.

ﻣﻌﺎﻳﻴﺮ ﻣﻌﺮﻓﺔ ﻓﻌﺎﻟﻴﺔ ﺍﻟﻤﺎﺩﺓ ﻻﻋﺘﺒﺎﺭﻫﺎ "ﺍﻛﺘﺸﺎﻑ ﺩﻭﺍﺀ" )1( NCE Library ﺗﻌﺘﺒﺮ ﺍﻟﻤﺎﺩﺓ ﺟﺪﻳﺪﺓ ، ﻓﺒﺎﻟﺘﺎﻟﻲ ﻻ ﻳﺴﺘﻄﻴﻊ ﺃﺤﺪ ﺑﺎﻟﻤﻄﺎﻟﺒﺔ ﺑﺄﻲ ﻣﻠﻜﻴﺔ ﻓﻜﺮﻳﺔ ﻟﻠﻤﺎﺩﺓ. ﻭﺍﻟﺘﺄﻜﺪ ﻣﻦ ﺃﻨﻪ ﻻ ﻳﻮﺟﺪ ﻓﺮﻳﻖ ﻋﻤﻞ آﺨﺮ ﻳﺨﺘﺒﺮﻫﺎ ﻛﺪﻭﺍﺀ. )2( ﺍﻟﺬﻭﺑﺎﻧﻴﺔ ﻓﻲ ﺍﻟﻤﺎﺀ )3( ﺍﺧﺘﺒﺎﺭﺍﺕ ﺍﻟﺨﻼﻳﺎ ﺍﻟﻨﺴﻴﺠﻴﺔ ﺗﺸﻴﺮ ﺇﻟﻰ ﺍﻧﻪ ﺍﻟﻤﺮﻛﺐ ﻻ ﺳﻤﻴﺔ ﻟﻪ ، ﻭﻟﻪ ﻧﺸﺎﻁ ﺑﻴﻮﻟﻮﺟﻲ. )4( Log P / Log D )5( ﺛﺒﺎﺗﻴﺔ ﺍﻟﻤﺎﺩﺓ )6( Cell transport ؟؟؟؟؟؟؟؟ - ( animal ) Bioavaillability ﻛﻤﻴﺔ ﺍﻟﺪﻭﺍﺀ ﻓﻲ - ﺍﺳﺘﻘﺮﺍﺭﻳﺔ ( animal ) Efficacy ﺍﻟﺒﻼﺯﻣﺎ Plasma Binding ﺗﻔﺎﻋﻼﺕ ﺍﻟﻤﺎﺩﺓ ﺍﻟﺤﻴﻮﻳﺔ Metabolic stability ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ
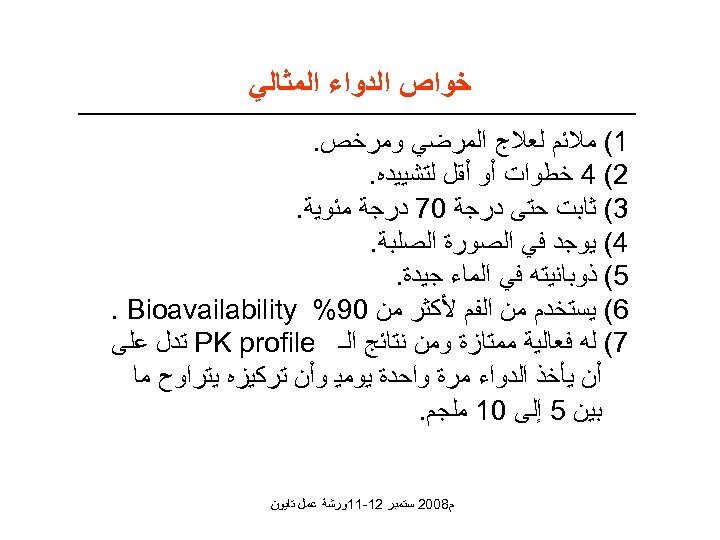
ﺧﻮﺍﺹ ﺍﻟﺪﻭﺍﺀ ﺍﻟﻤﺜﺎﻟﻲ 1( ﻣﻼﺋﻢ ﻟﻌﻼﺝ ﺍﻟﻤﺮﺿﻲ ﻭﻣﺮﺧﺺ. 2( 4 ﺧﻄﻮﺍﺕ ﺃﻮ ﺃﻘﻞ ﻟﺘﺸﻴﻴﺪﻩ. 3( ﺛﺎﺑﺖ ﺣﺘﻰ ﺩﺭﺟﺔ 07 ﺩﺭﺟﺔ ﻣﺌﻮﻳﺔ. 4( ﻳﻮﺟﺪ ﻓﻲ ﺍﻟﺼﻮﺭﺓ ﺍﻟﺼﻠﺒﺔ. 5( ﺫﻭﺑﺎﻧﻴﺘﻪ ﻓﻲ ﺍﻟﻤﺎﺀ ﺟﻴﺪﺓ. 6( ﻳﺴﺘﺨﺪﻡ ﻣﻦ ﺍﻟﻔﻢ ﻷﻜﺜﺮ ﻣﻦ 09% . Bioavailability 7( ﻟﻪ ﻓﻌﺎﻟﻴﺔ ﻣﻤﺘﺎﺯﺓ ﻭﻣﻦ ﻧﺘﺎﺋﺞ ﺍﻟـ PK profile ﺗﺪﻝ ﻋﻠﻰ ﺃﻦ ﻳﺄﺨﺬ ﺍﻟﺪﻭﺍﺀ ﻣﺮﺓ ﻭﺍﺣﺪﺓ ﻳﻮﻣﻴ ﻭﺃﻦ ﺗﺮﻛﻴﺰﻩ ﻳﺘﺮﺍﻭﺡ ﻣﺎ ﺑﻴﻦ 5 ﺇﻟﻰ 01 ﻣﻠﺠﻢ. ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ
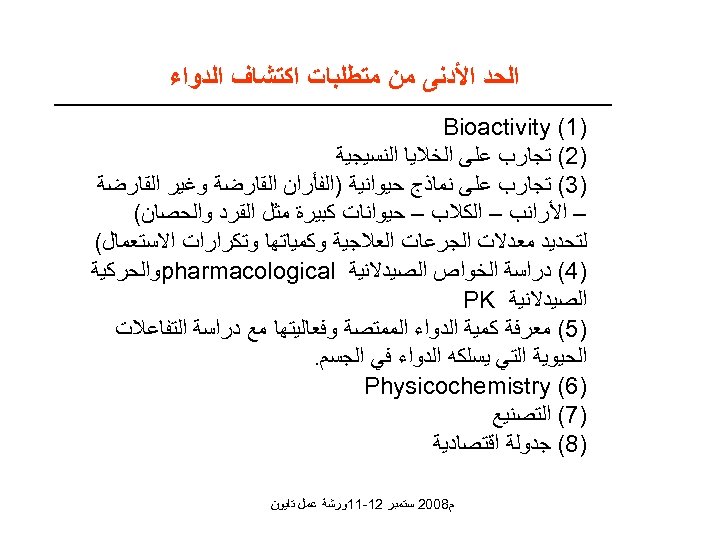
ﺍﻟﺤﺪ ﺍﻷﺪﻧﻰ ﻣﻦ ﻣﺘﻄﻠﺒﺎﺕ ﺍﻛﺘﺸﺎﻑ ﺍﻟﺪﻭﺍﺀ )1( Bioactivity )2( ﺗﺠﺎﺭﺏ ﻋﻠﻰ ﺍﻟﺨﻼﻳﺎ ﺍﻟﻨﺴﻴﺠﻴﺔ )3( ﺗﺠﺎﺭﺏ ﻋﻠﻰ ﻧﻤﺎﺫﺝ ﺣﻴﻮﺍﻧﻴﺔ )ﺍﻟﻔﺄﺮﺍﻥ ﺍﻟﻘﺎﺭﺿﺔ ﻭﻏﻴﺮ ﺍﻟﻘﺎﺭﺿﺔ – ﺍﻷﺮﺍﻧﺐ – ﺍﻟﻜﻼﺏ – ﺣﻴﻮﺍﻧﺎﺕ ﻛﺒﻴﺮﺓ ﻣﺜﻞ ﺍﻟﻘﺮﺩ ﻭﺍﻟﺤﺼﺎﻥ( ﻟﺘﺤﺪﻳﺪ ﻣﻌﺪﻻﺕ ﺍﻟﺠﺮﻋﺎﺕ ﺍﻟﻌﻼﺟﻴﺔ ﻭﻛﻤﻴﺎﺗﻬﺎ ﻭﺗﻜﺮﺍﺭﺍﺕ ﺍﻻﺳﺘﻌﻤﺎﻝ( )4( ﺩﺭﺍﺳﺔ ﺍﻟﺨﻮﺍﺹ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ pharmacological ﻭﺍﻟﺤﺮﻛﻴﺔ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ PK )5( ﻣﻌﺮﻓﺔ ﻛﻤﻴﺔ ﺍﻟﺪﻭﺍﺀ ﺍﻟﻤﻤﺘﺼﺔ ﻭﻓﻌﺎﻟﻴﺘﻬﺎ ﻣﻊ ﺩﺭﺍﺳﺔ ﺍﻟﺘﻔﺎﻋﻼﺕ ﺍﻟﺤﻴﻮﻳﺔ ﺍﻟﺘﻲ ﻳﺴﻠﻜﻪ ﺍﻟﺪﻭﺍﺀ ﻓﻲ ﺍﻟﺠﺴﻢ. )6( Physicochemistry )7( ﺍﻟﺘﺼﻨﻴﻊ )8( ﺟﺪﻭﻟﺔ ﺍﻗﺘﺼﺎﺩﻳﺔ ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ
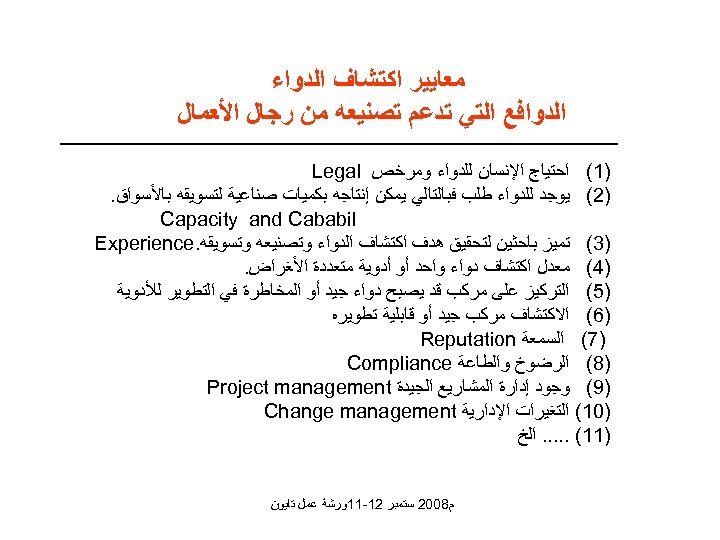
ﻣﻌﺎﻳﻴﺮ ﺍﻛﺘﺸﺎﻑ ﺍﻟﺪﻭﺍﺀ ﺍﻟﺪﻭﺍﻓﻊ ﺍﻟﺘﻲ ﺗﺪﻋﻢ ﺗﺼﻨﻴﻌﻪ ﻣﻦ ﺭﺟﺎﻝ ﺍﻷﻌﻤﺎﻝ )1( ﺍﺣﺘﻴﺎﺝ ﺍﻹﻧﺴﺎﻥ ﻟﻠﺪﻭﺍﺀ ﻭﻣﺮﺧﺺ Legal )2( ﻳﻮﺟﺪ ﻟﻠﺪﻭﺍﺀ ﻃﻠﺐ ﻓﺒﺎﻟﺘﺎﻟﻲ ﻳﻤﻜﻦ ﺇﻧﺘﺎﺟﻪ ﺑﻜﻤﻴﺎﺕ ﺻﻨﺎﻋﻴﺔ ﻟﺘﺴﻮﻳﻘﻪ ﺑﺎﻷﺴﻮﺍﻕ. Capacity and Cababil )3( ﺗﻤﻴﺰ ﺑﺎﺣﺜﻴﻦ ﻟﺘﺤﻘﻴﻖ ﻫﺪﻑ ﺍﻛﺘﺸﺎﻑ ﺍﻟﺪﻭﺍﺀ ﻭﺗﺼﻨﻴﻌﻪ ﻭﺗﺴﻮﻳﻘﻪ. Experience )4( ﻣﻌﺪﻝ ﺍﻛﺘﺸﺎﻑ ﺩﻭﺍﺀ ﻭﺍﺣﺪ ﺃﻮ ﺃﺪﻭﻳﺔ ﻣﺘﻌﺪﺩﺓ ﺍﻷﻐﺮﺍﺽ. )5( ﺍﻟﺘﺮﻛﻴﺰ ﻋﻠﻰ ﻣﺮﻛﺐ ﻗﺪ ﻳﺼﺒﺢ ﺩﻭﺍﺀ ﺟﻴﺪ ﺃﻮ ﺍﻟﻤﺨﺎﻃﺮﺓ ﻓﻲ ﺍﻟﺘﻄﻮﻳﺮ ﻟﻸﺪﻭﻳﺔ )6( ﺍﻻﻛﺘﺸﺎﻑ ﻣﺮﻛﺐ ﺟﻴﺪ ﺃﻮ ﻗﺎﺑﻠﻴﺔ ﺗﻄﻮﻳﺮﻩ )7( ﺍﻟﺴﻤﻌﺔ Reputation )8( ﺍﻟﺮﺿﻮﺥ ﻭﺍﻟﻄﺎﻋﺔ Compliance )9( ﻭﺟﻮﺩ ﺇﺩﺍﺭﺓ ﺍﻟﻤﺸﺎﺭﻳﻊ ﺍﻟﺠﻴﺪﺓ Project management )01( ﺍﻟﺘﻐﻴﺮﺍﺕ ﺍﻹﺩﺍﺭﻳﺔ Change management )11(. . . ﺍﻟﺦ ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

ﻏﻴﺮ ﻣﻔﻬﻮﻡ ﻣﻮﺍﺻﻔﺎﺕ ﺍﻟﻤﺮﻛﺒﺎﺕ ﺍﻟﺼﻐﻴﺮﺓ ﻛﺄﺪﻭﻳﺔ )1( ﺟﺰﻳﺌﺎﺕ ﺻﻐﻴﺮﺓ )2( SAR )3( 4 “ 5’s” Lipinski’s rule ﻣﻌﺪﻝ ﺍﻣﺘﺼﺎﺹ ﺍﻟﺪﻭﺍﺀ ﺑﺎﻟﺠﺴﻢ ﻣﻨﺢ ﺍﻟﻬﻴﺪﺭﻭﺟﻴﻦ ﺃﺼﻐﺮ ﻣﻦ 5 ﺍﺳﺘﻘﺒﺎﻝ ﺍﻟﻬﻴﺪﺭﻭﺟﻴﻦ ﺃﺼﻐﺮ ﻣﻦ 01 . M. Wt ﺃﺼﻐﺮ ﻣﻦ 005 log P ﺃﺼﻐﺮ ﻣﻦ 5 )4( ADME at early stage )5( ﺗﺼﻨﻴﻌﻪ ﺍﻗﺘﺼﺎﺩﻱ ﺑﻜﻤﻴﺎﺕ ﻛﺒﻴﺮﺓ )6( ﺟﺮﻋﺎﺗﻪ ﺗﺄﺨﺬ ﺑﺎﻟﻔﻢ )7( ﺗﺄﺜﻴﺮ ﻣﻌﺎﻛﺲ Adverse effect )8( ﻣﺮﻛﺐ ﻭﺍﺣﺪ ﻳﺘﻢ ﺗﺮﺧﻴﺼﻪ ﻣﻦ ﺧﻤﺴﺔ ﺇﻟﻰ ﻋﺸﺮﺓ آﻼﻑ ﻣﺮﻛﺐ ﻳﻤﻜﻦ ﺃﻦ ﻳﺤﺼﻞ ﻋﻠﻰ ﺗﺮﺧﻴﺺ ﻣﻦ ﻣﻨﻈﻤﺔ DFA ﻛﺪﻭﺍﺀ )9( ﻳﺘﻢ ﺗﺤﻀﻴﺮﻩ ﻓﻲ ﺃﻘﻞ ﻣﻦ 4 ﺧﻄﻮﺍﺕ. ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

ﺇﺳﺘﺮﺍﺗﻴﺠﻴﺔ ﺍﻟﻜﺸﻒ ﻋﻦ ﺍﻟﺪﻭﺍﺀ ﺍﻟﺘﻌﺮﻑ ﻋﻠﻰ ﻧﻮﻋﻴﺔ ﺍﻟﺪﻭﺍﺀ ﺍﻟﻤﺤﺘﺎﺝ ﻟﻪ ﺍﻹﻧﺴﺎﻥ ﻣﺜﻞ ﺍﻟﺴﺮﻃﺎﻥ – ﺃﻤﺮﺍﺽ ﺍﻟﺠﻬﺎﺯ ﺍﻟﻌﺼﺒﻲ– ﺃﻤﺮﺍﺽ ﺍﻷﻮﻋﻴﺔ ﺍﻟﻘﻠﺒﻴﺔ – Cardiovascular ﺃﻤﺮﺍﺽ ﺍﻟﻀﻐﻂ - Hypertension ﻣﺮﺽ ﻫﺸﺎﺷﺔ ﺍﻟﻌﻈﺎﻡ – Gastrointestinal ﺍﻻﻟﺘﻬﺎﺑﺎﺕ - Inflammation ﻣﺮﺽ ﻓﻘﺪﺍﻥ ﺍﻟﺬﺍﻛﺮﺓ – ﺍﻟﺒﻮﺍﻝ ﺍﻟﺴﻜﺮﻱ – ﺍﻟﺮﺑﻮ Asthma ﺍﻟﺒﺤﺚ ﻋﻦ ﻣﺎ ﻫﻲ ﺍﻟﻤﺮﻛﺒﺎﺕ ﺍﻟﺘﻲ ﺗﺼﻠﺢ ﻟﻠﻌﻼﺝ ﻣﻦ ﺿﻤﻦ ﺷﺮﻳﺤﺔ ﻛﺒﻴﺮﺓ ﻣﻦ ﺍﻟﻤﺮﻛﺒﺎﺕ. ﺍﻟﺒﺤﺚ ﻋﻦ آﻠﻴﺔ ﻋﻼﺝ ﺍﻟﻤﺮﺽ ﺑﺎﻟﺪﻭﺍﺀ ﻭﺻﻼﺣﻴﺔ ﺍﻟﺪﻭﺍﺀ ﺍﻟﻤﻜﺘﺸﻒ. ﺗﺤﺪﻳﺪ ﺃﻘﻞ ﻋﺪﺩ ﻣﻦ ﺍﻟﻤﺮﻛﺒﺎﺕ ﺍﻟﺘﻲ ﻳﻤﻜﻦ ﺃﻦ ﻳﺼﺒﺢ ﺑﻌﻀﻬﺎ ﺃﺪﻭﻳﺔ. ﺗﺠﺮﻯ ﺗﺠﺎﺭﺏ ﻣﺎ ﻗﺒﻞ ﻃﺒﻴﺔ. ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

ﺍﻟﺤﺪ ﺍﻷﺪﻧﻰ ﻣﻦ ﻣﺘﻄﻠﺒﺎﺕ ﺍﻛﺘﺸﺎﻑ ﺍﻟﺪﻭﺍﺀ 1 ﺗﺠﺎﺭﺏ ﻋﻠﻰ ﺍﻟﺨﻼﻳﺎ ﺍﻟﻨﺴﻴﺠﻴﺔ 2 ﺗﺠﺎﺭﺏ ﻋﻠﻰ ﻧﻤﺎﺫﺝ ﺣﻴﻮﺍﻧﻴﺔ )ﺍﻟﻔﺄﺮﺍﻥ ﺍﻟﻘﺎﺭﺿﺔ ﻭﻏﻴﺮ ﺍﻟﻘﺎﺭﺿﺔ – ﺍﻷﺮﺍﻧﺐ – ﺍﻟﻜﻼﺏ – ﺣﻴﻮﺍﻧﺎﺕ ﻛﺒﻴﺮﺓ ﻣﺜﻞ ﺍﻟﻘﺮﺩ ﻭﺍﻟﺤﺼﺎﻥ( ﻟﺘﺤﺪﻳﺪ ﻣﻌﺪﻻﺕ ﺍﻟﺠﺮﻋﺎﺕ ﺍﻟﻌﻼﺟﻴﺔ ﻭﻛﻤﻴﺎﺗﻬﺎ ﻭﺗﻜﺮﺍﺭﺍﺕ ﺍﻻﺳﺘﻌﻤﺎﻝ( 3 ﺩﺭﺍﺳﺔ ﺍﻟﺨﻮﺍﺹ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ cpharmacological ﻭﺍﻟﺤﺮﻛﻴﺔ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ PK 4 ﻣﻌﺮﻓﺔ ﻛﻤﻴﺔ ﺍﻟﺪﻭﺍﺀ ﺍﻟﻤﻤﺘﺼﺔ ﻭﻓﻌﺎﻟﻴﺘﻬﺎ ﻣﻊ ﺩﺭﺍﺳﺔ ﺍﻟﺘﻔﺎﻋﻼﺕ ﺍﻟﺤﻴﻮﻳﺔ ﺍﻟﺘﻲ ﻳﺴﻠﻜﻪ ﺍﻟﺪﻭﺍﺀ ﻓﻲ ﺍﻟﺠﺴﻢ. 5 Physicochemistry 6 ﺍﻟﺘﺼﻨﻴﻊ -7 ﺟﺪﻭﻟﺔ ﺍﻗﺘﺼﺎﺩﻳﺔ ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

ﻣﺼﺎﺩﺭ ﺍﻛﺘﺸﺎﻑ ﺍﻟﺪﻭﺍﺀ ﻻ ﺗﻮﺟﺪ ﺑﺮﺗﻮﻛﻮﻻﺕ ﻭ/ﺃﻮ ﺍﺳﺘﺮﺍﺗﻴﺠﻴﺎﺕ ﻭﺍﺿﺤﺔ ﺍﻟﻤﻌﺎﻟﻢ ﻹﺗﺒﺎﻋﻬﺎ ﻻﻛﺘﺸﺎﻑ ﺍﻟﺪﻭﺍﺀ ﺑﻞ ﺗﻮﺟﺪ ﻣﺼﺎﺩﺭ ﻣﺘﻨﻮﻋﺔ ﻣﻨﻬﺎ: ﺍﻟﺠﺎﻣﻌﺎﺕ - ﻣﺮﺍﻛﺰ ﺍﻷﺒﺤﺎﺙ – ﺍﻟﺤﻜﻮﻣﺎﺕ - ﺑﻌﺾ ﺍﻟﻤﺨﺘﺒﺮﺍﺕ ﻭ/ﺃﻮ ﺍﻟﻤﺘﺨﺼﺼﻴﻦ ﻗﺪ ﺗﺴﻬﻞ ﻋﻠﻰ ﺍﻟﺸﺮﻛﺎﺕ ﺗﺘﺒﻊ ﺑﻌﺾ ﺍﻟﻤﻮﺍﺩ ﻟﺘﺴﺘﺨﺪﻡ ﻛﻌﻼﺝ - ﺍﻟﺼﺪﻓﺔ ﻣﺼﺎﺩﺭ ﺍﻟﻤﻮﺍﺭﺩ ﺍﻟﻄﺒﻴﻌﻴﺔ ﻭ/ﺃﻮ ﺍﻟﻜﻴﻤﻴﺎﺋﻴﺔ ﺍﻟﺘﻲ ﻳﻤﻜﻦ ﺍﻛﺘﺸﺎﻑ ﺑﻌﻀﻬﺎ ﻛﺄﺪﻭﻳﺔ )1( ﺍﻟﻨﺒﺎﺗﺎﺕ ﺍﻟﺘﻲ ﺗﺴﺘﺨﺪﻡ ﻓﻲ ﺍﻟﻄﺐ ﺍﻟﺸﻌﺒﻲ ﻭﺃﺠﺮﻳﺖ ﻋﻠﻴﻬﺎ ﺑﻌﺾ ﺍﻟﺘﺠﺎﺭﺏ ﺍﻟﻌﻠﻤﻴﺔ ﻭﺗﻢ ﺇﺩﺭﺍﺟﻬﺎ ﺗﺤﺖ ﻗﺎﺋﻤﺔ ﺍﻟﻨﺒﺎﺗﺎﺕ ﺍﻟﻄﺒﻴﺔ. Natural Products )2( ﺍﻟﻤﻮﺍﺭﺩ ﺍﻟﻤﺎﺋﻴﺔ ﻓﻲ ﺍﻟﺒﺤﺎﺭ ﻭﺍﻷﻨﻬﺎﺭ ﻭﺍﻟﻤﺴﺘﻨﻘﻌﺎﺕ ﻭﻏﻴﺮﻫﺎ. Natural Products )3( ﺍﻟﺘﺸﻴﻴﺪ ﺍﻟﻜﻴﻤﻴﺎﺋﻲ ﻟﻠﻤﺮﻛﺒﺎﺕ ﺍﻟﺘﻲ ﺑﻌﻀﻬﺎ ﺑﺎﻟﻔﻌﻞ ﺗﻢ ﺍﺳﺘﻌﻤﺎﻟﻬﺎ ﻛﺄﺪﻭﻳﺔ ﻣﺮﺧﺼﺔ. Synthetic compounds )4( ﺍﻟﺘﺸﻴﻴﺪ ﺍﻟﺒﻴﻮﻟﻮﺟﻲ ﺧﻼﻝ ﻧﻤﻮ ﺑﻌﺾ ﺍﻟﻜﺎﺋﻨﺎﺕ ﺍﻟﺪﻗﻴﻘﺔ ﻭﺍﻟﺤﻴﻮﻳﺔ Proteomics )5( ﺍﻟﻜﻴﻤﻴﺎﺀ ﺍﻟﺘﻜﺎﻣﻠﻴﺔ Combinatorial chemistry )6( ﺍﻟﺼﺪﻓﺔ ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ
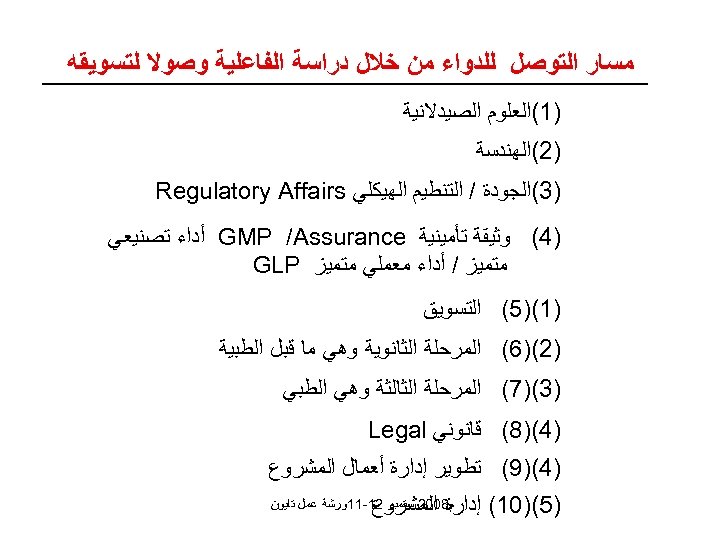
ﻣﺴﺎﺭ ﺍﻟﺘﻮﺻﻞ ﻟﻠﺪﻭﺍﺀ ﻣﻦ ﺧﻼﻝ ﺩﺭﺍﺳﺔ ﺍﻟﻔﺎﻋﻠﻴﺔ ﻭﺻﻮﻻ ﻟﺘﺴﻮﻳﻘﻪ )1(ﺍﻟﻌﻠﻮﻡ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ )2(ﺍﻟﻬﻨﺪﺳﺔ )3(ﺍﻟﺠﻮﺩﺓ / ﺍﻟﺘﻨﻄﻴﻢ ﺍﻟﻬﻴﻜﻠﻲ Regulatory Affairs )4( ﻭﺛﻴﻘﺔ ﺗﺄﻤﻴﻨﻴﺔ GMP /Assurance ﺃﺪﺍﺀ ﺗﺼﻨﻴﻌﻲ ﻣﺘﻤﻴﺰ / ﺃﺪﺍﺀ ﻣﻌﻤﻠﻲ ﻣﺘﻤﻴﺰ GLP )1()5( ﺍﻟﺘﺴﻮﻳﻖ )2()6( ﺍﻟﻤﺮﺣﻠﺔ ﺍﻟﺜﺎﻧﻮﻳﺔ ﻭﻫﻲ ﻣﺎ ﻗﺒﻞ ﺍﻟﻄﺒﻴﺔ )3()7( ﺍﻟﻤﺮﺣﻠﺔ ﺍﻟﺜﺎﻟﺜﺔ ﻭﻫﻲ ﺍﻟﻄﺒﻲ )4()8( ﻗﺎﻧﻮﻧﻲ Legal )4()9( ﺗﻄﻮﻳﺮ ﺇﺩﺍﺭﺓ ﺃﻌﻤﺎﻝ ﺍﻟﻤﺸﺮﻭﻉ )5()01( ﺇﺩﺍﺭﺓ ﺍﻟﻤﺸﺮﻭﻉ ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

ﻣﺨﺎﻃﺮ ﺍﻛﺘﺸﺎﻑ ﺍﻟﺪﻭﺍﺀ )1( ﺗﻜﻠﻔﺔ ﺍﺳﺘﻤﺮﺍﺭﻳﺔ ﺍﻻﺧﺘﺒﺎﺭﺍﺕ ﻭﻣﻄﺎﻟﺒﺔ ﻣﻨﻈﻤﺔ ﺍﻟﺪﻭﺍﺀ ﻭﺍﻟﻐﺬﺍﺀ ﻟﻤﺴﺘﻨﺪﺍﺕ ﺗﺜﺒﺖ ﺳﻼﻣﺔ ﻭﻋﺪﻡ ﺳﻤﻴﺔ ﺍﻟﺪﻭﺍﺀ ﺍﻟﻤﻜﺘﺸﻒ. )2( ﻣﻌﺪﻝ ﺗﺴﻮﻳﻖ ﺍﻟﺪﻭﺍﺀ ﻓﻲ ﺍﻷﺴﻮﺍﻕ ﻭﺩﺭﺍﺳﺔ ﺍﻟﺠﺪﻭﺓ ﻟﺘﺤﺪﻳﺪ ﻣﻌﺪﻝ ﺍﻟﺮﺑﺢ ﺇﻥ ﻭﺟﺪ )3( ﻋﺪﻡ ﺍﻟﺘﻤﻜﻦ ﻣﻦ ﺍﻟﺤﺼﻮﻝ ﻋﻠﻰ ﺍﻟﺘﺮﺧﻴﺺ ﻣﻦ FDA )4( ﺻﻼﺣﻴﺔ ﺍﻻﺳﺘﻤﺮﺍﺭﻳﺔ ﻓﻲ ﺍﺳﺘﻌﻤﺎﻝ ﺍﻟﺪﻭﺍﺀ )5( ﻣﻌﺪﻝ ﺍﻗﺘﺼﺎﺩﻳﺎﺕ ﺗﺼﻨﻴﻊ ﺍﻟﺪﻭﺍﺀ ﻣﻘﺎﺑﻞ ﺳﻌﺔ ﺍﺣﺘﻴﺎﺝ ﺍﻟﺴﻮﻕ ﻟﻠﺪﻭﺍﺀ API )6( ﻣﺼﺪﺭ IP = Intellective Properties )7( ﻗﺎﺑﻠﻴﺔ ﺍﻟﺪﻭﺍﺀ ﻟﻠﺘﻄﻮﻳﺮ )8( ﺳﻬﻮﻟﺔ ﺍﺳﺘﻌﻤﺎﻝ ﺍﻟﻤﺮﺿﻰ ﻟﻠﺪﻭﺍﺀ )9( ﺍﺳﺘﺮﺍﺗﻴﺠﻴﺎﺕ ﺍﻟﻤﺸﺎﺭﻛﺔ ﻓﻲ ﺇﻧﺸﺎﺀ ﺷﺮﻛﺎﺕ ﺍﻟﺪﻭﺍﺀ ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

ﻣﺘﻄﻠﺒﺎﺕ ﻣﻨﻈﻤﺔ ﺍﻟﺪﻭﺍﺀ ﻭﺍﻟﻐﺬﺍﺀ ﻟﻠﺤﺼﻮﻝ ﻋﻠﻰ ﺗﺮﺧﻴﺺ ﺩﻭﺍﺋﻲ ﺗﺘﻮﻗﻊ ﻣﻨﻈﻤﺔ ﺍﻟﺪﻭﺍﺀ ﻭﺍﻟﻐﺬﺍﺀ FDA ﺃﻦ ﺃﻲ ﺗﺮﺧﻴﺺ ﻳﺼﺪﺭ ﻣﻦ ﻣﻨﻈﻤﺘﻬﺎ ﻳﺤﺘﺎﺝ ﺇﻟﻰ ﺛﻤﺎﻧﻴﺔ ﻭﻧﺼﻒ ﺳﻨﺔ ﻟﻠﺤﺼﻮﻝ ﻋﻠﻰ ﺗﺮﺧﻴﺺ ﻹﺟﺮﺍﺀ: )1( ﺍﻟﺪﺭﺍﺳﺎﺕ ﺍﻟﻤﻌﻤﻠﻴﺔ )2( ﺍﻻﺧﺘﺒﺎﺭﺍﺕ ﻋﻠﻰ ﺣﻴﻮﺍﻧﺎﺕ ﺍﻟﺘﺠﺎﺭﺏ )3( ﺍﻻﺧﺘﺒﺎﺭﺍﺕ ﺍﻟﺴﺮﻳﺮﻳﺔ ﺍﻟﻄﺒﻴﺔ ﻋﻠﻰ ﻣﺮﺿﻰ ﻣﺘﻄﻮﻋﻴﻦ ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

ﺍﻟﻤﺼﺎﺩﺭ ﺍﻟﺘﻲ ﻳﻤﻜﻨﻬﺎ ﻗﻴﺎﺩﺓ ﺩﻓﺔ ﺍﻛﺘﺸﺎﻑ ﺍﻟﺪﻭﺍﺀ )1( ﺍﻟﻤﺼﺎﺩﺭ ﺍﻟﻤﺎﻟﻴﺔ. )2( ﺍﻟﺨﺒﺮﺍﺀ. )3( ﺗﻮﻓﺮ ﺍﻟﻤﻮﺍﺭﺩ ﺍﻷﻮﻟﻴﺔ ﺍﻟﻄﺒﻴﻌﻴﺔ ﺑﺎﻟﺪﻭﻟﺔ. )4( ﺗﻮﻓﺮ ﺍﻹﻣﻜﺎﻧﺎﺕ ﺍﻟﺒﺤﺜﻴﺔ. )5( ﺗﻮﻓﺮ ﺍﻟﻤﺴﺘﻠﺰﻣﺎﺕ ﻭﺍﻟﻜﻴﻤﺎﻭﻳﺎﺕ ﻭﺍﻷﺠﻬﺰﺓ ﺍﻟﺒﺤﺜﻴﺔ. )6( ﺗﻮﻓﺮ ﻓﻨﻴﻴﻦ ﻣﺘﻤﻴﺰﻳﻦ. )7( ﺗﻮﻓﺮ ﺍﻻﺳﺘﻘﺮﺍﺭ ﺍﻟﺒﺤﺜﻲ ﺑﻮﺿﻊ ﺃﻨﻈﻤﺔ ﺗﺴﻬﻞ ﻋﻤﻠﻴﺔ ﺗﻨﻔﻴﺬ ﺍﻟﺒﺤﺚ ﺍﻟﻌﻠﻤﻲ. )8( ﺗﻮﻓﺮ ﻣﻮﺍﺩ ﻓﻌﺎﻟﺔ ﻭﺃﻤﺎﻧﺔ )ﻏﻴﺮ ﺳﺎﻣﺔ( ﺗﺪﻓﻊ ﻗﻴﺎﺩﺓ ﺍﻟﻌﻤﻞ ﺍﻟﺒﺤﺜﻲ ﻟﻠﻮﺻﻮﻝ ﻟﻠﺪﻭﺍﺀ. )9( ﺗﻮﻓﺮ ﻣﺰﺍﺭﻉ ﻓﺄﺮﺍﻥ ﺍﻟﺘﺠﺎﺭﺏ ﺍﻟﻨﻤﻮﺫﺟﻴﺔ )01( ﺗﻮﻓﺮ ﺍﻟﻤﺼﺎﺩﺭ ﺍﻟﺘﺼﻨﻴﻌﻴﺔ ﻭﺗﻮﻓﺮ ﺍﻟﺠﻮﺩﺓ ﺗﻌﻤﻞ ﻋﻠﻰ ﻗﻴﺎﺩﺓ ﺩﻓﺔ ﺍﻛﺘﺸﺎﻑ ﺍﻟﺪﻭﺍﺀ ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

ﺻﻴﺪﻟﺔ ﺍﻟﺴﻤﻴﺔ Pharmacotoxicokinetic ﺍﻻﻣﺘﺼﺎﺻﻴﺔ ﺍﻟﺪﻭﺍﺀ ﻓﻲ ﺍﻟﺠﺴﻢ ﺗﻮﺯﻳﻊ ﺍﻟﺪﻭﺍﺀ ﺩﺍﺧﻞ ﺍﻟﺠﺴﻢ ﺍﻟﺘﻔﺎﻋﻼﺕ ﺍﻟﻜﻴﻤﻴﺎﺋﻴﺔ ﺍﻟﺤﻴﻮﻳﺔ ﺳﻤﻴﺔ ﺍﻟﺪﻭﺍﺀ ﺑﺎﻟﺠﺴﻢ ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ
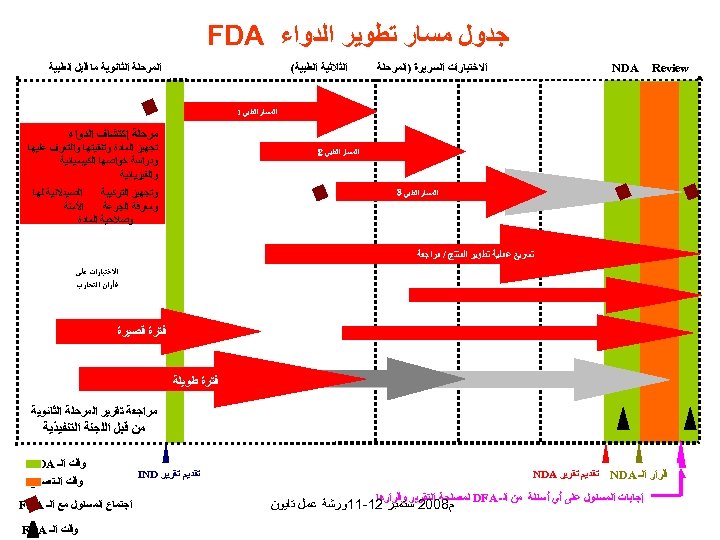
ﺟﺪﻭﻝ ﻣﺴﺎﺭ ﺗﻄﻮﻳﺮ ﺍﻟﺪﻭﺍﺀ FDA Review ﺍﻻﺧﺘﺒﺎﺭﺍﺕ ﺍﻟﺴﺮﻳﺮﺓ )ﺍﻟﻤﺮﺣﻠﺔ NDA ﺍﻟﻤﺮﺣﻠﺔ ﺍﻟﺜﺎﻧﻮﻳﺔ ﻣﺎ ﻗﺒﻞ ﺍﻟﻄﺒﻴﺔ ﺍﻟﺜﻼﺛﻴﺔ ﺍﻟﻄﺒﻴﺔ( ﺍﻟﻤﺴﺎﺭ ﺍﻟﻄﺒﻲ 1 ﻣﺮﺣﻠﺔ ﺇﻛﺘﺸﺎﻑ ﺍﻟﺪﻭﺍﺀ ﺗﺠﻬﻴﺰ ﺍﻟﻤﺎﺩﺓ ﻭﺗﻨﻘﻴﺘﻬﺎ ﻭﺍﻟﺘﻌﺮﻑ ﻋﻠﻴﻬﺎ ﻭﺩﺭﺍﺳﺔ ﺧﻮﺍﺻﻬﺎ ﺍﻟﻜﻴﺒﻤﻴﺎﺋﻴﺔ ﻭﺍﻟﻔﻴﺰﻳﺎﺋﻴﺔ ﺍﻟﻤﺴﺎﺭ ﺍﻟﻄﺒﻲ 2 ﺍﻟﺼﻴﺪﻻﻧﻴﺔ ﻟﻬﺎ ﻭﺗﺠﻬﻴﺰ ﺍﻟﺘﺮﻛﻴﺒﺔ ﺍﻷﻤﻨﺔ ﻭﻣﻌﺮﻓﺔ ﺍﻟﺠﺮﻋﺔ ﻭﺻﻼﺣﻴﺔ ﺍﻟﻤﺎﺩﺓ ﺍﻟﻤﺴﺎﺭ ﺍﻟﻄﺒﻲ 3 ﺗﺴﺮﻳﻊ ﻋﻤﻠﻴﺔ ﺗﻄﻮﻳﺮ ﺍﻟﻤﻨﺘﺞ / ﻣﺮﺍﺟﻌﺔ ﺍﻻﺧﺘﺒﺎﺭﺍﺕ ﻋﻠﻰ ﻓﺄﺮﺍﻥ ﺍﻟﺘﺠﺎﺭﺏ ﻓﺘﺮﺓ ﻗﺼﻴﺮﺓ ﻓﺘﺮﺓ ﻃﻮﻳﻠﺔ ﻣﺮﺍﺟﻌﺔ ﺗﻘﺮﻳﺮ ﺍﻟﻤﺮﺣﻠﺔ ﺍﻟﺜﺎﻧﻮﻳﺔ ﻣﻦ ﻗﺒﻞ ﺍﻟﻠﺠﻨﺔ ﺍﻟﺘﻨﻔﻴﺬﻳﺔ ﻗﺮﺍﺭ ﺍﻟـ NDA ﺗﻘﺪﻳﻢ ﺗﻘﺮﻳﺮ NDA ﻟﻤﺼﻠﺤﺔ ﺍﻟﺘﻘﺮﻳﺮ ﻭﻗﺮﺍﺭﻫﺎ ﺇﺟﺎﺑﺎﺕ ﺍﻟﻤﺴﺌﻮﻝ ﻋﻠﻰ ﺃﻲ ﺃﺴﺌﻠﺔ ﻣﻦ ﺍﻟـ DFA ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ ﺗﻘﺪﻳﻢ ﺗﻘﺮﻳﺮ IND ﻭﻗﺖ ﺍﻟـ FDA ﻭﻗﺖ ﺍﻟـﺘﺼﻨﻴﻊ ﺍﺟﺘﻤﺎﻉ ﺍﻟﻤﺴﺌﻮﻝ ﻣﻊ ﺍﻟـ FDA ﻭﻗﺖ ﺍﻟـ FDA
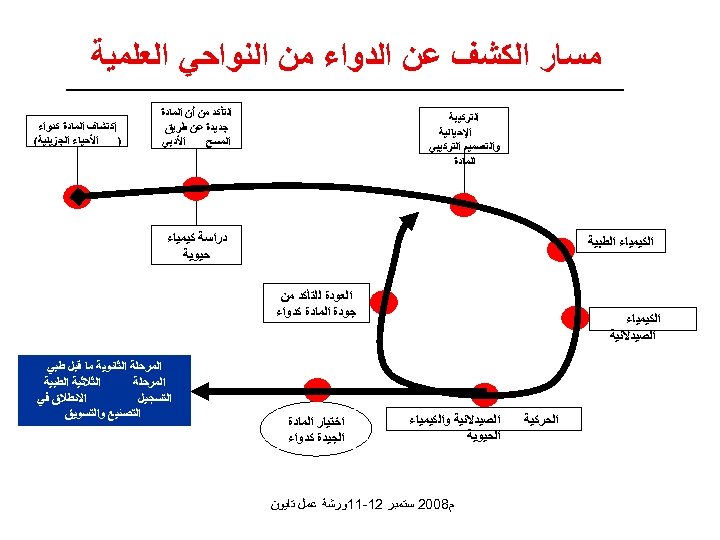
ﻣﺴﺎﺭ ﺍﻟﻜﺸﻒ ﻋﻦ ﺍﻟﺪﻭﺍﺀ ﻣﻦ ﺍﻟﻨﻮﺍﺣﻲ ﺍﻟﻌﻠﻤﻴﺔ ﺍﻟﺘﺄﻜﺪ ﻣﻦ ﺃﻦ ﺍﻟﻤﺎﺩﺓ ﺟﺪﻳﺪﺓ ﻋﻦ ﻃﺮﻳﻖ ﺍﻷﺪﺑﻲ ﺍﻟﻤﺴﺢ ﺍﻟﺘﺮﻛﻴﺒﺔ ﺍﻹﺣﻴﺎﺋﻴﺔ ﻭﺍﻟﺘﺼﻤﻴﻢ ﺍﻟﺘﺮﻛﻴﺒﻲ ﻟﻠﻤﺎﺩﺓ ﺇﻛﺘﺸﺎﻑ ﺍﻟﻤﺎﺩﺓ ﻛﺪﻭﺍﺀ ) ﺍﻷﺤﻴﺎﺀ ﺍﻟﺠﺰﻳﺌﻴﺔ( ﺩﺭﺍﺳﺔ ﻛﻴﻤﻴﺎﺀ ﺣﻴﻮﻳﺔ ﺍﻟﻜﻴﻤﻴﺎﺀ ﺍﻟﻄﺒﻴﺔ ﺍﻟﻌﻮﺩﺓ ﻟﻠﺘﺄﻜﺪ ﻣﻦ ﺟﻮﺩﺓ ﺍﻟﻤﺎﺩﺓ ﻛﺪﻭﺍﺀ ﺍﻟﻜﻴﻤﻴﺎﺀ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ ﺍﻟﺤﺮﻛﻴﺔ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ ﻭﺍﻟﻜﻴﻤﻴﺎﺀ ﺍﻟﺤﻴﻮﻳﺔ ﺍﺧﺘﻴﺎﺭ ﺍﻟﻤﺎﺩﺓ ﺍﻟﺠﻴﺪﺓ ﻛﺪﻭﺍﺀ ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ ﺍﻟﻤﺮﺣﻠﺔ ﺍﻟﺜﺎﻧﻮﻳﺔ ﻣﺎ ﻗﺒﻞ ﻃﺒﻲ ﺍﻟﺜﻼﺛﻴﺔ ﺍﻟﻄﺒﻴﺔ ﺍﻟﻤﺮﺣﻠﺔ ﺍﻻﻧﻄﻼﻕ ﻓﻲ ﺍﻟﺘﺴﺠﻴﻞ ﺍﻟﺘﺼﻨﻴﻊ ﻭﺍﻟﺘﺴﻮﻳﻖ
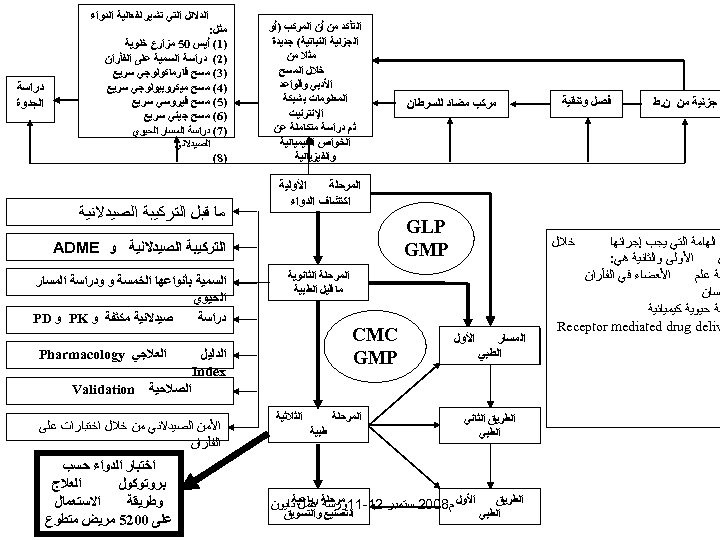
ﺟﺰﺋﻴﺔ ﻣﻦ ﻥ. ﻁ ﻓﺼﻞ ﻭﺗﻨﻘﻴﺔ ﻣﺮﻛﺐ ﻣﻀﺎﺩ ﻟﻠﺴﺮﻃﺎﻥ ﺍﻟﺘﺄﻜﺪ ﻣﻦ ﺃﻦ ﺍﻟﻤﺮﻛﺐ )ﺃﻮ ﺍﻟﺠﺰﺋﻴﺔ ﺍﻟﻨﺒﺎﺗﻴﺔ( ﺟﺪﻳﺪﺓ ﻣﺜﻼ ﻣﻦ ﺧﻼﻝ ﺍﻟﻤﺴﺢ ﺍﻷﺪﺑﻲ ﻭﻗﻮﺍﻋﺪ ﺍﻟﻤﻌﻠﻮﻣﺎﺕ ﺑﺸﺒﻜﺔ ﺍﻹﻧﺘﺮﻧﻴﺖ ﺛﻢ ﺩﺭﺍﺳﺔ ﻣﺘﻜﺎﻣﻠﺔ ﻋﻦ ﺍﻟﺨﻮﺍﺹ ﺍﻟﻜﻴﻤﻴﺎﺋﻴﺔ ﻭﺍﻟﻔﻴﺰﻳﺎﺋﻴﺔ ﺍﻷﻮﻟﻴﺔ ﺍﻟﻤﺮﺣﻠﺔ ﺍﻛﺘﺸﺎﻑ ﺍﻟﺪﻭﺍﺀ ﺕ ﺍﻟﻬﺎﻣﺔ ﺍﻟﺘﻲ ﻳﺠﺐ ﺇﺟﺮﺍﺋﻬﺎ ﺍﻷﻮﻟﻰ ﻭﺍﻟﺜﺎﻧﻴﺔ ﻫﻲ: ﻦ ﺍﻷﻌﻀﺎﺀ ﻓﻲ ﺍﻟﻔﺄﺮﺍﻥ ﺳﺔ ﻋﻠﻢ ﻧﺴﺎﻥ ﺳﺔ ﺣﻴﻮﻳﺔ ﻛﻴﻤﻴﺎﺋﻴﺔ Receptor mediated drug deliv GLP GMP ﺧﻼﻝ ﺍﻟﻤﺴﺎﺭ ﺍﻟﻄﺮﻳﻖ ﺍﻟﻄﺒﻲ ﻣﺜﻞ: )1( ﺃﻴﺲ 05 ﻣﺰﺍﺭﻉ ﺧﻠﻮﻳﺔ )2( ﺩﺭﺍﺳﺔ ﺍﻟﺴﻤﻴﺔ ﻋﻠﻰ ﺍﻟﻔﺄﺮﺍﻥ )3( ﻣﺴﺢ ﻓﺎﺭﻣﺎﻛﻮﻟﻮﺟﻲ ﺳﺮﻳﻊ )4( ﻣﺴﺢ ﻣﻴﻜﺮﻭﺑﻴﻮﻟﻮﺟﻲ ﺳﺮﻳﻊ )5( ﻣﺴﺢ ﻓﻴﺮﻭﺳﻲ ﺳﺮﻳﻊ )6( ﻣﺴﺢ ﺟﻴﻨﻲ ﺳﺮﻳﻊ )7( ﺩﺭﺍﺳﺔ ﺍﻟﻤﺴﺎﺭ ﺍﻟﺤﻴﻮﻱ ﺍﻟﺼﻴﺪﻻﻧﻲ )8( ﻣﺎ ﻗﺒﻞ ﺍﻟﺘﺮﻛﻴﺒﺔ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ CMC GMP ﺍﻟﺴﻤﻴﺔ ﺑﺄﻨﻮﺍﻋﻬﺎ ﺍﻟﺨﻤﺴﺔ ﻭ ﻭﺩﺭﺍﺳﺔ ﺍﻟﻤﺴﺎﺭ ﺍﻟﺤﻴﻮﻱ ﺻﻴﺪﻻﻧﻴﺔ ﻣﻜﺜﻔﺔ ﻭ PK ﻭ PD ﺩﺭﺍﺳﺔ ﺍﻟﺪﻟﻴﻞ Index ﺍﻟﻌﻼﺟﻲ Pharmacology ﺍﻟﺼﻼﺣﻴﺔ ﺍﻟﻄﺮﻳﻖ ﺍﻟﺜﺎﻧﻲ ﺍﻟﻄﺒﻲ ﺍﻟﻄﺮﻳﻖ ﺍﻟﻄﺒﻲ ﺩﺭﺍﺳﺔ ﺍﻟﺠﺪﻭﺓ ﺍﻟﺘﺮﻛﻴﺒﺔ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ ﻭ ADME ﺍﻟﻤﺮﺣﻠﺔ ﺍﻟﺜﺎﻧﻮﻳﺔ ﻣﺎ ﻗﻴﻞ ﺍﻟﻄﺒﻴﺔ ﺍﻷﻮﻝ ﺍﻟﺪﻻﺋﻞ ﺍﻟﺘﻲ ﺗﺸﻴﺮ ﻟﻔﻌﺎﻟﻴﺔ ﺍﻟﺪﻭﺍﺀ ﺍﻟﺜﻼﺛﻴﺔ ﺍﻟﻤﺮﺣﻠﺔ ﻃﺒﻴﺔ ﻣﺮﺣﻠﺔ ﺭﺑﺎﻋﻴﺔﺗﺎﻳﻮﻥ ﺍﻷﻮﻝ ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺍﻟﺘﺼﻨﻴﻊ ﻭﺍﻟﺘﺴﻮﻳﻖ Validation ﺍﻷﻤﻦ ﺍﻟﺼﻴﺪﻻﻧﻲ ﻣﻦ ﺧﻼﻝ ﺍﺧﺘﺒﺎﺭﺍﺕ ﻋﻠﻰ ﺍﻟﻔﺄﺮﺍﻥ ﺍﺧﺘﺒﺎﺭ ﺍﻟﺪﻭﺍﺀ ﺣﺴﺐ ﺍﻟﻌﻼﺝ ﺑﺮﻭﺗﻮﻛﻮﻝ ﺍﻻﺳﺘﻌﻤﺎﻝ ﻭﻃﺮﻳﻘﺔ ﻋﻠﻰ 0025 ﻣﺮﻳﺾ ﻣﺘﻄﻮﻉ
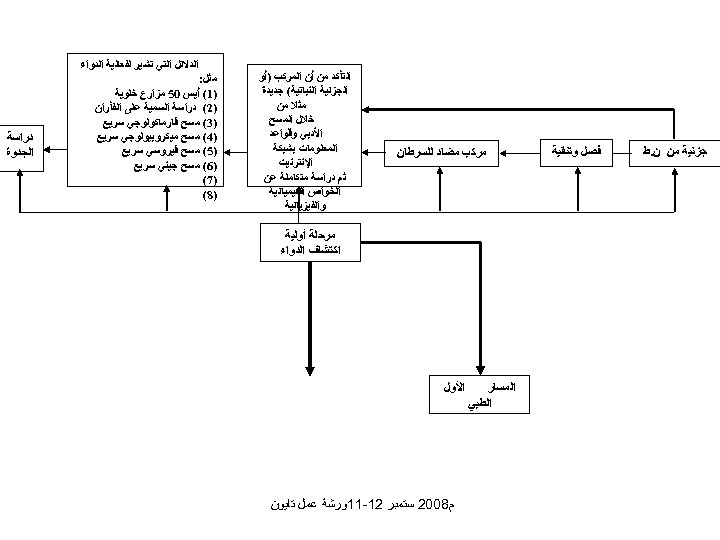
ﺟﺰﺋﻴﺔ ﻣﻦ ﻥ. ﻁ ﻓﺼﻞ ﻭﺗﻨﻘﻴﺔ ﻣﺮﻛﺐ ﻣﻀﺎﺩ ﻟﻠﺴﺮﻃﺎﻥ ﺍﻟﺘﺄﻜﺪ ﻣﻦ ﺃﻦ ﺍﻟﻤﺮﻛﺐ )ﺃﻮ ﺍﻟﺠﺰﺋﻴﺔ ﺍﻟﻨﺒﺎﺗﻴﺔ( ﺟﺪﻳﺪﺓ ﻣﺜﻼ ﻣﻦ ﺧﻼﻝ ﺍﻟﻤﺴﺢ ﺍﻷﺪﺑﻲ ﻭﻗﻮﺍﻋﺪ ﺍﻟﻤﻌﻠﻮﻣﺎﺕ ﺑﺸﺒﻜﺔ ﺍﻹﻧﺘﺮﻧﻴﺖ ﺛﻢ ﺩﺭﺍﺳﺔ ﻣﺘﻜﺎﻣﻠﺔ ﻋﻦ ﺍﻟﺨﻮﺍﺹ ﺍﻟﻜﻴﻤﻴﺎﺋﻴﺔ ﻭﺍﻟﻔﻴﺰﻳﺎﺋﻴﺔ ﻣﺮﺣﻠﺔ ﺃﻮﻟﻴﺔ ﺍﻛﺘﺸﺎﻑ ﺍﻟﺪﻭﺍﺀ ﺍﻟﻤﺴﺎﺭ ﺍﻟﻄﺒﻲ ﺍﻷﻮﻝ ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ ﺍﻟﺪﻻﺋﻞ ﺍﻟﺘﻲ ﺗﺸﻴﺮ ﻟﻔﻌﺎﻟﻴﺔ ﺍﻟﺪﻭﺍﺀ ﻣﺜﻞ: )1( ﺃﻴﺲ 05 ﻣﺰﺍﺭﻉ ﺧﻠﻮﻳﺔ )2( ﺩﺭﺍﺳﺔ ﺍﻟﺴﻤﻴﺔ ﻋﻠﻰ ﺍﻟﻔﺄﺮﺍﻥ )3( ﻣﺴﺢ ﻓﺎﺭﻣﺎﻛﻮﻟﻮﺟﻲ ﺳﺮﻳﻊ )4( ﻣﺴﺢ ﻣﻴﻜﺮﻭﺑﻴﻮﻟﻮﺟﻲ ﺳﺮﻳﻊ )5( ﻣﺴﺢ ﻓﻴﺮﻭﺳﻲ ﺳﺮﻳﻊ )6( ﻣﺴﺢ ﺟﻴﻨﻲ ﺳﺮﻳﻊ )7( )8( ﺩﺭﺍﺳﺔ ﺍﻟﺠﺪﻭﺓ
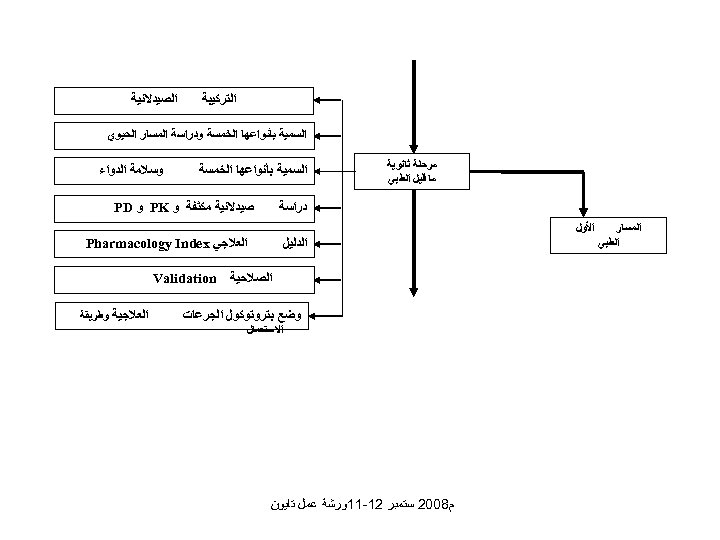
ﺍﻟﺘﺮﻛﻴﺒﺔ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ ﺍﻟﺴﻤﻴﺔ ﺑﺄﻨﻮﺍﻋﻬﺎ ﺍﻟﺨﻤﺴﺔ ﻭﺩﺭﺍﺳﺔ ﺍﻟﻤﺴﺎﺭ ﺍﻟﺤﻴﻮﻱ ﻣﺮﺣﻠﺔ ﺛﺎﻧﻮﻳﺔ ﻣﺎ ﻗﻴﻞ ﺍﻟﻄﺒﻲ ﺍﻟﺴﻤﻴﺔ ﺑﺄﻨﻮﺍﻋﻬﺎ ﺍﻟﺨﻤﺴﺔ ﺩﺭﺍﺳﺔ ﺍﻟﻤﺴﺎﺭ ﺍﻟﻄﺒﻲ ﻭﺳﻼﻣﺔ ﺍﻟﺪﻭﺍﺀ ﺻﻴﺪﻻﻧﻴﺔ ﻣﻜﺜﻔﺔ ﻭ PK ﻭ PD ﺍﻷﻮﻝ ﺍﻟﻌﻼﺟﻲ Pharmacology Index ﺍﻟﺪﻟﻴﻞ ﺍﻟﺼﻼﺣﻴﺔ Validation ﻭﺿﻊ ﺑﺘﺮﻭﺗﻮﻛﻮﻝ ﺍﻟﺠﺮﻋﺎﺕ ﺍﻻﺳﺘﻌﻤﺎﻝ ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ ﺍﻟﻌﻼﺟﻴﺔ ﻭﻃﺮﻳﻘﺔ
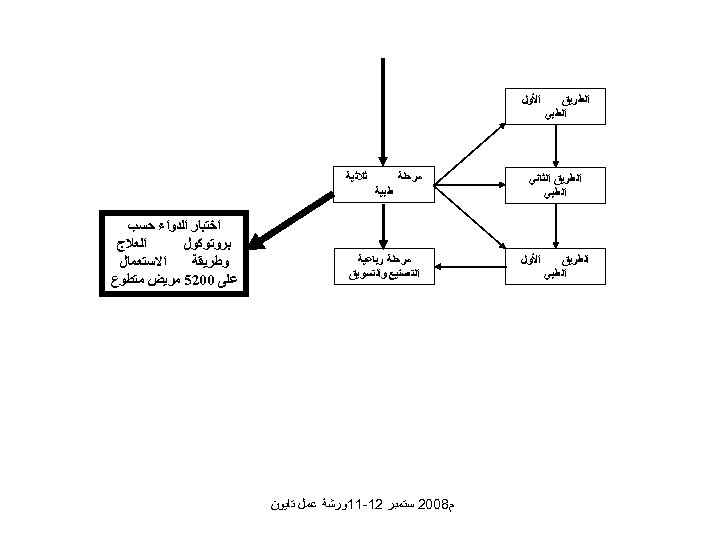
ﺍﻟﻄﺮﻳﻖ ﺍﻟﻄﺒﻲ ﺍﻷﻮﻝ ﺍﻟﻄﺮﻳﻖ ﺍﻟﺜﺎﻧﻲ ﺍﻟﻄﺒﻲ ﺍﻟﻄﺮﻳﻖ ﺍﻟﻄﺒﻲ ﺍﻷﻮﻝ ﺛﻼﺛﻴﺔ ﻣﺮﺣﻠﺔ ﻃﺒﻴﺔ ﻣﺮﺣﻠﺔ ﺭﺑﺎﻋﻴﺔ ﺍﻟﺘﺼﻨﻴﻊ ﻭﺍﻟﺘﺴﻮﻳﻖ ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ ﺍﺧﺘﺒﺎﺭ ﺍﻟﺪﻭﺍﺀ ﺣﺴﺐ ﺍﻟﻌﻼﺝ ﺑﺮﻭﺗﻮﻛﻮﻝ ﺍﻻﺳﺘﻌﻤﺎﻝ ﻭﻃﺮﻳﻘﺔ ﻋﻠﻰ 0025 ﻣﺮﻳﺾ ﻣﺘﻄﻮﻉ
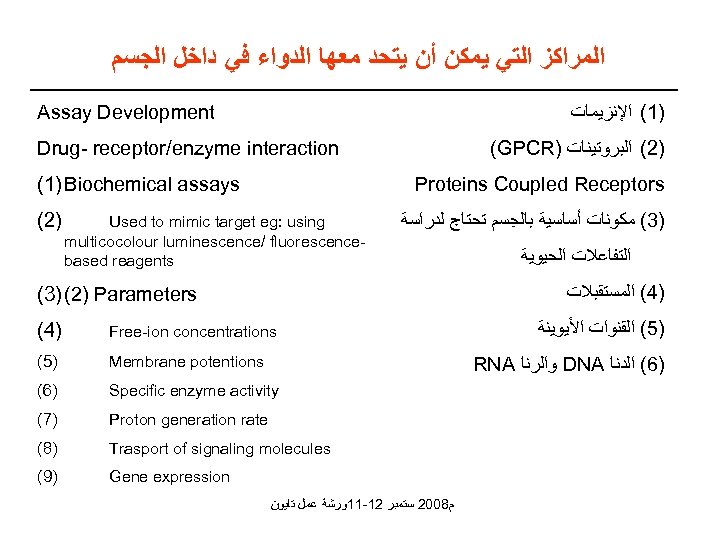
ﺍﻟﻤﺮﺍﻛﺰ ﺍﻟﺘﻲ ﻳﻤﻜﻦ ﺃﻦ ﻳﺘﺤﺪ ﻣﻌﻬﺎ ﺍﻟﺪﻭﺍﺀ ﻓﻲ ﺩﺍﺧﻞ ﺍﻟﺠﺴﻢ )1( ﺍﻹﻧﺰﻳﻤﺎﺕ Assay Development (GPCR) )2( ﺍﻟﺒﺮﻭﺗﻴﻨﺎﺕ Drug- receptor/enzyme interaction (1) Biochemical assays (2) Proteins Coupled Receptors Used to mimic target eg: using multicocolour luminescence/ fluorescencebased reagents )3( ﻣﻜﻮﻧﺎﺕ ﺃﺴﺎﺳﻴﺔ ﺑﺎﻟﺠﺴﻢ ﺗﺤﺘﺎﺝ ﻟﺪﺭﺍﺳﺔ ﺍﻟﺘﻔﺎﻋﻼﺕ ﺍﻟﺤﻴﻮﻳﺔ )4( ﺍﻟﻤﺴﺘﻘﺒﻼﺕ (3) (2) Parameters (4) Free-ion concentrations (5) Membrane potentions (6) Specific enzyme activity (7) Proton generation rate (8) Trasport of signaling molecules (9) Gene expression )5( ﺍﻟﻘﻨﻮﺍﺕ ﺍﻷﻴﻮﻳﻨﺔ RNA ﻭﺍﻟﺮﻧﺎ DNA )6( ﺍﻟﺪﻧﺎ ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

ﺍﻟﻤﺤﺎﺿﺮﺓ 2 ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ
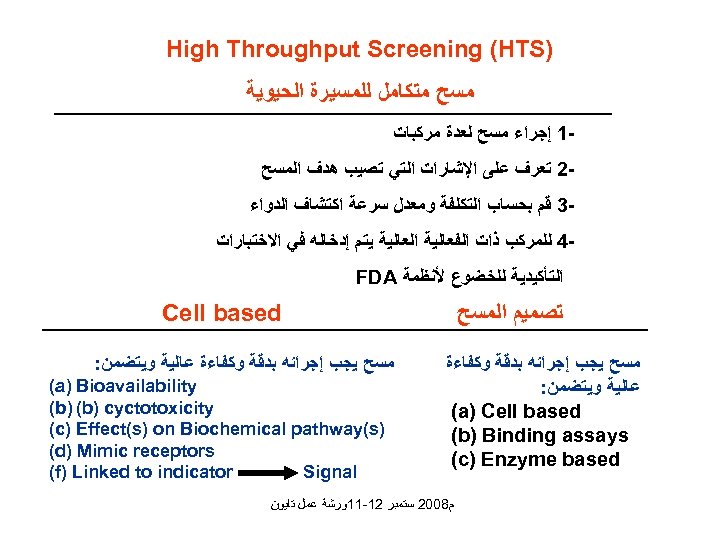
) High Throughput Screening (HTS ﻣﺴﺢ ﻣﺘﻜﺎﻣﻞ ﻟﻠﻤﺴﻴﺮﺓ ﺍﻟﺤﻴﻮﻳﺔ 1 ﺇﺟﺮﺍﺀ ﻣﺴﺢ ﻟﻌﺪﺓ ﻣﺮﻛﺒﺎﺕ 2 ﺗﻌﺮﻑ ﻋﻠﻰ ﺍﻹﺷﺎﺭﺍﺕ ﺍﻟﺘﻲ ﺗﺼﻴﺐ ﻫﺪﻑ ﺍﻟﻤﺴﺢ 3 ﻗﻢ ﺑﺤﺴﺎﺏ ﺍﻟﺘﻜﻠﻔﺔ ﻭﻣﻌﺪﻝ ﺳﺮﻋﺔ ﺍﻛﺘﺸﺎﻑ ﺍﻟﺪﻭﺍﺀ 4 ﻟﻠﻤﺮﻛﺐ ﺫﺍﺕ ﺍﻟﻔﻌﺎﻟﻴﺔ ﺍﻟﻌﺎﻟﻴﺔ ﻳﺘﻢ ﺇﺩﺧﺎﻟﻪ ﻓﻲ ﺍﻻﺧﺘﺒﺎﺭﺍﺕ ﺍﻟﺘﺄﻜﻴﺪﻳﺔ ﻟﻠﺨﻀﻮﻉ ﻷﻨﻈﻤﺔ FDA Cell based ﺗﺼﻤﻴﻢ ﺍﻟﻤﺴﺢ ﻣﺴﺢ ﻳﺠﺐ ﺇﺟﺮﺍﺋﻪ ﺑﺪﻗﺔ ﻭﻛﻔﺎﺀﺓ ﻋﺎﻟﻴﺔ ﻭﻳﺘﻀﻤﻦ: (a) Cell based (b) Binding assays (c) Enzyme based ﻣﺴﺢ ﻳﺠﺐ ﺇﺟﺮﺍﺋﻪ ﺑﺪﻗﺔ ﻭﻛﻔﺎﺀﺓ ﻋﺎﻟﻴﺔ ﻭﻳﺘﻀﻤﻦ: (a) Bioavailability (b) cyctotoxicity ) (c) Effect(s) on Biochemical pathway(s (d) Mimic receptors (f) Linked to indicator Signal ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

ﺍﺧﺘﺒﺎﺭ ﺍﻟﺨﻼﻳﺎ ﺍﻟﻨﺴﻴﺠﻴﺔ ﺑﺎﺳﺘﺨﺪﺍﻡ : Caco-2 Cells ﻃﺒﺎﻋﺔ ﻣﻦ ﺻﻔﺤﺔ 81 ﻭ 91 ﺍﻟﻤﺤﺎﺿﺮﺓ 2 ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

ﺍﻛﺘﺸﺎﻑ ﺍﻟﻤﺴﺢ ﺍﻟﺒﻴﻮﻟﻮﺟﻲ Discovery Bio-Screens ﻃﺒﺎﻋﺔ ﻣﻦ ﺻﻔﺤﺔ 22 ﺍﻟﻤﺤﺎﺿﺮﺓ 2 ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

ﺍﻟﻤﺴﺢ ﺍﻟﺒﻴﻮﻟﻮﺟﻲ Bio-Screens ﻧﺼﺎﺋﺢ ﻳﻔﻀﻞ ﺍﻻﻧﺘﺒﺎﻩ ﻟﻬﺎ ﻃﺒﺎﻋﺔ ﻣﻦ ﺻﻔﺤﺔ 22 ﺍﻟﻤﺤﺎﺿﺮﺓ 2 ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

ﺍﺧﺘﻴﺎﺭ ﺍﻷﺪﻭﻳﺔ ﺍﻟﻤﻜﺘﺸﻔﺔ ﺑﺎﻟﺘﺄﻜﺪ ﻣﻦ ﺍﻟﺘﺠﺎﺭﺏ ﻭ/ﺃﻮ ﺇﻋﺎﺩﺓ ﺑﻌﺾ ﺍﻟﺘﺠﺎﺭﺏ ﺻﻔﺤﺔ 03 ﺍﻟﻤﺤﺎﺿﺮﺓ 2 ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

ﻧﺼﺎﺋﺢ ﺇﺗﺒﺎﻋﻬﺎ ﻋﻨﺪ ﺍﻟﺘﻮﺻﻞ ﻟﺪﻭﺍﺀ ﻟﻪ ﺗﺄﺜﺮﺍﺕ ﺑﻴﻮﻟﻮﺟﻴﺔ Biologics Candidates ﺻﻔﺤﺔ 23 ﺍﻟﻤﺤﺎﺿﺮﺓ 2 ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

3 ﺍﻟﻤﺤﺎﺿﺮﺓ Pre-clinical: Keys: (1)Pharmaceutics (2) Formulation (3) CMC Issues ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

Preclinical Development Main Goals = Pre formulation & Formulation. ﻣﻦ ﻧﺘﺎﺋﺞ ﺗﺠﺎﺭﺏ ﺍﻟﻤﺤﺎﻭﺭ ﺍﻷﺴﺎﺳﻴﺔ ﺧﻼﻝ ﺍﻟﻤﺮﺣﻠﺔ : ﺍﻟﺜﺎﻧﻮﻳﺔ ﻣﺎ ﻗﺒﻞ ﺍﻟﻄﺒﻴﺔ ﻧﺘﻮﺻﻞ ﺇﻟﻲ = Pharmacology = Study & Toxicology (toxicity and safety pharmacology) through animal testing. : )1( ﺗﺤﺪﻳﺪ ﺍﻟﺠﺮﻋﺎﺕ ﺍﻟﻌﻼﺟﻴﺔ ﺍﻟﺘﻲ = Determination a drug’s pharmacodynamics PD ) ﺃ ( ﺗﻜﻮﻥ آﻤﻨﺔ ﻋﻠﻰ ﺍﻟﺼﺤﺔ (2) = Determination a drug’s pharmacokinetics PK )ﺏ( ﺗﻌﺎﻟﺞ ﺍﻟﻤﺮﺽ ﺧﻼﻝ ﻓﺘﺮﺓ ﻣﻨﺎﺳﺒﺔ (3) = ADME ( For prediction of oral absorption in humans & Determination of mechanisms of intestinal absorption )2( ﻛﻤﻴﺔ ﺍﻟﺠﺮﻋﺎﺕ ﺣﺴﺐ ﺍﻟﻌﻤﺮ ﻭﺍﻟﻮﺯﻥ . )3( ﻃﺮﻳﻘﺔ ﺗﻌﺎﻃﻴﻬﺎ . )4( ﻓﺘﺮﺍﺕ ﺗﻌﺎﻃﻴﻬﺎ = Bioavailability studies. = Metabolites in biological matrices. = Preparation & analysis of the drug. = Silico Modeling )5( ﺷﺮﻭﻁ ﺍﻻﺳﺘﻌﻤﺎﻝ ﺑﻤﻌﻨﻰ ﺗﺤﺪﻳﺪ ﺍﻟﻔﺌﺔ ﺍﻟﻤﺮﻳﻀﺔ ﺍﻟﺘﻲ ﻳﻤﻜﻦ ﻣﻌﺎﻟﺠﺘﻬﺎ . )6( ﻣﻌﻮﻗﺎﺕ ﺍﻻﺳﺘﻌﻤﺎﻝ )7( ﻣﻌﺮﻓﺔ ﻣﺪﻯ ﺍﺭﺗﻴﺎﺡ ﺍﻟﻤﺮﺿﻰ ﻋﻨﺪ ﺗﻨﺎﻭﻟﻬﻢ ﺍﻟﺪﻭﺍﺀ ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ
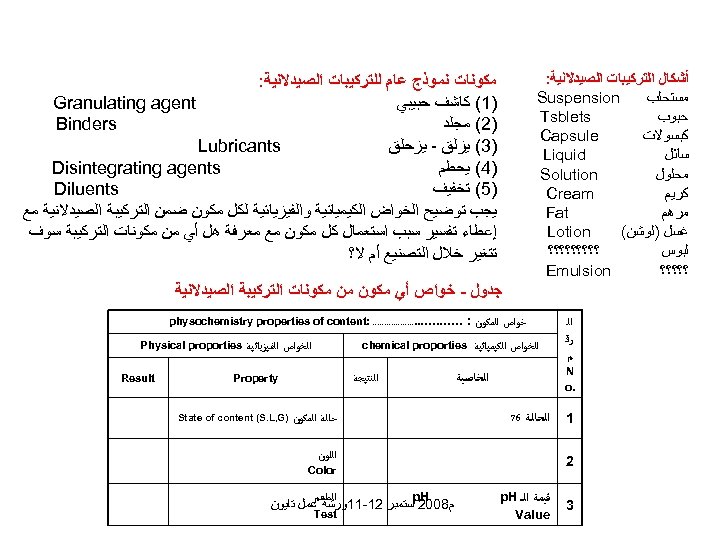
ﺃﺸﻜﺎﻝ ﺍﻟﺘﺮﻛﻴﺒﺎﺕ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ: Suspension ﻣﺴﺘﺤﻠﺐ Tsblets ﺣﺒﻮﺏ Capsule ﻛﺒﺴﻮﻻﺕ Liquid ﺳﺎﺋﻞ Solution ﻣﺤﻠﻮﻝ Cream ﻛﺮﻳﻢ Fat ﻣﺮﻫﻢ Lotion ﻏﺴﻞ )ﻟﻮﺷﻦ( ؟؟؟؟؟ ﻟﺒﻮﺱ Emulsion ؟؟؟؟؟ ﻣﻜﻮﻧﺎﺕ ﻧﻤﻮﺫﺝ ﻋﺎﻡ ﻟﻠﺘﺮﻛﻴﺒﺎﺕ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ: Granulating agent )1( ﻛﺎﺷﻒ ﺣﺒﻴﺒﻲ Binders )2( ﻣﺠﻠﺪ Lubricants )3( ﻳﺰﻟﻖ - ﻳﺰﺣﻠﻖ Disintegrating agents )4( ﻳﺤﻄﻢ Diluents )5( ﺗﺨﻔﻴﻒ ﻳﺠﺐ ﺗﻮﺿﻴﺢ ﺍﻟﺨﻮﺍﺽ ﺍﻟﻜﻴﻤﻴﺎﺋﻴﺔ ﻭﺍﻟﻔﻴﺰﻳﺎﺋﻴﺔ ﻟﻜﻞ ﻣﻜﻮﻥ ﺿﻤﻦ ﺍﻟﺘﺮﻛﻴﺒﺔ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ ﻣﻊ ﺇﻋﻄﺎﺀ ﺗﻔﺴﻴﺮ ﺳﺒﺐ ﺍﺳﺘﻌﻤﺎﻝ ﻛﻞ ﻣﻜﻮﻥ ﻣﻊ ﻣﻌﺮﻓﺔ ﻫﻞ ﺃﻲ ﻣﻦ ﻣﻜﻮﻧﺎﺕ ﺍﻟﺘﺮﻛﻴﺒﺔ ﺳﻮﻑ ﺗﺘﻐﻴﺮ ﺧﻼﻝ ﺍﻟﺘﺼﻨﻴﻊ ﺃﻢ ﻻ؟ ﺟﺪﻭﻝ - ﺧﻮﺍﺹ ﺃﻲ ﻣﻜﻮﻥ ﻣﻦ ﻣﻜﻮﻧﺎﺕ ﺍﻟﺘﺮﻛﻴﺒﺔ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ ﺍﻟ ﺮﻗ ﻢ ﺧﻮﺍﺹ ﺍﻟﻤﻜﻮﻥ : ﺍﻟﺨﻮﺍﺹ ﺍﻟﻜﻴﻤﻴﺎﺋﻴﺔ N . o 1 chemical proporties ﺍﻟﺨﺎﺻﻴﺔ ﺍﻟﺤﺎﻟﺔ 67 ﺍﻟﺨﻮﺍﺹ ﺍﻟﻔﻴﺰﻳﺎﺋﻴﺔ ﺍﻟﻨﺘﻴﺠﺔ ﺣﺎﻟﺔ ﺍﻟﻤﻜﻮﻥ ) State of content (S. L, G Color ﻗﻴﻤﺔ ﺍﻟـ p. H Value Physical proporties Property ﺍﻟﻠﻮﻥ 2 3 . . . . : physochemistry properties of content ﺍﻟﻄﻌﻢ p. H ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ Test Result
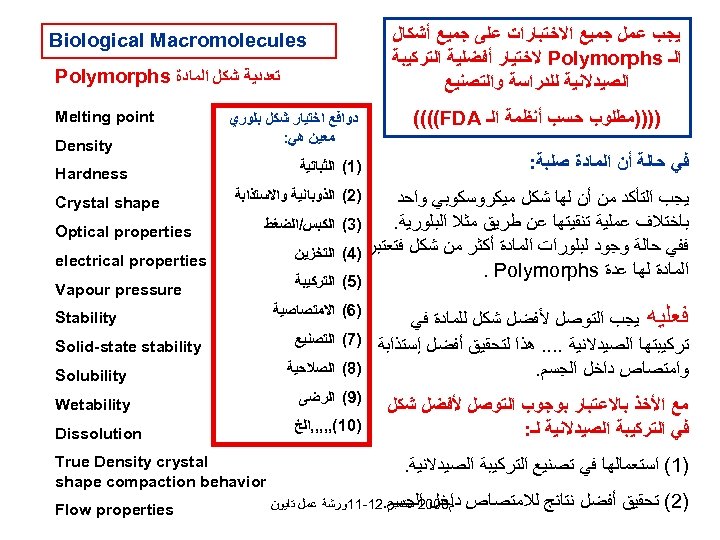
ﻳﺠﺐ ﻋﻤﻞ ﺟﻤﻴﻊ ﺍﻻﺧﺘﺒﺎﺭﺍﺕ ﻋﻠﻰ ﺟﻤﻴﻊ ﺃﺸﻜﺎﻝ ﺍﻟـ Polymorphs ﻻﺧﺘﻴﺎﺭ ﺃﻔﻀﻠﻴﺔ ﺍﻟﺘﺮﻛﻴﺒﺔ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ ﻟﻠﺪﺭﺍﺳﺔ ﻭﺍﻟﺘﺼﻨﻴﻊ ))))ﻣﻄﻠﻮﺏ ﺣﺴﺐ ﺃﻨﻈﻤﺔ ﺍﻟـ ((((FDA ﻓﻲ ﺣﺎﻟﺔ ﺃﻦ ﺍﻟﻤﺎﺩﺓ ﺻﻠﺒﺔ: Biological Macromolecules ﺗﻌﺪﺩﻳﺔ ﺷﻜﻞ ﺍﻟﻤﺎﺩﺓ Polymorphs ﺩﻭﺍﻓﻊ ﺍﺧﺘﻴﺎﺭ ﺷﻜﻞ ﺑﻠﻮﺭﻱ ﻣﻌﻴﻦ ﻫﻲ: )1( ﺍﻟﺜﺒﺎﺗﻴﺔ Density Hardness )2( ﺍﻟﺬﻭﺑﺎﻧﻴﺔ ﻭﺍﻻﺳﺘﺬﺍﺑﺔ ﻳﺠﺐ ﺍﻟﺘﺄﻜﺪ ﻣﻦ ﺃﻦ ﻟﻬﺎ ﺷﻜﻞ ﻣﻴﻜﺮﻭﺳﻜﻮﺑﻲ ﻭﺍﺣﺪ ﺑﺎﺧﺘﻼﻑ ﻋﻤﻠﻴﺔ ﺗﻨﻘﻴﺘﻬﺎ ﻋﻦ ﻃﺮﻳﻖ ﻣﺜﻼ ﺍﻟﺒﻠﻮﺭﻳﺔ. )3( ﻓﻔﻲ ﺣﺎﻟﺔ ﻭﺟﻮﺩ ﻟﺒﻠﻮﺭﺍﺕ ﺍﻟﻤﺎﺩﺓ ﺃﻜﺜﺮ ﻣﻦ ﺷﻜﻞ ﻓﺘﻌﺘﺒﺮ )4( ﺍﻟﻤﺎﺩﺓ ﻟﻬﺎ ﻋﺪﺓ . Polymorphs Melting point ﺍﻟﻜﺒﺲ/ﺍﻟﻀﻐﻂ Crystal shape Optical properties ﺍﻟﺘﺨﺰﻳﻦ electrical properties )5( ﺍﻟﺘﺮﻛﻴﺒﺔ Vapour pressure ﻓﻌﻠﻴﻪ ﻳﺠﺐ ﺍﻟﺘﻮﺻﻞ ﻷﻔﻀﻞ ﺷﻜﻞ ﻟﻠﻤﺎﺩﺓ ﻓﻲ ﺗﺮﻛﻴﺒﺘﻬﺎ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ. . ﻫﺬﺍ ﻟﺘﺤﻘﻴﻖ ﺃﻔﻀﻞ ﺇﺳﺘﺬﺍﺑﺔ )7( )8( ﻭﺍﻣﺘﺼﺎﺹ ﺩﺍﺧﻞ ﺍﻟﺠﺴﻢ. )6( ﺍﻻﻣﺘﺼﺎﺻﻴﺔ ﻣﻊ ﺍﻷﺨﺬ ﺑﺎﻻﻋﺘﺒﺎﺭ ﺑﻮﺟﻮﺏ ﺍﻟﺘﻮﺻﻞ ﻷﻔﻀﻞ ﺷﻜﻞ ﻓﻲ ﺍﻟﺘﺮﻛﻴﺒﺔ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ ﻟـ: ﺍﻟﺘﺼﻨﻴﻊ ﺍﻟﺼﻼﺣﻴﺔ )9( ﺍﻟﺮﺿﻰ )01(, , , ﺍﻟﺦ )1( ﺍﺳﺘﻌﻤﺎﻟﻬﺎ ﻓﻲ ﺗﺼﻨﻴﻊ ﺍﻟﺘﺮﻛﻴﺒﺔ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ. )2( ﺗﺤﻘﻴﻖ ﺃﻔﻀﻞ ﻧﺘﺎﺋﺞ ﻟﻼﻣﺘﺼﺎﺹ ﺩﺍﺧﻞ ﺍﻟﺠﺴﻢ ﻡ 8002 ﺳﺘﻤﺒﺮ. 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ Stability Solid-state stability Solubility Wetability Dissolution True Density crystal shape compaction behavior Flow properties
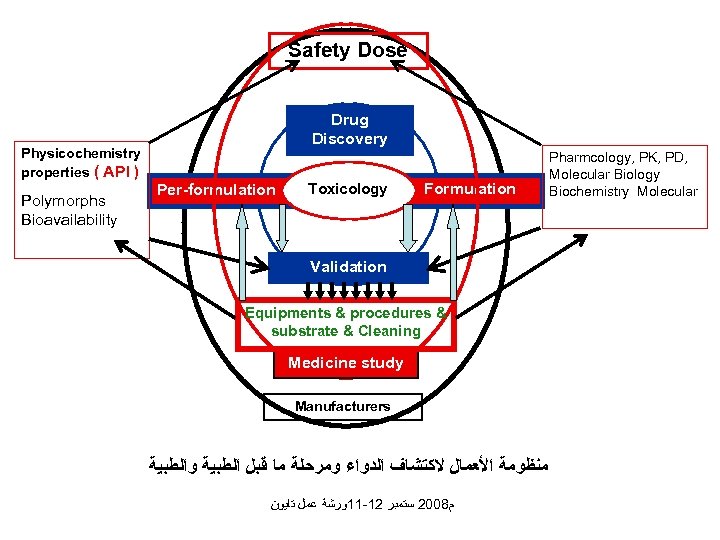
Safety Dose Physicochemistry properties ( API ) Polymorphs Bioavailability Drug Discovery Per-formulation Toxicology Formulation Validation Equipments & procedures & substrate & Cleaning Medicine study Manufacturers ﻣﻨﻈﻮﻣﺔ ﺍﻷﻌﻤﺎﻝ ﻻﻛﺘﺸﺎﻑ ﺍﻟﺪﻭﺍﺀ ﻭﻣﺮﺣﻠﺔ ﻣﺎ ﻗﺒﻞ ﺍﻟﻄﺒﻴﺔ ﻭﺍﻟﻄﺒﻴﺔ ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ Pharmcology, PK, PD, Molecular Biology Biochemistry Molecular

ﻳﻔﻀﻞ ﺗﺤﺪﻳﺪ : ﺍﻟﺠﺮﻋﺔ ﺍﻟﻌﻼﺟﻴﺔ ﻓﻲ ﺍﻟﺘﺮﻛﻴﺒﺔ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ Route & frequency of administration for patient compliance ﻳﺘﻢ ﺍﻟﺘﻮﺻﻞ ﻟﻠﺠﺮﻋﺔ ﻣﻦ ﻧﺘﺎﺋﺞ ﺍﻷﺮﺑﻌﺔ ﺩﺭﺍﺳﺎﺕ ﺍﻟﺘﺎﻟﻴﺔ: )1( ﻣﻌﺮﻓﺔ ﺍﻟﺨﻮﺍﺹ ﺍﻟﻔﻴﺰﻭﻛﻴﻤﻴﺎﺋﻴﺔ ﻟﻠﻤﺎﺩﺓ ﺍﻟﻌﻼﺟﻴﺔ ﺍﻟﻨﻘﻴﺔ ﺛﻢ ﻓﻲ ﺗﺮﻛﻴﺒﺘﻬﺎ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ ﺗﺴﺎﻋﺪ ﻓﻲ ﺍﻟﺘﻮﺻﻞ ﻟﻠﺘﺮﻛﻴﺒﺔ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ ﺍﻟﻤﺜﻠﻰ(. ) )2( ﻣﻌﺮﻓﺔ ﺍﻟﺨﻮﺍﺹ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ ﻟﻠﺪﻭﺍﺀ )ﺗﺄﺜﻴﺮ ﻭﺟﻬﺪ ﺍﻟﺪﻭﺍﺀ ﻋﻠﻰ ﺍﻟﺠﺴﻢ ﻟﻤﻌﺮﻓﺔ ﻛﻤﻴﺔ ﺍﻟﺠﺮﻋﺔ ﺍﻟﻌﻼﺟﻴﺔ ﻭﻣﻌﺮﻓﺔ ﺍﻟﻮﺍﺟﻬﺔ ﺍﻟﻌﻼﺟﻴﺔ(. )3( ﻣﻌﺮﻓﺔ ﺍﻟﺤﺮﻛﻴﺔ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ ﻟﻠﺪﻭﺍﺀ )ﻛﻤﻴﺔ ﺍﻟﺪﻭﺍﺀ ﺍﻟﻤﻤﺘﺼﺔ ﻭﺗﻮﺯﻳﻌﻬﺎ ﻭﺗﻔﺎﻋﻠﻪ ﺍﻷﻴﻀﻲ ﺑﺎﻟﺠﺴﻢ ﺑﺠﺎﻧﺐ ﻣﻌﺮﻓﺔ ﻧﻮﺍﺗﺞ ﺗﻜﺴﻴﺮ ﺍﻟﺪﻭﺍﺀ ﺑﻌﺪ ﻣﻌﺎﻟﺠﺘﻪ ﻟﻠﻤﺮﺽ ﺑﻤﻌﻨﻲ ﻣﻌﺮﻓﺔ ﻣﺴﺎﺭ ﺗﺤﻮﻝ ﺍﻟﺪﻭﺍﺀ ﻟﻤﻮﺍﺩ ﺃﻮﻟﻴﺔ ﺗﺨﺮﺝ ﻣﻦ ﺍﻟﺠﺴﻢ( ﻋﻦ ﻃﺮﻳﻖ ﻗﻴﺎﺱ ﻣﻌﺪﻝ ﻛﻤﻴﺔ ﺍﻟﺪﻭﺍﺀ ﺣﺮﻛﻴﺎ ﺑﺎﻟﺪﻡ. )4( ﻣﻌﺮﻓﺔ ﺍﻟﺪﻳﻨﻤﻜﺔ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ ﻟﻠﺪﻭﺍﺀ )ﻣﻌﺮﻓﺔ آﻠﻴﺔ ﺃﺴﺎﺱ ﻋﻤﻞ ﺍﻟﺪﻭﺍﺀ ﻟﻠﻌﻼﺝ ﺑﺎﻟﺠﺴﻢ ﺑﻤﻌﻨﻰ ﻛﻴﻔﻴﺔ ﺣﺪﻭﺙ ﺍﻟﺘﺪﺍﺧﻞ ﺍﻟﻌﻼﺟﻲ ﺑﻴﻦ ﺍﻟﻤﺮﺽ ﻭﺍﻟﺪﻭﺍﺀ ﻭﻛﻴﻔﻴﺔ ﺗﻮﺯﻳﻊ ﺟﺮﻋﺎﺕ ﺍﻟﻌﻼﺝ ﺣﺴﺐ ﻧﻮﻉ ﺍﻟﺠﻨﺲ ﻭﺍﻟﻮﺯﻥ ﻭ. . ﺍﻟﺦ( ﺍﺳﺘﺮﺍﺗﻴﺠﻴﺔ ﺗﺤﺪﻳﺪ ﺍﻟﺠﺮﻋﺎﺕ ﺍﻟﻌﺎﺟﻴﺔ ﻭﻋﺪﺩ ﻣﺮﺍﺕ ﺗﻨﺎﻭﻟﻬﺎ ﻭﻛﻴﻔﻴﺔ ﺗﻨﺎﻭﻟﻬﺎ ﺗﻌﺘﺒﺮ ﺍﻟﺠﺮﻋﺔ ﺍﻟﻌﻼﺟﻴﺔ ﻣﺘﻤﻴﺰﺓ ﻓﻲ ﺣﺎﻟﺔ ﻭﺻﻮﻟﻬﺎ ﻟﻠﺠﺰﺀ ﺍﻟﻤﺼﺎﺏ ﺑﺎﻟﺠﺴﻢ ﻭﻛﻤﻴﺘﻬﺎ ﻣﻨﺎﺳﺒﺔ ﻟﻠﻌﻼﺝ. ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

4 ﺍﻟﻤﺤﺎﺿﺮﺓ Pre-clinical: Keys: - Basic Pharmacology PD - Pharmacodynamics what the drug dose to the body ? -Principles of Pharmacokinetics & ADME PK what the body dose to the drug ? - Bioavailability - Dose selection and species selection - Role of metabolites in safety - Pro drugs ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

ﻣﺼﻄﻠﺤﺎﺕ ﻋﻠﻤﻴﺔ ﻭﺗﻌﺮﻳﻒ ﻛﻞ ﻣﻨﻬﺎ ﺍﻻﻣﺘﺼﺎﺻﻴﺔ : Absorption ﺗﻌﺒﺮ ﻋﻦ ﻣﺴﺎﺭ ﺩﺧﻮﻝ ﺍﻟﺪﻭﺍﺀ ﻟﺪﺍﺧﻞ ﺍﻟﺠﺴﻢ. ﺍﻟﺘﻮﺯﻳﻊ : Distribution ﻫﻮ ﺗﻮﺯﻳﻊ ﺍﻟﺠﺮﻋﺔ ﺍﻟﻌﻼﺟﻴﺔ ﻋﻠﻰ ﺟﻤﻴﻊ ﺳﻮﺍﺋﻞ ﻭﺃﻨﺴﺠﺔ ﺍﻟﺠﺴﻢ. ﺍﻟﺘﻔﺎﻋﻞ ﺍﻷﻴﻀﻲ : Metabolism ﺗﺤﻮﺭ ﻏﻴﺮ ﻋﻜﻮﺱ ﻟﻠﻤﺮﻛﺐ ﺍﻷﺒﻮﻱ ﺇﻟﻲ ﺍﻷﺒﻨﺎﺀ ﺧﻼﻝ ﺍﻟﺘﻔﺎﻋﻞ ﺍﻷﻴﻀﻲ. ﺗﺼﻔﻴﺔ ﺇﻓﺮﺍﺯﻳﺔ : Excretion ﻫﻲ ﺣﺬﻑ ﺍﻟﻤﻮﺍﺩ )ﻏﻴﺮ ﺍﻟﻤﺮﻏﻮﺏ ﺑﻬﺎ ﺑﺎﻟﺠﺴﻢ( ﻣﻦ ﺍﻟﺠﺴﻢ. ﻓﻲ ﺑﻌﺾ ﺍﻟﺤﺎﻻﺕ ﺍﻟﻨﺎﺩﺭﺓ ﻳﺤﺪﺙ ﺗﺠﻤﻊ ﻟﻠﺪﻭﺍﺀ ﺑﻴﻦ ﺍﻧﺴﺠﺔ ﺍﻟﺠﺴﻢ. ﺍﻻﻣﺘﺼﺎﺻﻴﺔ )ﺑﺎﺳﺘﺜﻨﺎﺀ ﺗﻨﺎﻭﻝ ﺍﻟﺠﺮﻋﺔ ﻋﻦ ﻃﺮﻳﻖ ﺍﻟﻮﺭﻳﺪ( ﻳﺠﺐ ﻋﻠﻰ ﺍﻟﻤﺮﻛﺐ ﺃﻦ ﻳﻌﺒﺮ ﻣﻦ ﺧﻼﻝ ﺍﻻﻏﺸﻴﺔ ﺍﻟﺨﻠﻮﻳﺔ ﻟﻴﺼﻞ ﺇﻟﻰ ﺍﻟﻤﺠﺎﻝ ﺍﻟﻤﺮﺍﺩ ﻋﻼﺟﻪ ﺑﺎﻟﺠﺴﻢ ﻭﻫﺬﺍ ﻳﺤﺪﺙ ﻋﻠﻰ ﻣﺮﺍﺣﻞ ﻫﻲ: )1( Passive diffusion )2( ﺑﺴﻬﻞ ﻋﻤﻠﻴﺔ ﺍﻻﻧﺘﺸﺎﺭ Facilitated diffusion )3( ﻧﺸﻄﺔ ﻋﻤﻠﻴﺔ ﺍﻻﻧﺘﻘﺎﻝ Active transport )4( ) Pinocytosis (engulfing of fluids by cell ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

ﺍﻟﺘﻮﺯﻳﻊ Distribution ﻛﻤﻴﺔ ﺍﻟﺪﻭﺍﺀ ﻓﻲ ﺍﻟﺠﺴﻢ ﺗﺮﻛﻴﺰ ﺍﻟﺪﻭﺍﺀ ﻓﻲ ﺍﻟﺪﻡ ﺣﻴﺚ ﺃﻦ Vd ﻫﻲ ﺍﻟﻘﻴﻤﺔ ﺍﻟﻨﻈﺮﻳﺔ = Vd Volume distribution ﻭﻋﻨﺪﻣﺎ ﺗﻜﻮﻥ ﻋﺎﻟﻴﺔ ﻳﺬﻭﺏ ﺍﻟﺪﻭﺍﺀ ﻓﻲ ﺍﻟﻠﻴﺒﻴﺪﺍﺕ ﻭﻋﻨﺪﻣﺎ ﺗﻜﻮﻥ ﻣﻨﺨﻔﻀﺔ ﻻ ﻳﺬﻭﺏ ﺍﻟﺪﻭﺍﺀ ﻓﻲ ﺍﻟﻠﻴﺒﻴﺪﺍﺕ ﺗﻔﺎﻋﻞ ﺃﻴﻀﻲ Metaboliso )1( ﻳﻌﺘﺒﺮ ﺍﻟﻜﺒﺪ ﺍﻟﻤﺮﻛﺰ ﺍﻷﺴﺎﺳﻲ ﻟﻠﻌﻤﻠﻴﺎﺕ ﺍﻷﻴﻀﻴﺔ ﻓـ: ﺍﻟﻄﻮﺭ : I ﺗﻔﺎﻋﻼﺕ ﺃﻴﻀﻴﺔ ﺍﻷﻜﺴﺪﺓ ﻭﺍﻻﺧﺘﺰﺍﻝ ﻭﺍﻟﺘﻤﻴﺀ ﻣﻦ ﺧﻼﻝ ﻧﻤﺎﺫﺝ ﻣﻦ ﺍﻟﺘﻔﺎﻋﻼﺕ ﺍﻷﻴﻀﻴﺔ ﻓﻲ ﺍﻟﺨﺎﺭﺝ : In. Vitro Metabolism models Hepatocytes Liver slices ﻣﻴﺮﻭﺯﻭﻣﺎﺕ ﺍﻟﻜﺒﺪ Liver microsomes ﺇﻧﺰﻳﻢ 054 -. P ﺍﻟﻄﻮﺭ : II ﺇﺿﺎﻓﺔ ﺃﻮ ﺍﻗﺘﺮﺍﻥ ﺍﻟﺪﻭﺍﺀ ﺑﺎﻟﻤﺠﻤﻮﻋﺎﺕ ﺍﻟﻜﺤﻮﻟﻴﺔ ﻭﺍﻻﻣﻴﻨﻴﺔ ﻭﺍﻟﺜﻴﻮﻛﺤﻮﻟﻴﺔ ﻟﺘﻘﻞ ﺫﻭﺑﺎﻧﻴﺘﻪ ﻭﺗﺼﻔﻴﺘﻪ ﺍﻹﻓﺮﺍﺯﻩ ﻋﻦ ﻃﺮﻳﻖ ﺍﻟﻜﻠﻴﺔ ﺃﻮ ﺍﻟﺠﻠﺪ ﺃﻮ ﻓﺘﺤﺔ ﺍﻟﺸﺮﺝ ﺃﻮ ﺍﻟﺒﻮﻝ ﺃﻮ ﺍﻟﻤﺴﺘﻘﻴﻢ ﺃﻮ ﺍﻟﺮﺋﺔ ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ Variable correlation to in vivo ) (rat

PD ? what the drug dose to the body TOOLS: FREE ENERGY MAPS ﺗﺄﺜﻴﺮ ﺍﻟﺪﻭﺍﺀ ﺍﻟﻔﺴﻴﻮﻟﻮﺟﻲ ﻭﺍﻟﺤﻴﻮﻱ ﻛﻴﻤﺎﻭﻱ ﻋﻠﻰ ﺍﻟﺠﺴﻢ ﻭ/ﺃﻮ ﺍﻟﻜﺎﺋﻨﺎﺕ ﺍﻟﺪﻗﻴﻘﺔ ﺍﻟﺠﺮﺛﻮﻣﻴﺔ ﻭ/ﺃﻮ ﺍﻟﻄﻔﻴﻠﻴﺎﺕ ﻓﻲ ﺍﻟﺠﺴﻢ ﻣﻌﺮﻓﺔ آﻠﻴﺎﺕ ﻛﻴﻔﻴﺔ ﺗﺄﺜﻴﺮ ﺍﻟﺪﻭﺍﺀ ﻋﻠﻰ ﺍﻟﻤﺮﺽ ﺑﺎﻟﺠﺴﻢ. ﻛﻴﻔﻴﺔ ﺣﺪﻭﺙ ﺍﻻﻣﺘﺼﺎﺹ ﺑﺎﻟﺠﺴﻢ ﻳﺘﺨﻠﺺ ﺍﻟﺠﺴﻢ ﻣﻦ ﺍﻟﺪﻭﺍﺀ )ﻣﻌﺮﻓﺔ ﻣﻌﺪﻝ ﺑﺪﺍﻳﺔ ﺗﺄﺜﻴﺮ ﺍﻟﺪﻭﺍﺀ ﻋﻠﻰ ﺍﻟﺠﺴﻢ( ﻣﺪﺓ ﺗﺄﺜﻴﺮ ﺍﻟﺪﻭﺍﺀ )ﺗﺤﻮﻝ ﺍﻟﻤﺎﺩﺓ ﺣﻴﻮﻳﺎ ﻓﻲ ﺍﻟﺠﺴﻢ(. ﺗﺄﺜﻴﺮ ﻭﻣﺴﺎﺭ ﺍﻟﺘﺨﻠﺺ ﺍﻷﻴﻀﻲ ﻟﻠﺪﻭﺍﺀ, ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

PD Information about Pharmacodynmics )1( ﺃﻐﻠﺐ ﺍﻷﺪﻭﻳﺔ ﺗﺘﻔﺎﻋﻞ ﻛﻤﺨﺎﻟﺐ ﺑﺎﺭﺗﺒﺎﻃﻬﺎ ﻣﻊ ﻣﺴﺘﻘﺒﻼﺕ ﺍﻟﺠﺴﻢ ﻟﺘﻘﺪﻳﺮ ﺍﻟﺘﺄﺜﻴﺮﺍﺕ ﺍﻟﺨﻠﻮﻳﺔ Determine cellular effects )2( ﻋﻨﺪﻣﺎ ﻳﺤﺪﺙ ﺍﺭﺗﺒﺎﻁ ﺍﻟﺪﻭﺍﺀ ﻛﻤﺨﻠﺐ ﻣﻊ ﺍﻟﻤﺴﺘﻘﺒﻞ ﻗﺪ ﻳﺤﺪﺙ ﻟﻠﻤﺴﺘﻘﺒﻞ ﺃﺤﺪ ﺛﻼﺛﺔ ﺗﺄﺜﻴﺮﺍﺕ ﻫﻲ: )1( ﻳﻌﻤﻞ ﺍﻟﻤﺴﺘﻘﺒﻞ ﺑﺴﻂ ﻃﺒﻴﻌﻲ ﻭﻳﻄﻠﻖ ﻋﻠﻴﻪ ﺑﺎﺳﻢ agonist )2( ﻳﻔﻘﺪ ﺍﻟﻤﺴﺘﻘﺒﻞ ﺗﺄﺜﻴﺮﻩ ﺗﻤﺎﻣ ﻭﻳﻄﻠﻖ ﻋﻠﻴﻪ ﺑﺎﺳﻢ Antagonist )3( ﻳﻌﻤﻞ ﺍﻟﻤﺴﺘﻘﺒﻞ ﺑﺸﻜﻞ ﻋﻜﺴﻲ ﻋﻦ ﻋﻤﻠﻪ ﺍﻟﻄﺒﻴﻌﻲ ﻭﻳﻄﻠﻖ ﻋﻠﻴﻪ ﺑﺎﺳﻢ inverse agonist )3( ﻓﻲ ﺍﻷﺴﺎﺱ ﺍﻟﺼﻴﺪﻻﻧﻲ ﻳﻄﻤﺢ ﻓﻲ ﺍﻟﺤﺼﻮﻝ ﻋﻠﻰ ﺗﺮﻛﻴﺰ ﻋﺎﻟﻰ ﻣﻦ ﺍﻟﺪﻭﺍﺀ ﻓﻲ ﺑﻼﺯﻣﺎ ﺍﻟﺪﻡ ﻟﻴﺘﻮﺻﻞ ﺇﻟﻲ ﻣﺴﺘﻮﻳﺎﺕ ﻋﺎﻟﻴﺔ ﻣﻦ ﺍﺳﺘﺠﺎﺑﺔ ﺍﻟﺠﺴﻢ ﻟﻠﺪﻭﺍﺀ ﻭﻳﻘﻮﻡ ﺑﺪﺭﺍﺳﺔ ﻫﺬﻩ ﺍﻻﺳﺘﺠﺎﺑﺎﺕ ﻭﺗﺤﻠﻴﻞ ﻧﺘﺎﺋﺠﻬﺎ. )4( ﻓﻲ ﺍﻟﺤﻘﻴﻘﺔ ، ﺗﻮﺟﺪ ﻋﻮﺍﻣﻞ ﻛﺜﻴﺮﺓ ﺗﺆﺜﺮ ﻋﻠﻰ ﺑﻘﺎﺀ ﺗﺮﻛﻴﺰ ﻋﺎﻟﻰ ﻣﻦ ﺍﻟﺪﻭﺍﺀ ﻓﻲ ﺑﻼﺯﻣﺎ ﺍﻟﺪﻡ ﻣﻨﻬﺎ: • ﻋﻮﺍﻣﻞ ﺍﻟﺤﺮﻛﻴﺔ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ: ﻭﻫﻲ ﺗﻌﻤﻞ ﻋﻠﻰ ﺗﻘﺪﻳﺮ ﺗﺮﻛﻴﺰ ﺍﻟﺪﻭﺍﺀ ﻓﻲ ﺑﻼﺯﻣﺎ ﺍﻟﺪﻡ ، ﻭﻟﺤﺪﻭﺙ ﺍﻟﺘﻔﺎﻋﻼﺕ ﺍﻷﻴﻀﻴﺔ ﺍﻟﺘﻲ ﺗﻌﻤﻞ ﻋﻠﻰ ﺍﺳﺘﻬﻼﻙ ﺗﺮﻛﻴﺰ ﺍﻟﺪﻭﺍﺀ ﺑﺎﺳﺘﻤﺮﺍﺭ ﻭﺍﻟﺘﻨﻈﻴﻒ ﺍﻹﻓﺮﺍﺯﻱ ﻹﺧﺮﺍﺝ ﺍﻟﺪﻭﺍﺀ ﺃﻮ ﻧﺘﺎﺋﺞ ﺗﻜﺴﻴﺮﻩ ﺇﻟﻰ ﺧﺎﺭﺝ ﺍﻟﺠﺴﻢ ، excretory clearance ﻻ ﻳﻤﻜﻦ ﺍﻟﺘﻮﺻﻞ ﻟﻘﻴﻢ ﺗﺮﻛﻴﺰ ﺍﻟﺪﻭﺍﺀ ﺑﺒﻼﺯﻣﺎ ﺍﻟﺪﻡ ﺑﺸﻜﻞ ﻣﻄﻠﻖ. )1( ﺍﻟﻌﻮﺍﻣﻞ ﺍﻟﺠﻴﻨﻴﺔ ) : (Genetic factors ﻓﻲ ﺣﺎﻟﺔ ﺣﺪﻭﺙ ﻣﺜﻞ ﻫﺬﻩ ﺍﻟﻌﻮﺍﻣﻞ ﻧﻼﺣﻆ ﺗﺒﺪﻳﻞ ﻓﻲ ﺍﻟﺘﻔﺎﻋﻼﺕ ﺍﻷﻴﻀﻴﺔ ﺃﻮ ﺗﺒﺪﻳﻞ ﺗﺄﺜﻴﺮ ﺍﻟﺪﻭﺍﺀ ﺍﻷﻴﻀﻲ ﻧﺘﻴﺠﺔ ﻇﻬﻮﺭ ﺃﻌﺮﺍﺽ ﻋﻠﻰ ﺍﻟﻤﺮﺿﻰ ﻣﺒﺎﺷﺮﺓ ﻣﻤﺎ ﻳﺄﻜﺪ ﻭﺟﻮﺩ ﺍﻣﺘﺼﺎﺹ ﻭﺗﺄﺜﻴﺮ ﺍﻟﺠﺮﻋﺔ ﺍﻟﻌﻼﺟﻴﺔ ﻋﻠﻰ ﺍﻟﺠﺴﻢ. ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

Therapeutic Index ﺍﻟﺪﻟﻴﻞ ﺍﻟﻌﻼﺟﻲ ) The amount that gives an effect (Effective dose The amount that gives more adverse effects = Therapeutic Index ﻓﻲ ﺣﺎﻟﺔ ﻗﻴﻤﺔ ﺍﻟﺪﻟﻴﻞ ﺍﻟﻌﻼﺟﻲ ﺗﻜﻮﻥ ﺻﻐﻴﺮﺓ ﻓﻴﺠﺐ ﺍﻻﻧﺘﺒﺎﻩ ﻭﻣﺮﺍﻗﺒﺔ ﺍﻟﺤﺎﻟﺔ ﺍﻟﻤﺮﺿﻴﺔ ﺧﻼﻝ ﺗﻨﺎﻭﻟﻪ ﺍﻟﺠﺮﻋﺔ ﺍﻟﻌﻼﺟﻴﺔ ﺑﺎﺳﺘﻤﺮﺍﺭ ، ﻭﺫﻟﻚ ﺑﺘﺤﻠﻴﻞ ﺗﺮﻛﻴﺰ ﺍﻟﺪﻭﺍﺀ ﺑﺎﻟﺪﻡ ﺑﺼﻮﺭﺓ ﻣﺴﺘﻤﺮﺓ ، ﺑﺤﻜﻢ ﺍﺣﺘﻤﺎﻟﻴﺔ ﺣﺪﻭﺙ ﻋﺪﻡ ﺗﺄﺜﻴﺮ ﺍﻟﺪﻭﺍﺀ ﻋﻠﻰ ﺍﻟﻤﺮﺽ ﺃﻮ ﺣﺪﻭﺙ ﺗﺄﺜﻴﺮ ﻣﻌﺎﻛﺲ ﻟﺘﺄﺜﻴﺮ ﺍﻟﺪﻭﺍﺀ ﻧﺘﻴﺠﺔ ﻗﺮﺏ ﺍﻟﺠﺮﻋﺔ ﺍﻟﻌﻼﺟﻴﺔ ﻣﻦ ﺍﻟﺠﺮﻋﺔ ﺍﻟﻤﻔﺮﻃﺔ ﺍﻟﺘﻲ ﻗﺪ ﺗﻌﻄﻲ ﻧﺘﻴﺠﺔ ﻋﻜﺴﻴﺔ ﺿﺎﺭﺓ ﺑﺎﻟﻤﺮﻳﺾ. ﺃﻐﻠﺐ ﺃﺪﻭﻳﺔ ﻋﻼﺝ ﺍﻟﺴﺮﻃﺎﻥ ﻟﻬﺎ ﻗﻴﻤﺔ ﺍﻟﺪﻟﻴﻞ ﺍﻟﻌﻼﺟﻲ ﺻﻐﻴﺮﺓ ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

ﻓﺎﻋﻠﻴﺔ ﺍﻟﺪﻭﺍﺀ ﻗﺪ ﺗﺤﺪﺙ ﻧﺘﻴﺠﺔ: ﺃﻐﻠﺐ ﺍﻷﺪﻭﻳﺔ ﻗﺪ ﻳﻜﻮﻥ ﻟﻬﺎ ﺃﺤﺪ ﺍﻟﺘﺄﺜﻴﺮﺍﺕ ﺍﻟﺘﺎﻟﻴﺔ: )1( ﺣﺪﻭﺙ ﺗﺸﻮﻳﻪ ﻟﻠﺠﺪﺍﺭ ﺍﻟﺨﻠﻮﻱ ﻟﻠﻤﺮﺽ. : Mimic effect )2( ﺗﻔﺎﻋﻞ ﻛﻴﻤﻴﺎﺋﻲ ﻣﻊ ﺍﻟﻤﺮﺽ. physiological/biochemical processes )3( ﺍﺗﺤﺎﺩ ﺍﻟﺪﻭﺍﺀ ﻣﻊ ﺑﺮﻭﺗﻴﻨﺎﺕ ﺍﻹﻧﺰﻳﻤﺎﺕ. ﻣﺴﺎﺭﺍﺕ ﺗﺆﺜﺮ ﻓﻲ ﺍﻷﻌﻀﺎﺀ ﻭ/ﺃﻮ ﺗﻔﺎﻋﻞ ﻛﻴﻤﺎﻭﻱ ﺣﻴﻮﻱ )4( ﺍﺗﺤﺎﺩ ﻣﻊ ﺗﺮﻛﻴﺐ ﺍﻟﺒﺮﻭﺗﻴﻦ ﻣﻌﻴﻦ. : Inhibit normal inhibit pathological processes in animal )5( ﺍﺗﺤﺎﺩ ﻣﻊ ﺍﻟﺒﺮﻭﺗﻴﻨﺎﺕ ﺍﻟﺤﺎﻣﻠﺔ. ﻣﺴﺎﺭﺍﺕ ﺗﺜﺒﻴﻂ ﺍﻷﻤﺮﺍﺽ ﻓﻲ ﺣﻴﻮﺍﻧﺎﺕ ﺍﻟﺘﺠﺎﺭﺏ )6( ﺍﺗﺤﺎﺩ ﻣﻊ ﺍﻟﻘﻨﻮﺍﺕ ﺍﻷﻴﻮﻧﻴﺔ. : Inhibit vital processes )7( ﺍﻻﺭﺗﺒﺎﻁ ﻣﻊ ﺍﻟﻤﺴﺘﻘﺒﻼﺕ ﻣﺜﻞ: (a ﻣﺴﺘﻘﺒﻼﺕ ﻫﻮﺭﻣﻮﻧﻴﺔ. of endo- & ecto- parasites and (b ﻣﺴﺘﻘﺒﻼﺕ ﺍﻟﺨﻼﻳﺎ ﺍﻟﻌﺼﺒﻴﺔ ﺍﻟﻤﻌﺪﻟﺔ. Neuromodulator (c ﻣﺴﺘﻘﺒﻼﺕ ﺍﻟﺨﻼﻳﺎ ﺍﻟﻌﺼﺒﻴﺔ ﺍﻟﻤﺮﺳﻠﺔ. Neurotransmitter microbial organisms ﻣﺴﺎﺭﺍﺕ ﺗﺜﺒﻴﻂ ﺃﻨﺸﻄﺔ ﺍﻷﻌﻀﺎﺀ ﺍﻟﺤﻴﻮﻳﺔ ﻓﻲ ﺃﻨﻮﺍﻉ ﺍﻟﻄﻔﻴﻠﻴﺎﺕ ﻭﺍﻟﻜﺎﺋﻨﺎﺕ ﺍﻟﺤﻴﺔ ﺍﻟﺠﺮﺛﻮﻣﻴﺔ. ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

ﺗﺄﺜﻴﺮﺍﺕ ﺍﻷﺪﻭﻳﺔ ﺗﺘﻤﺮﻛﺰ ﻓﻲ ﺃﺮﺑﻌﺔ ﺍﺗﺠﺎﻫﺎﺕ ﺣﻴﺚ ﺗﺆﺪﻱ ﺇﻟﻲ: )1( ﺣﺪﻭﺙ ﻛآﺒﺔ ﻓﻲ ﺍﻟﻨﻔﺲ Depressing )2( ﺗﻨﺸﻴﻂ ﻓﻌﺎﻟﻴﺔ ﺃﻌﻀﺎﺀ ﺍﻟﺠﺴﻢ Stimulating )3( ﺗﺤﻄﻢ ﺍﻟﺨﻼﻳﺎ Destroying cells )4( ﺗﺴﺘﺒﺪﻝ ﺍﻟﻤﻮﺍﺩ Replacing substances ﺗﺄﺜﻴﺮﺍﺕ ﺍﻟﺪﻭﺍﺀ ﻏﻴﺮ ﺍﻟﻤﺮﻏﻮﺏ ﺣﺪﻭﺛﻬﺎ ﺑﺎﻟﺠﺴﻢ: )1(ﺍﻟﺰﻳﺎﺩﺓ ﺍﻟﻤﺤﺘﻤﻠﺔ ﻟﻠﻄﻔﺮﺍﺕ ﺍﻟﺴﺮﻃﺎﻧﻴﺔ Carcinogenic activity )2(ﺣﺸﺪ ﻣﻦ ﺍﻟﺘﺄﺜﻴﺮﺍﺕ ﺍﻟﺘﻠﻘﺎﺋﻴﺔ ﺍﻟﻤﺼﻨﻔﺔ ﺍﻟﺘﻲ ﺭﺑﻤﺎ ﺗﺤﺪﺙ ﺃﻀﺮﺍﺭ ﺑﺼﺤﺔ ﺍﻟﺠﺴﻢ. )3(ﺗﺪﺍﺧﻼﺕ ﻣﺜﻞ ﺇﺿﺎﻓﺎﺕ – ﻣﻀﺎﻋﻔﺎﺕ – ﺗﺤﻮﻝ ﻏﺬﺍﺋﻲ ﺃﻴﻀﻲ. )4(ﺣﺪﻭﺙ ﺣﺚ ﻓﺴﻴﻮﻟﻮﺟﻲ ﺿﺎﺭ ﺑﺎﻟﺠﺴﻢ ﺃﻮ ﺣﺪﻭﺙ ﺃﻤﺮﺍﺽ ﻣﺰﻣﻨﺔ ﻏﻴﺮ ﻋﺎﺩﻳﺔ. ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

PK ? what the body dose to the drug ﺩﺭﺍﺳﺔ ﺍﻟﺤﺮﻛﻴﺔ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ ﺗﻌﺘﻤﺪ ﻋﻠﻰ ﺗﺒﻌﻴﺔ ﺍﻟﻮﻗﺖ )ﺃﻲ ﺍﺳﺘﻬﻼﻙ ﺍﻟﻤﺎﺩﺓ ﺍﻟﺪﻭﺍﺋﻴﺔ ﻣﻊ ﻣﺮﻭﺭ ﺍﻟﻮﻗﺖ( ﺃﻮ ﺍﻟﺬﻱ ﻳﺤﺪﺩ ﻣﺼﻴﺮ ﺍﻟﻤﺎﺩﺓ ﺍﻟﺪﻭﺍﺋﻴﺔ ﺍﻟﻤﻌﻄﺎﺓ ﻣﻦ ﺍﻟﺨﺮﺍﺝ ﻟﻠﻜﺎﺋﻦ ﺍﻟﺤﻲ ﺧﻮﺍﺹ ﺍﻟﺪﻭﺍﺀ ﺍﻟﺤﺮﻛﻴﺔ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ ﺗﺘﺄﺜﺮ ﺑﻌﺾ ﺍﻟﻌﻨﺎﺻﺮ ﻣﺜﻞ: )1( ﻧﻮﻋﻴﺔ ﻣﻜﺎﻥ ﺇﻋﻄﺎﺀ ﺍﻟﺪﻭﺍﺀ Site of administration )2( ﺗﺮﻛﻴﺰ ﺍﻟﺪﻭﺍﺀ ﺍﻟﻤﻌﻄﺎﺓ ﻛﻤﺎ ﻳﻤﻜﻦ ﺃﻦ ﻳﺆﺜﺮﺍﻥ ﻋﻠﻰ ﻣﻌﺪﻝ ﺍﻣﺘﺼﺎﺹ ﺍﻟﺪﻭﺍﺀ ﻓﻲ ﺍﻟﺠﺴﻢ. ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

PK/PD Impact on Dose Design ﺍﻟﻤﺆﺜﺮﺍﺕ ﻋﻠﻰ ﺗﺼﻤﻴﻢ ﺍﻟﺠﺮﻋﺔ )1( ﻟﻠﺤﺼﻮﻝ ﻋﻠﻰ ﺍﻟﻌﻼﺝ ﺍﻟﻤﻨﺸﻮﺩ ﻳﺘﻢ ﺍﺳﺘﻌﻤﺎﻝ ﻛﻤﻴﺔ ﻣﻨﺎﺳﺒﺔ ﻣﻦ ﺍﻟﺠﺮﻋﺔ ﺍﻟﻌﻼﺟﻴﺔ ﺍﻟﻔﻌﺎﻟﺔ ﺍﻟﺘﻲ ﻳﺠﺐ ﺃﻦ ﺗﻤﺘﺺ ﻭﺗﻨﺘﻘﻞ ﻟﻠﻨﺎﺣﻴﺔ ﺍﻟﻤﺮﺿﻴﺔ ﺑﺎﻟﺠﺴﻢ ﻓﻲ ﻭﻗﺖ ﻣﻨﺎﺳﺐ. )2( ﻳﺠﺐ ﻣﻌﺮﻓﺔ ﺃﻮﻗﺎﺕ ﺗﻨﺎﻭﻝ ﺍﻟﺠﺮﻋﺎﺕ ﺍﻟﻌﻼﺟﻴﺔ )ﻭﺑﺎﻟﻜﻤﻴﺔ ﺍﻟﻔﻌﺎﻟﺔ ﺍﻟﻤﻄﻠﻮﺑﺔ( ﻟﻜﻲ ﻳﻜﻮﻥ ﻣﻌﺪﻝ ﺗﻌﺎﻗﺐ ﺗﺮﻛﻴﺰ ﺍﻟﺠﺮﻋﺔ ﻓﻲ ﺍﻟﺠﺴﻢ ﻳﺘﻮﺍﺀﻡ ﻣﻊ ﺍﻟﻜﻤﻴﺔ ﺍﻟﻤﻨﺎﺳﺒﺔ ﻟﻠﻌﻼﺝ ﺑﻤﻌﺪﻝ ﺯﻣﻨﻲ ﺗﻌﺎﻗﺒﻲ ﺣﺘﻰ ﺷﻔﺎﺀ ﺍﻟﻤﺮﻳﺾ ﺑﺈﺫﻥ ﺍﻟﻠﻪ. )3( ﺍﻟﺪﻭﺍﺀ ﻳﺠﺐ ﺃﻦ ﻳﺘﻢ ﺗﺠﻬﻴﺰﻩ ﻓﻲ ﺗﺮﻛﻴﺒﺔ ﺻﻴﺪﻻﻧﻴﺔ ﺗﺤﺘﻮﻱ ﻋﻠﻰ ﺍﻟﺠﺮﻋﺔ ﺍﻟﻌﻼﺟﻴﺔ ﺍﻟﻤﻨﺎﺳﺒﺔ ﻭﺫﻟﻚ ﻟﻮﺟﻮﺩ ﻋﻼﻗﺔ ﺑﻴﻦ ﺍﻻﺧﺘﺒﺎﺭﺍﺕ ﺧﺎﺭﺝ ﺍﻟﺠﺴﻢ in vitro testing ﻭ ﺍﻟﺤﻴﻮﻳﺔ ﺍﻟﻤﺘﻮﻓﺮﺓ Bioavailability ﺃﻮ ﺍﻟﻘﻮﺓ ﺍﻟﺒﻴﻮﻟﻮﺟﻴﺔ . potency biological )4( ﻣﺜﺎﻝ : ﺭﺑﻤﺎ ﻳﺤﺪﺙ ﻟﻠﻤﺮﻳﺀ oesophageal ﻭﺟﺪﺍﺭ ﺍﻟﻤﻌﺪﺓ ﺍﻟﺨﻠﻮﻱ gastric mucosal injury ﺃﻀﺮﺍﺭ ﻓﻲ ﺣﺎﻟﺔ ﺍﺳﺘﻌﻤﺎﻝ ﺗﺮﻛﻴﺒﺔ ﺻﻴﺪﻻﻧﻴﺔ ﻏﻴﺮ ﻣﻨﺎﺳﺒﺔ ﻟﻠﻌﻼﺝ. ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ
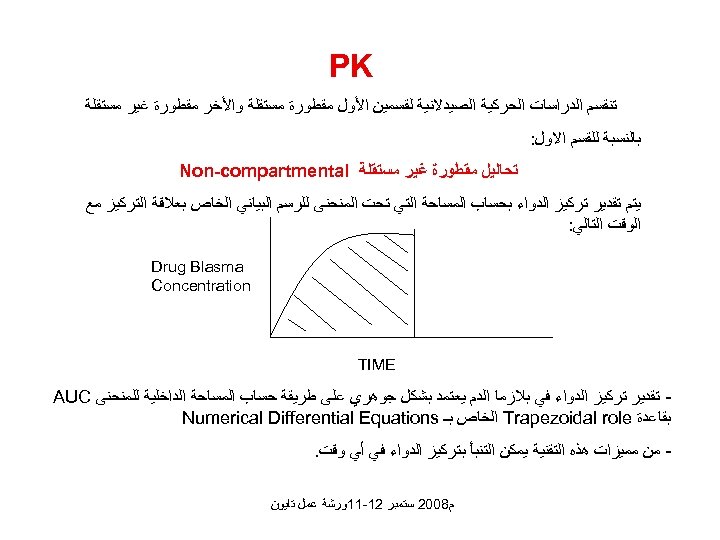
PK ﺗﻨﻘﺴﻢ ﺍﻟﺪﺭﺍﺳﺎﺕ ﺍﻟﺤﺮﻛﻴﺔ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ ﻟﻘﺴﻤﻴﻦ ﺍﻷﻮﻝ ﻣﻘﻄﻮﺭﺓ ﻣﺴﺘﻘﻠﺔ ﻭﺍﻷﺨﺮ ﻣﻘﻄﻮﺭﺓ ﻏﻴﺮ ﻣﺴﺘﻘﻠﺔ ﺑﺎﻟﻨﺴﺒﺔ ﻟﻠﻘﺴﻢ ﺍﻻﻭﻝ: ﺗﺤﺎﻟﻴﻞ ﻣﻘﻄﻮﺭﺓ ﻏﻴﺮ ﻣﺴﺘﻘﻠﺔ Non-compartmental ﻳﺘﻢ ﺗﻘﺪﻳﺮ ﺗﺮﻛﻴﺰ ﺍﻟﺪﻭﺍﺀ ﺑﺤﺴﺎﺏ ﺍﻟﻤﺴﺎﺣﺔ ﺍﻟﺘﻲ ﺗﺤﺖ ﺍﻟﻤﻨﺤﻨﻰ ﻟﻠﺮﺳﻢ ﺍﻟﺒﻴﺎﻧﻲ ﺍﻟﺨﺎﺹ ﺑﻌﻼﻗﺔ ﺍﻟﺘﺮﻛﻴﺰ ﻣﻊ ﺍﻟﻮﻗﺖ ﺍﻟﺘﺎﻟﻲ: Drug Blasma Concentration TIME ﺗﻘﺪﻳﺮ ﺗﺮﻛﻴﺰ ﺍﻟﺪﻭﺍﺀ ﻓﻲ ﺑﻼﺯﻣﺎ ﺍﻟﺪﻡ ﻳﻌﺘﻤﺪ ﺑﺸﻜﻞ ﺟﻮﻫﺮﻱ ﻋﻠﻰ ﻃﺮﻳﻘﺔ ﺣﺴﺎﺏ ﺍﻟﻤﺴﺎﺣﺔ ﺍﻟﺪﺍﺧﻠﻴﺔ ﻟﻠﻤﻨﺤﻨﻰ AUC ﺑﻘﺎﻋﺪﺓ Trapezoidal role ﺍﻟﺨﺎﺹ ﺑـ Numerical Differential Equations ﻣﻦ ﻣﻤﻴﺰﺍﺕ ﻫﺬﻩ ﺍﻟﺘﻘﻨﻴﺔ ﻳﻤﻜﻦ ﺍﻟﺘﻨﺒﺄ ﺑﺘﺮﻛﻴﺰ ﺍﻟﺪﻭﺍﺀ ﻓﻲ ﺃﻲ ﻭﻗﺖ. ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

ﺗﺤﺎﻟﻴﻞ ﻣﻘﻄﻮﺭﺓ ﻣﺴﺘﻘﻠﺔ Compartmental ﺗﺴﺘﺨﺪﻡ ﻧﻤﺎﺫﺝ ﺣﺮﻛﻴﺔ ﻟﻮﺻﻒ ﻭﺍﻟﺘﻨﺒﺆ ﺑﻤﻨﺤﻨﻰ ﺍﻟﺘﺮﻛﻴﺰ ﻭﺍﻟﻮﻗﺖ. ﺍﻟﺪﺭﺍﺳﺔ ﺍﻟﺤﺮﻛﻴﺔ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ ﺗﺘﺸﺎﺑﻪ ﻟﺤﺪ ﻛﺒﻴﺮ ﻣﻊ ﺍﻟﺪﺭﺍﺳﺎﺕ ﺍﻟﺤﺮﻛﻴﺔ ﻓﻲ ﻓﺮﻭﻉ ﺍﻟﻌﻠﻢ ﺍﻷﺨﺮﻯ. - ﻣﻦ ﺍﻟﺼﻌﺐ ﺗﻄﻮﻳﺮ ﺃﻮ ﺍﻟﻮﺻﻮﻝ ﻟﻨﻤﻮﺫﺝ ﺣﺮﻛﻲ ﻣﻌﺘﻤﺪ. ﻗﺎﻋﺪﺓ ﻧﻤﺎﺫﺝ ﺣﻴﻮﺍﻧﻴﺔ ﺍﻟﺘﺠﺎﺭﺏ ﺭﺑﻤﺎ ﻧﻌﻢ ﻭﺭﺑﻤﺎ ﻻ ﺇﻣﻜﺎﻧﻴﺔ ﺍﻟﺘﻨﺒﺆ ﺑﺎﻟﺠﺮﻋﺔ ﺍﻻﻣﻨﺔ ، ﻭﺣﻘﻴﻘﺔ ﺍﻟﺘﻔﺎﻋﻞ ﺍﻷﻴﻀﻲ ﺃﻮ ﺧﻮﺍﺹ PK ﻓﻲ ﺍﻹﻧﺴﺎﻥ. ﺍﻟﻤﺼﺎﺩﻗﺔ ﻋﻠﻰ ﺩﻗﺔ ﻭﺳﻼﻣﺔ ﺍﺳﺘﺮﺍﺗﻴﺠﻴﺔ ﺍﻟﺘﺤﺎﻟﻴﻞ ﻟﻠﺪﻭﺍﺀ ﻭ/ﺃﻮ ﺍﻟﺘﻔﺎﻋﻼﺕ ﺍﻷﻴﻀﻴﺔ ﻓﻲ ﺍﻟﺒﻼﺯﻣﺎ. ﺟﺰﺋﻴﺔ ﻣﻦ ﺍﻟﻤﺼﺎﺩﻗﺔ ﻫﻲ ﺍﻛﺘﺸﺎﻑ ﺩﺭﺍﺳﺔ ﺍﻟـ p. K ﺍﻟﻤﺒﺪﺋﻴﺔ. ﺩﺭﺍﺳﺎﺕ ﺍﻟـ PK ﻻ ﺗﺴﺘﺨﺪﻡ ﻟﻠﻤﺴﺢ ﺍﻟﺒﻴﻮﻟﻮﺟﻲ ﺑﻞ ﻟﺤﺬﻑ ﺍﻟﺪﻭﺍﺀ ﺍﻟﺮﺩﻳﺀ ) ﺑﺴﺒﺐ ﻻ ﺗﻌﻮﺯ ﺍﻟﻔﻌﺎﻟﻴﺔ ﻭﺍﻟﺴﻤﻴﺔ ﻟﻌﻮﺍﻣﻞ ﺩﺭﺍﺳﺎﺕ ﺍﻟـ PK ﻭ ﺍﻟﺘﻲ ﺗﺠﻬﺰ ﻟﻤﻔﺘﺎﺡ ﺗﻄﻮﻳﺮ ﺍﻟﺪﺭﺍﺳﺎﺕ ﺍﻟﻄﺒﻴﺔ ﺍﻟﻜﻴﻤﻴﺎﺋﻴﺔ. - ﺭﺑﻤﺎ ﻳﻤﻜﻦ ﺑﺮﻫﻨﺔ ﺍﻟﺘﻔﺎﻋﻼﺕ ﺍﻷﻴﻀﻴﺔ ﺑﺸﻜﻞ ﺃﻔﻀﻞ ) ﺍﻷﻤﺎﻥ ، ﺍﻟﻔﻌﺎﻟﻴﺔ( ﻳﻔﻀﻞ ﺍﺳﺘﻌﻤﺎﻝ ﺗﻘﻨﻴﺎﺕ ﺣﺪﻳﺜﺔ ﻻﻛﺘﺸﺎﻑ ﺍﻷﺪﻭﻳﺔ ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ
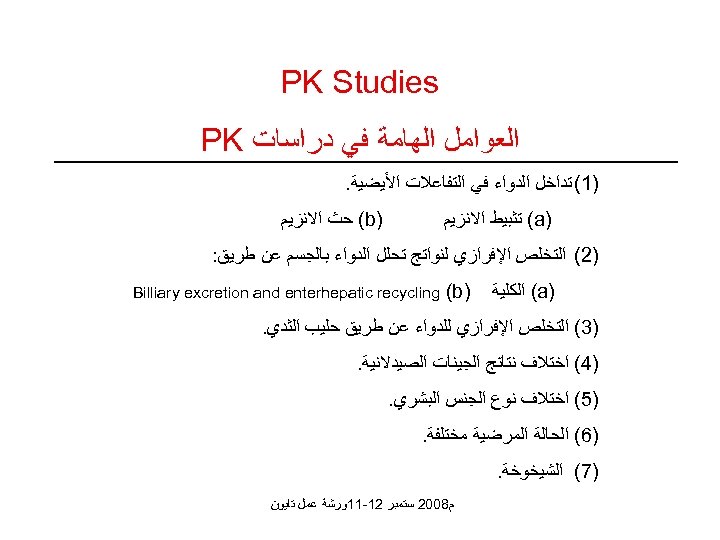
PK Studies ﺍﻟﻌﻮﺍﻣﻞ ﺍﻟﻬﺎﻣﺔ ﻓﻲ ﺩﺭﺍﺳﺎﺕ PK )1( ﺗﺪﺍﺧﻞ ﺍﻟﺪﻭﺍﺀ ﻓﻲ ﺍﻟﺘﻔﺎﻋﻼﺕ ﺍﻷﻴﻀﻴﺔ. ) (b ﺣﺚ ﺍﻻﻧﺰﻳﻢ ) (a ﺗﺜﺒﻴﻂ ﺍﻻﻧﺰﻳﻢ )2( ﺍﻟﺘﺨﻠﺺ ﺍﻹﻓﺮﺍﺯﻱ ﻟﻨﻮﺍﺗﺞ ﺗﺤﻠﻞ ﺍﻟﺪﻭﺍﺀ ﺑﺎﻟﺠﺴﻢ ﻋﻦ ﻃﺮﻳﻖ: ) (a ﺍﻟﻜﻠﻴﺔ ) ( b Billiary excretion and enterhepatic recycling )3( ﺍﻟﺘﺨﻠﺺ ﺍﻹﻓﺮﺍﺯﻱ ﻟﻠﺪﻭﺍﺀ ﻋﻦ ﻃﺮﻳﻖ ﺣﻠﻴﺐ ﺍﻟﺜﺪﻱ. )4( ﺍﺧﺘﻼﻑ ﻧﺘﺎﺋﺞ ﺍﻟﺠﻴﻨﺎﺕ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ. )5( ﺍﺧﺘﻼﻑ ﻧﻮﻉ ﺍﻟﺠﻨﺲ ﺍﻟﺒﺸﺮﻱ. )6( ﺍﻟﺤﺎﻟﺔ ﺍﻟﻤﺮﺿﻴﺔ ﻣﺨﺘﻠﻔﺔ. )7( ﺍﻟﺸﻴﺨﻮﺧﺔ. ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

ADME Studies ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

ADME in Drug Selection )1( ﺭﺑﻤﺎ ﺗﺘﻨﺒﺄ ﺩﺭﺍﺳﺎﺕ ﺍﻷﺪﻣﻲ ) (ADME ﺇﻟﻲ ﺃﻦ ﻛﻴﻨﻮﻧﺔ ﺍﻟﻤﺎﺩﺓ ﺍﻟﻜﻴﻤﻴﺎﺋﻴﺔ ﺍﻟﺠﺪﻳﺪﺓ ) (NCE ﻟﻬﺎ ﻧﻔﺲ ﺍﻟﺨﻮﺍﺹ ﻓﻲ ﺍﻹﻧﺴﺎﻥ. )2( ﺍﻟﺤﻴﻮﻳﺔ ﺍﻟﻤﺘﻮﻓﺮﺓ Bioavailability )3( ﺃﻔﻀﻞ ﺟﻬﺎﺯ ﻣﻌﻠﻦ Optimal systemic exposure )1( ﺗﺼﻔﻴﺔ ﺍﻟﺠﺴﻢ ﻣﻦ ﺍﻟﺪﻭﺍﺀ ﺑﺸﻜﻞ ﻣﻨﺨﻔﺾ Low clearance )2( ﺍﻟﺤﻴﻮﻳﺔ ﺍﻟﻤﺘﻮﻓﺮﺓ ﺍﻟﻤﻌﻄﺎﺓ ﺑﺎﻟﻔﻢ ﺟﻴﺪﺓ Good oral bioavailability )3( ﺍﻟﺤﺼﻮﻝ ﻋﻠﻰ ﺃﻔﻀﻞ ﻧﺼﻒ ﻋﻤﺮ )4( ﻗﺒﻮﻝ ﺍﻟﺘﻔﺎﻋﻼﺕ ﺍﻷﻴﻀﻴﺔ ﻓﻲ ﺍﻟﺪﺭﺍﺳﺎﺕ ﻗﺒﻞ ﺍﻟﻄﺒﻴﺔ ﻭﻓﻲ ﺍﻹﻧﺴﺎﻥ. )4( ﺍﺗﺰﺍﻥ ﻧﺼﻒ ﺍﻟﻌﻤﺮ ﺑﻴﻦ ﺗﺼﻔﻴﺔ ﺍﻟﺠﺴﻢ ﻣﻦ ﺍﻟﺪﻭﺍﺀ Clearance ﻋﻦ ﻃﺮﻳﻖ ﺍﻟﺘﻔﺎﻋﻼﺕ ﺍﻷﻴﻀﻴﺔ ﻭﺍﻟﺘﺨﻠﺺ ﺍﻻﻓﺮﺍﺯﻱ ﻣﻦ ﺍﻟﺪﻭﺍﺀ ﻧﻔﺴﻪ (Excretion of ) unchanged drug ﺗﺼﻤﻴﻢ ﺩﻭﺍﺋﻲ ﻣﻌﻘﻮﻝ Rational drug design ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ )5(
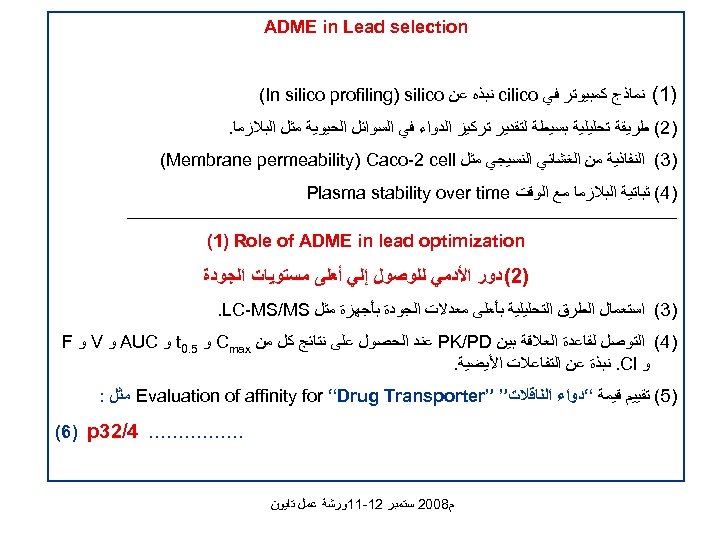
ADME in Lead selection )1( ﻧﻤﺎﺫﺝ ﻛﻤﺒﻴﻮﺗﺮ ﻓﻲ cilico ﻧﺒﺬﻩ ﻋﻦ (In silico profiling) silico )2( ﻃﺮﻳﻘﺔ ﺗﺤﻠﻴﻠﻴﺔ ﺑﺴﻴﻄﺔ ﻟﺘﻘﺪﻳﺮ ﺗﺮﻛﻴﺰ ﺍﻟﺪﻭﺍﺀ ﻓﻲ ﺍﻟﺴﻮﺍﺋﻞ ﺍﻟﺤﻴﻮﻳﺔ ﻣﺜﻞ ﺍﻟﺒﻼﺯﻣﺎ. )3( ﺍﻟﻨﻔﺎﺫﻳﺔ ﻣﻦ ﺍﻟﻐﺸﺎﺋﻲ ﺍﻟﻨﺴﻴﺠﻲ ﻣﺜﻞ (Membrane permeability) Caco-2 cell )4( ﺛﺒﺎﺗﻴﺔ ﺍﻟﺒﻼﺯﻣﺎ ﻣﻊ ﺍﻟﻮﻗﺖ Plasma stability over time _______________________________________________________ (1) Role of ADME in lead optimization )2( ﺩﻭﺭ ﺍﻷﺪﻣﻲ ﻟﻠﻮﺻﻮﻝ ﺇﻟﻲ ﺃﻌﻠﻰ ﻣﺴﺘﻮﻳﺎﺕ ﺍﻟﺠﻮﺩﺓ )3( ﺍﺳﺘﻌﻤﺎﻝ ﺍﻟﻄﺮﻕ ﺍﻟﺘﺤﻠﻴﻠﻴﺔ ﺑﺄﻌﻠﻰ ﻣﻌﺪﻻﺕ ﺍﻟﺠﻮﺩﺓ ﺑﺄﺠﻬﺰﺓ ﻣﺜﻞ . LC-MS/MS )4( ﺍﻟﺘﻮﺻﻞ ﻟﻘﺎﻋﺪﺓ ﺍﻟﻌﻼﻗﺔ ﺑﻴﻦ PK/PD ﻋﻨﺪ ﺍﻟﺤﺼﻮﻝ ﻋﻠﻰ ﻧﺘﺎﺋﺞ ﻛﻞ ﻣﻦ Cmax ﻭ 5. 0 t ﻭ AUC ﻭ V ﻭ F ﻭ . Cl ﻧﺒﺬﺓ ﻋﻦ ﺍﻟﺘﻔﺎﻋﻼﺕ ﺍﻷﻴﻀﻴﺔ. )5( ﺗﻘﻴﻴﻢ ﻗﻴﻤﺔ “ﺩﻭﺍﺀ ﺍﻟﻨﺎﻗﻼﺕ” ” Evaluation of affinity for “Drug Transporter ﻣﺜﻞ : . …………… 4/23 (6) p ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ
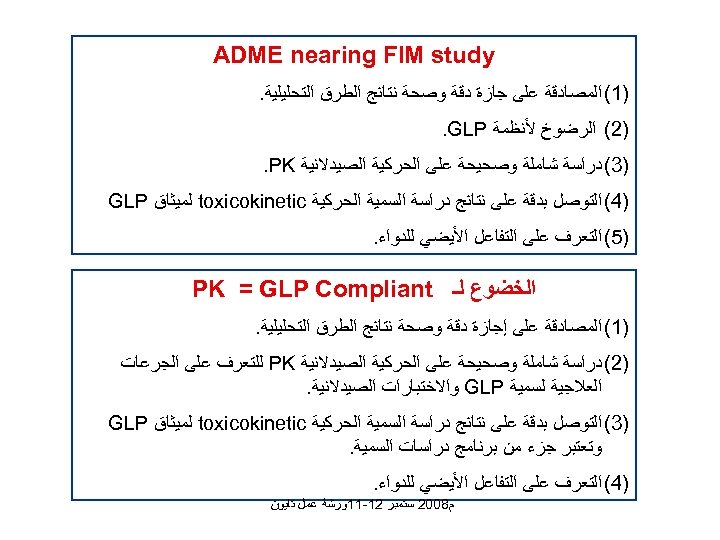
ADME nearing FIM study )1( ﺍﻟﻤﺼﺎﺩﻗﺔ ﻋﻠﻰ ﺟﺎﺯﺓ ﺩﻗﺔ ﻭﺻﺤﺔ ﻧﺘﺎﺋﺞ ﺍﻟﻄﺮﻕ ﺍﻟﺘﺤﻠﻴﻠﻴﺔ. )2( ﺍﻟﺮﺿﻮﺥ ﻷﻨﻈﻤﺔ . GLP )3( ﺩﺭﺍﺳﺔ ﺷﺎﻣﻠﺔ ﻭﺻﺤﻴﺤﺔ ﻋﻠﻰ ﺍﻟﺤﺮﻛﻴﺔ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ . PK )4( ﺍﻟﺘﻮﺻﻞ ﺑﺪﻗﺔ ﻋﻠﻰ ﻧﺘﺎﺋﺞ ﺩﺭﺍﺳﺔ ﺍﻟﺴﻤﻴﺔ ﺍﻟﺤﺮﻛﻴﺔ toxicokinetic ﻟﻤﻴﺜﺎﻕ GLP )5( ﺍﻟﺘﻌﺮﻑ ﻋﻠﻰ ﺍﻟﺘﻔﺎﻋﻞ ﺍﻷﻴﻀﻲ ﻟﻠﺪﻭﺍﺀ. ﺍﻟﺨﻀﻮﻉ ﻟـ PK = GLP Compliant )1( ﺍﻟﻤﺼﺎﺩﻗﺔ ﻋﻠﻰ ﺇﺟﺎﺯﺓ ﺩﻗﺔ ﻭﺻﺤﺔ ﻧﺘﺎﺋﺞ ﺍﻟﻄﺮﻕ ﺍﻟﺘﺤﻠﻴﻠﻴﺔ. )2( ﺩﺭﺍﺳﺔ ﺷﺎﻣﻠﺔ ﻭﺻﺤﻴﺤﺔ ﻋﻠﻰ ﺍﻟﺤﺮﻛﻴﺔ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ PK ﻟﻠﺘﻌﺮﻑ ﻋﻠﻰ ﺍﻟﺠﺮﻋﺎﺕ ﺍﻟﻌﻼﺟﻴﺔ ﻟﺴﻤﻴﺔ GLP ﻭﺍﻻﺧﺘﺒﺎﺭﺍﺕ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ. )3( ﺍﻟﺘﻮﺻﻞ ﺑﺪﻗﺔ ﻋﻠﻰ ﻧﺘﺎﺋﺞ ﺩﺭﺍﺳﺔ ﺍﻟﺴﻤﻴﺔ ﺍﻟﺤﺮﻛﻴﺔ toxicokinetic ﻟﻤﻴﺜﺎﻕ GLP ﻭﺗﻌﺘﺒﺮ ﺟﺰﺀ ﻣﻦ ﺑﺮﻧﺎﻣﺞ ﺩﺭﺍﺳﺎﺕ ﺍﻟﺴﻤﻴﺔ. )4( ﺍﻟﺘﻌﺮﻑ ﻋﻠﻰ ﺍﻟﺘﻔﺎﻋﻞ ﺍﻷﻴﻀﻲ ﻟﻠﺪﻭﺍﺀ. ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

ADME scheme 4/61 P ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

ﺍﻟﺪﻭﺍﺀ ﺍﻟﻤﺮﺗﺒﻂ Pro Drug )1( ﻣﻄﻠﻮﺏ ﻣﻌﺮﻓﺔ ﺍﻟﺘﻔﺎﻋﻼﺕ ﺍﻻﻳﻀﻴﺔ ﺍﻟﻤﺘﺤﻮﺭﻩ ﻟﻠﺪﻭﺍﺀ ﺑﺎﻟﺠﺴﻢ ﺑﻌﺪ ﺍﻟﺘﺄﻜﺪ ﻣﻦ ﻓﻌﺎﻟﻴﺔ ﺍﻟﺪﻭﺍﺀ ﻋﻨﺪ ﺗﻌﺎﻃﻴﻪ ﻟﻠﻜﺎﺋﻦ ﺍﻟﺤﻲ ﺗﺤﺖ ﺍﻟﺪﺭﺍﺳﺔ. )2( ﻣﻄﻠﻮﺏ ﺗﺼﻤﻴﻢ ﻛﻞ ﻣﻦ: ﺫﻭﺑﺎﻧﻴﺔ ﻣﺘﻘﺪﻣﺔ – ﺻﻴﻐﺔ ﺍﻟﺠﺮﻋﺔ ﺍﻟﺼﺤﻴﺤﺔ – ﻣﺴﺎﺭ ﺗﻌﺎﻃﻲ ﺍﻟﺪﻭﺍﺀ. . IV admin - . Increase lipid solubility for absorption - ) Biostability for transport ( e. g. acid – enzume - . Site-specific action of superior potency - ﻳﻄﻴﻞ ﺗﺤﺮﺭ ﺍﻟﺪﻭﺍﺀ. Prolonged drug release - ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

ﺍﻟﻤﺤﺎﺿﺮﺓ 5 ﺃﻤﺎﻥ ﺻﻴﺪﻻﻧﻲ & ﺗﻘﻴﻴﻢ ﺍﻟﺴﻤﻴﺔ Safety Pharmacology & Toxicology Evaluation ﻣﻠﺨﺺ ﺍﻟﻤﺤﺎﺿﺮﺓ: ﻣﺎ ﻫﻲ ﺍﻟﺴﻤﻴﺔ ؟ ? ” What is “toxicity - ﺍﻟﺪﺭﺍﺳﺎﺕ ﻓﻲ ﺍﻟﺪﺍﺧﻞ ﻭﺍﻟﺨﺎﺭﺝ In vitro & In vivo studies ﻧﻤﺎﺫﺝ ﺣﻴﻮﺍﻧﺎﺕ ﺍﻟﺘﺠﺎﺭﺏ ﻭﺍﻟﺠﺮﻋﺎﺕ ﺍﻟﻤﺨﺘﺎﺭﺓ ﻟﻠﺪﺭﺍﺳﺎﺕ. ﺩﺭﺍﺳﺔ ﺻﻴﺪﻻﻧﻴﺔ ﺃﻤﻨﺔ. ﺍﻟﻬﺪﻑ ﻫﻮ ﺗﻮﺻﻴﻞ ﺍﻟﻤﻨﺘﺞ ﺇﻟﻲ ﺍﻟﺴﻮﻕ ﻭﻟﺘﺤﻘﻴﻖ ﻫﺬﺍ ﺍﻟﻬﺪﻑ ﻧﺤﺘﺎﺝ ﻟﻨﺘﺎﺋﺞ ﺗﺪﻋﻢ ﺩﺭﺍﺳﺔ ﺍﻟﻤﻨﺘﺞ ﻓﻲ ﺍﻻﻧﺴﺎﻥ ﺗﺼﻤﻴﻢ ﺍﻟﺪﺭﺍﺳﺔ. ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

Preclinical Development )1( ﻣﻦ ﺍﻟﺪﺭﺍﺳﺎﺕ ﺍﻟﻬﺎﻣﺔ ﻫﻲ ﻣﻌﺮﻓﺔ ﺳﻤﻴﺔ ﺍﻟﺪﻭﺍﺀ ﺑﺠﺎﻧﺐ ﻣﻌﺮﻓﺔ ﺃﻲ ﻋﻀﻮ ﻓﻲ ﺍﻟﺠﺴﻢ ﺍﻟﻤﺤﺘﺎﺝ ﻟﻬﺬﺍ ﺍﻟﺪﻭﺍﺀ. ﻣﻊ ﻣﺮﺍﻋﺎﺓ ﻫﻞ ﺗﻮﺟﺪ ﺇﻣﻜﺎﻧﻴﺔ ﻋﻠﻰ ﺍﻟﻤﺪﻯ ﺍﻟﻄﻮﻳﻞ ﺣﺪﻭﺙ ﻃﻔﺮﺓ ﺟﻴﻨﻴﺔ ﺗﺆﺪﻱ ﻷﻤﺮﺍﺽ ﺳﺮﻃﺎﻧﻴﺔ ﺃﻮ ﺳﻤﻴﺔ ﺗﺆﺜﺮ ﻋﻠﻰ ﺗﻜﺎﺛﺮ ﺍﻻﻧﺴﺎﻥ. )2( ﻓﻲ ﺣﺎﻟﺔ ﺗﺄﻜﻴﺪ ﺍﻟﺪﺭﺍﺳﺎﺕ ﻗﺒﻞ ﺍﻟﻄﺒﻴﺔ ﺑﺄﻦ ﺍﻟﺪﻭﺍﺀ ﻣﺘﻤﻴﺰ ﺍﻟﺠﻮﺩﺓ ﻭﻏﻴﺮ ﺳﺎﻡ ، ﻓﺒﺎﻟﺘﺎﻟﻲ ﻳﻤﻜﻦ ﺍﻋﺘﺒﺎﺭ ﺻﺎﻟﺢ ﻹﺟﺮﺍﺀ ﺍﻟﺘﺠﺎﺭﺏ ﺍﻟﻄﺒﻴﺔ ﻓﻲ ﺍﻻﻧﺴﺎﻥ. )3( ﻣﻴﺜﺎﻕ ﺻﻼﺣﻴﺔ ﻧﺘﺎﺋﺞ ﺍﻻﺧﺘﺒﺎﺭﺍﺕ ﻋﻠﻰ ﻧﻤﺎﺫﺝ ﺣﻴﻮﺍﻧﻴﺔ ﻳﺘﻄﻠﺐ ﺇﺟﺮﺍﺀ ﺍﻟﺘﺠﺎﺭﺏ ﻋﻠﻰ ﻧﻮﻋﻴﻦ ﻣﻦ ﻓﺄﺮﺍﻥ ﺍﻟﺘﺠﺎﺭﺏ ﻗﺎﺭﺿﺔ rodent ﻭﻏﻴﺮ ﻗﺎﺭﺿﺔ non-rodent ﻣﺜﻞ murine ﻭ ، canine ﻛﺬﻟﻚ ﻳﻤﻜﻦ ﺍﺳﺘﻌﻤﺎﻝ ﻓﺄﺮﺍﻥ primate ﻭ . porcine ﻭﺑﺎﻟﻨﺴﺒﺔ ﻟﻐﻴﺮ ﺍﻟﻘﺎﺭﺿﺔ ﺍﻷﺮﺍﻧﺐ. )4( ﻣﻦ ﺍﻟﻀﺮﻭﺭﻱ ﻣﻌﺮﻓﺔ ﻣﻌﺪﻝ ﺳﻤﻴﺔ ﺍﻟﺪﻭﺍﺀ. )5( ﻳﺘﻄﻠﺐ ﺣﺴﺎﺏ ﻛﻞ ﻣﻦ ﻛﻤﻴﺔ ﺍﻟﺠﺮﻋﺔ ﺍﻟﻤﻌﺎﻟﺠﺔ ﺍﻟﻌﻠﻴﺎ ﺍﻟﻤﺴﻤﻮﺡ ﺑﻬﺎ MTD ﻭﻛﻤﻴﺔ ﺍﻟﺪﻭﺍﺀ ﻓﻲ ﺑﻼﺯﻣﺎ ﺍﻟﺪﻡ ﺑﺤﺴﺎﺏ AUC ﺿﻤﻦ ﺩﺭﺍﺳﺎﺕ . PK ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

Functional & Morphological effects of Drug )1( ﻧﻤﻂ ﺍﺳﺘﻌﻤﺎﻝ ﺍﻟﺪﻭﺍﺀ ﻭﻫﻴﺌﺔ ﺗﺮﻛﻴﺒﺘﻪ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ )2( ﺍﻟﻌﻀﻮ ﺍﻟﻤﺮﺍﺩ ﻋﻼﺟﻪ ﻭﺗﺄﺜﻴﺮ ﺍﻟﺪﻭﺍﺀ ﻋﻠﻴﻪ. )3( ﻛﻤﻴﺔ ﺍﻟﺠﺮﻋﺔ ﺍﻟﻤﺴﻤﻮﺡ ﺑﻬﺎ ﻭﻋﻼﻗﺎﺗﻬﺎ. )4( ﻧﻮﻉ ﺍﻟﺠﻨﺲ ﻭﺗﺄﺜﻴﺮ ﺍﻟﺪﻭﺍﺀ ﻋﻠﻴﻪ. )5( ﻗﺎﺑﻠﻴﺔ ﺍﻹﻧﻌﻜﺎﺱ Latency & progression & reversibility Characterize Biopharmaceutic & Pharmakinetic )1( ﺍﻻﺧﺘﺒﺎﺭﺍﺕ ﻓﻲ ﺍﻟﺪﺍﺧﻞ ﻋﻠﻰ ﻧﻤﺎﺫﺝ ﻣﻦ ﺣﻴﻮﺍﻧﺎﺕ ﺍﻟﺘﺠﺎﺭﺏ )2( ﺩﺭﺍﺳﺎﺕ ﻋﻠﻰ ﺃﻌﻀﺎﺀ ﻓﺄﺮﺍﻥ ﺍﻟﺘﺠﺎﺭﺏ ﻣﺜﻞ ﺍﻟﻜﺒﺪ، ﺍﻟﻜﻠﻴﺔ، ﺍﻻﻣﻌﺎﺀ، ﺍﻟﻘﻠﺐ، hind limb )3( ﺩﺭﺍﺳﺎﺕ ﻋﻠﻰ ﻧﻤﻮﺫﺝ ﺍﻣﺘﺼﺎﺹ ﺍﻟﻄﺒﻘﺔ ﺍﻷﻮﻟﻴﺔ ﻓﻲ ﺗﺠﺮﺑﺔ ﺍﻟﻤﺰﺍﺭﻉ ﺍﻟﺨﻠﻮﻳﺔ Caco-2 cell )4( ﺗﺠﺎﺭﺏ ﻣﻴﻜﺮﻭﺳﻮﻡ microsomes ﻛﺒﺪ ﺍﻟﺤﻴﻮﺍﻥ ﻭﺍﻻﻧﺴﺎﻥ. ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

ICH Guidelines on Safety Studies . Carcinogenicity )1( ﺍﻟﻄﻔﺮﺍﺕ ﺍﻟﺠﻴﻨﻴﺔ . Genotoxicity )2( ﺍﻟﺴﻤﻴﺔ ﺍﻟﺠﻴﻨﻴﺔ Toxicokinetics & PK )3( ﺍﻟﺴﻤﻴﺔ ﺍﻟﺤﺮﻛﻴﺔ ﻭﺍﻟﺤﺮﻛﻴﺔ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ Toxicity testing )4( ﺍﺧﺘﺒﺎﺭﺍﺕ ﺍﻟﺴﻤﻴﺔ . Reproductive Toxicology )5( ﺳﻤﻴﺔ ﺍﻟﺘﻜﺎﺛﺮ Biotechnology products )6( ﻣﻨﺘﺠﺎﺕ ﺍﻟﺘﻘﻨﻴﺎﺕ ﺍﻟﺤﻴﻮﻳﺔ Pharmacology studies )7( ﺍﻟﺪﺭﺍﺳﺎﺕ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

Species Selection )1( ﻳﺘﻢ ﺇﺟﺮﺍﺀ ﺍﻟﺘﺠﺎﺭﺏ ﻋﻠﻰ ﻧﻮﻋﻴﻦ ﻣﻦ ﻓﺄﺮﺍﻥ ﺍﻟﺘﺠﺎﺭﺏ ﺃﺤﺪﻫﺎ ﻗﺎﺭﺽ ﻭﺍﻷﺨﺮ ﻏﻴﺮ ﻗﺎﺭﺽ. )2( ﻏﺎﻟﻴﺎ ﻣﺎ ﻳﺴﺘﺨﺪﻡ ﺍﻟﻔﺄﺮﺍﻥ Rats ﻛﻘﻮﺍﺭﺽ ، ﺃﻤﺎ Mouse ﺗﺴﺘﺨﺪﻡ ﻓﻲ ﺇﺛﺒﺎﺕ ﺍﻟﻤﻔﺎﻫﻴﻢ proof-of-concept ﻭﺩﺭﺍﺳﺎﺕ ﺍﻟﺤﺮﻛﻴﺔ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ ﺍﻟﻤﺒﺪﺋﻴﺔ. )3( ﺗﺘﻄﻠﺐ ﺍﻟﺪﺭﺍﺳﺎﺕ ﻋﻠﻰ ﺍﻟﻜﻼﺏ ﻭﺍﻟﻘﺮﻭﺩ ﺭﻋﺎﻳﺔ ﺧﺎﺻﺔ ﻭﻣﺮﺍﻗﺒﺔ ﺩﻭﺍﺋﻴﺔ ﻣﺴﺘﻤﺮﺓ ﺗﻌﺘﻤﺪ ﻋﻠﻰ: )1( ﻣﺪﻯ ﺍﻻﺳﺘﺠﺎﺑﺔ ﻟﻠﺪﻭﺍﺀ. )2( ﺩﺭﺍﺳﺎﺕ ﺍﻻﺩﻣﻲ ADME ﻋﻠﻰ ﺍﻟﺤﻴﻮﺍﻥ ﻭﻣﺎ ﻳﻘﺎﺑﻠﻬﺎ ﻋﻠﻰ ﺍﻻﻧﺴﺎﻥ. )4( ﻣﻦ ﺍﻟﺼﻌﺐ ﺍﻟﺘﻨﺒﻴﺀ ﺑﻤﻌﺪﻝ ﺃﻤﻦ ﺍﻟﺪﻭﺍﺀ ﻋﻠﻰ ﺍﻻﻧﺴﺎﻥ ﻋﻠﻰ ﺿﻮﺀ ﻣﻌﻈﻢ ﻧﺘﺎﺋﺞ ﺍﻟﺘﺠﺎﺭﺏ ﺍﻟﺘﻲ ﺗﺠﺮﻯ ﻋﻠﻰ ﺃﺠﻨﺎﺱ ﺍﻟﺤﻴﻮﺍﻧﺎﺕ ﺍﻟﺤﺴﺎﺳﺔ. ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ
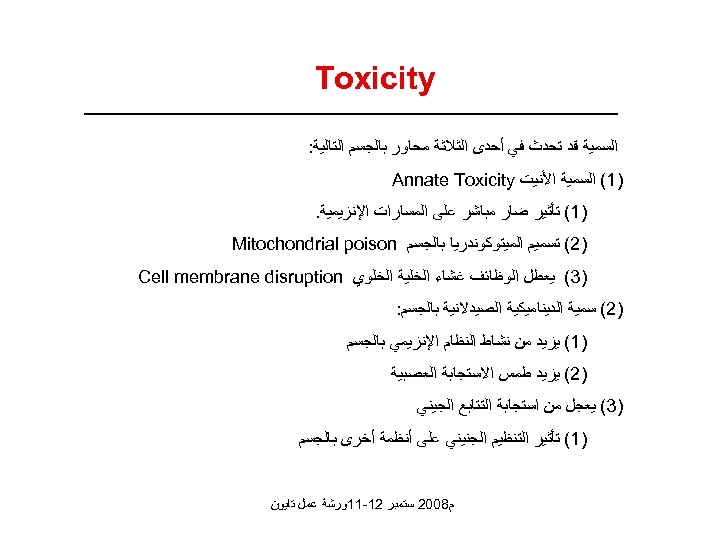
Toxicity ﺍﻟﺴﻤﻴﺔ ﻗﺪ ﺗﺤﺪﺙ ﻓﻲ ﺃﺤﺪﻯ ﺍﻟﺜﻼﺛﺔ ﻣﺤﺎﻭﺭ ﺑﺎﻟﺠﺴﻢ ﺍﻟﺘﺎﻟﻴﺔ: )1( ﺍﻟﺴﻤﻴﺔ ﺍﻷﻨﻴﺖ Annate Toxicity )1( ﺗﺄﺜﻴﺮ ﺿﺎﺭ ﻣﺒﺎﺷﺮ ﻋﻠﻰ ﺍﻟﻤﺴﺎﺭﺍﺕ ﺍﻹﻧﺰﻳﻤﻴﺔ. )2( ﺗﺴﻤﻴﻢ ﺍﻟﻤﻴﺘﻮﻛﻮﻧﺪﺭﻳﺎ ﺑﺎﻟﺠﺴﻢ Mitochondrial poison )3( ﻳﻌﻄﻞ ﺍﻟﻮﻇﺎﺋﻒ ﻏﺸﺎﺀ ﺍﻟﺨﻠﻴﺔ ﺍﻟﺨﻠﻮﻱ Cell membrane disruption )2( ﺳﻤﻴﺔ ﺍﻟﺪﻳﻨﺎﻣﻴﻜﻴﺔ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ ﺑﺎﻟﺠﺴﻢ: )1( ﻳﺰﻳﺪ ﻣﻦ ﻧﺸﺎﻁ ﺍﻟﻨﻈﺎﻡ ﺍﻹﻧﺰﻳﻤﻲ ﺑﺎﻟﺠﺴﻢ )2( ﻳﺰﻳﺪ ﻃﻤﺲ ﺍﻻﺳﺘﺠﺎﺑﺔ ﺍﻟﻌﺼﺒﻴﺔ )3( ﻳﻌﺠﻞ ﻣﻦ ﺍﺳﺘﺠﺎﺑﺔ ﺍﻟﺘﺘﺎﺑﻊ ﺍﻟﺠﻴﻨﻲ )1( ﺗﺄﺜﻴﺮ ﺍﻟﺘﻨﻈﻴﻢ ﺍﻟﺠﻨﻴﻨﻲ ﻋﻠﻰ ﺃﻨﻈﻤﺔ ﺃﺨﺮﻯ ﺑﺎﻟﺠﺴﻢ ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

Pharmacology / Toxicology Data )1(ﺗﻌﺘﻤﺪ ﺍﻟﺪﺭﺍﺳﺎﺕ ﻋﻠﻰ ﻋﺪﺩ ﻭﻣﺪﺓ ﺇﺟﺮﺍﺀ ﺍﻟﺘﺠﺎﺭﺏ ﺍﺳﺘﻨﺎﺩﺍ ﻟﺒﺮﻭﺗﻮﻛﻮﻝ ﺍﻟﺒﺤﺚ ﻟـ: )1(ﻋﻤﻞ ﺗﺠﺎﺭﺏ ﺩﺭﺍﺳﺎﺕ ﺍﻟﺠﺮﻋﺔ ﺍﻟﺪﻭﺍﺋﻴﺔ ﺑﺸﻜﻞ ﻣﻨﻔﺮﺩ ﺃﻮ ﺇﻋﺎﺩﺗﻬﺎ ﻣﺮﺓ ﺃﺨﺮﻯ. )2(ﺑﺮﻧﺎﻣﺞ ﺗﻌﺎﻃﻲ ﺍﻟﺪﻭﺍﺀ ﺑﺸﻜﻞ ﻣﺘﻘﻄﻊ ﺃﻮ ﻳﻮﻣﻴ ﺧﻼﻝ ﺳﻨﺔ. )2( ﺍﻟﺪﺭﺍﺳﺔ ﻋﻠﻰ: )1(ﻣﺘﻄﻮﻋﻴﻦ ﻏﻴﺮ ﻣﺮﺿﻰ / ﻣﺮﺿﻲ ﻣﺼﺎﺑﻴﻦ ﺑﺄﻤﺮﺍﺽ ﺧﻄﻴﺮﺓ serious disease ﺃﻮ ﺃﻤﺮﺍﺽ ﺧﻄﻴﺮﺓ ﺗﻬﺪﺩ ﺣﻴﺎﺗﻬﻢ ﺑﺎﻟﻜﺎﻣﻞ . life-threatening ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ
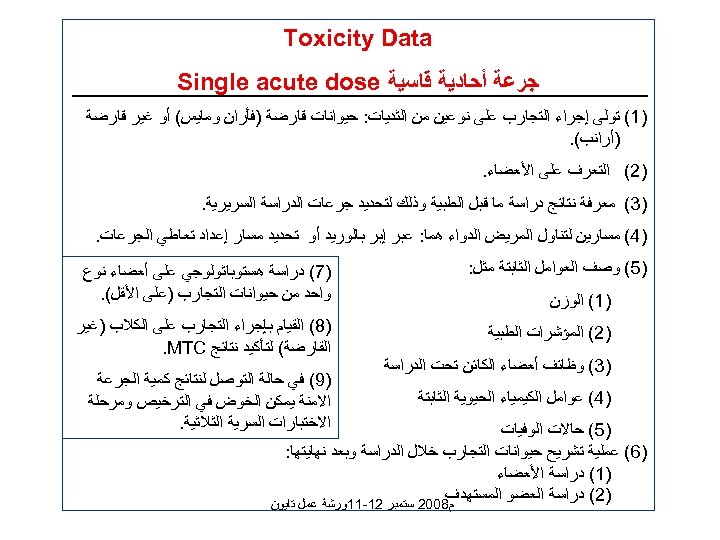
Toxicity Data ﺟﺮﻋﺔ ﺃﺤﺎﺩﻳﺔ ﻗﺎﺳﻴﺔ Single acute dose )1( ﺗﻮﻟﻰ ﺇﺟﺮﺍﺀ ﺍﻟﺘﺠﺎﺭﺏ ﻋﻠﻰ ﻧﻮﻋﻴﻦ ﻣﻦ ﺍﻟﺜﺪﻳﺎﺕ: ﺣﻴﻮﺍﻧﺎﺕ ﻗﺎﺭﺿﺔ )ﻓﺄﺮﺍﻥ ﻭﻣﺎﻳﺲ( ﺃﻮ ﻏﻴﺮ ﻗﺎﺭﺿﺔ )ﺃﺮﺍﻧﺐ(. )2( ﺍﻟﺘﻌﺮﻑ ﻋﻠﻰ ﺍﻷﻌﻀﺎﺀ. )3( ﻣﻌﺮﻓﺔ ﻧﺘﺎﺋﺞ ﺩﺭﺍﺳﺔ ﻣﺎ ﻗﺒﻞ ﺍﻟﻄﺒﻴﺔ ﻭﺫﻟﻚ ﻟﺘﺤﺪﻳﺪ ﺟﺮﻋﺎﺕ ﺍﻟﺪﺭﺍﺳﺔ ﺍﻟﺴﺮﻳﺮﻳﺔ. )4( ﻣﺴﺎﺭﻳﻦ ﻟﺘﻨﺎﻭﻝ ﺍﻟﻤﺮﻳﺾ ﺍﻟﺪﻭﺍﺀ ﻫﻤﺎ: ﻋﺒﺮ ﺇﺑﺮ ﺑﺎﻟﻮﺭﻳﺪ ﺃﻮ ﺗﺤﺪﻳﺪ ﻣﺴﺎﺭ ﺇﻋﺪﺍﺩ ﺗﻌﺎﻃﻲ ﺍﻟﺠﺮﻋﺎﺕ. )5( ﻭﺻﻒ ﺍﻟﻌﻮﺍﻣﻞ ﺍﻟﺜﺎﺑﺘﺔ ﻣﺜﻞ: )1( ﺍﻟﻮﺯﻥ )2( ﺍﻟﻤﺆﺸﺮﺍﺕ ﺍﻟﻄﺒﻴﺔ )3( ﻭﻇﺎﺋﻒ ﺃﻌﻀﺎﺀ ﺍﻟﻜﺎﺋﻦ ﺗﺤﺖ ﺍﻟﺪﺭﺍﺳﺔ )4( ﻋﻮﺍﻣﻞ ﺍﻟﻜﻴﻤﻴﺎﺀ ﺍﻟﺤﻴﻮﻳﺔ ﺍﻟﺜﺎﺑﺘﺔ )7( ﺩﺭﺍﺳﺔ ﻫﺴﺘﻮﺑﺎﺛﻮﻟﻮﺟﻲ ﻋﻠﻰ ﺃﻌﻀﺎﺀ ﻧﻮﻉ ﻭﺍﺣﺪ ﻣﻦ ﺣﻴﻮﺍﻧﺎﺕ ﺍﻟﺘﺠﺎﺭﺏ )ﻋﻠﻰ ﺍﻷﻘﻞ(. )8( ﺍﻟﻘﻴﺎﻡ ﺑﺈﺟﺮﺍﺀ ﺍﻟﺘﺠﺎﺭﺏ ﻋﻠﻰ ﺍﻟﻜﻼﺏ )ﻏﻴﺮ ﺍﻟﻘﺎﺭﺿﺔ( ﻟﺘﺄﻜﻴﺪ ﻧﺘﺎﺋﺞ . MTC )9( ﻓﻲ ﺣﺎﻟﺔ ﺍﻟﺘﻮﺻﻞ ﻟﻨﺘﺎﺋﺞ ﻛﻤﻴﺔ ﺍﻟﺠﺮﻋﺔ ﺍﻻﻣﻨﺔ ﻳﻤﻜﻦ ﺍﻟﺨﻮﺽ ﻓﻲ ﺍﻟﺘﺮﺧﻴﺺ ﻭﻣﺮﺣﻠﺔ ﺍﻻﺧﺘﺒﺎﺭﺍﺕ ﺍﻟﺴﺮﻳﺔ ﺍﻟﺜﻼﺛﻴﺔ. )5( ﺣﺎﻻﺕ ﺍﻟﻮﻓﻴﺎﺕ )6( ﻋﻤﻠﻴﺔ ﺗﺸﺮﻳﺢ ﺣﻴﻮﺍﻧﺎﺕ ﺍﻟﺘﺠﺎﺭﺏ ﺧﻼﻝ ﺍﻟﺪﺭﺍﺳﺔ ﻭﺑﻌﺪ ﻧﻬﺎﻳﺘﻬﺎ: )1( ﺩﺭﺍﺳﺔ ﺍﻷﻌﻀﺎﺀ )2( ﺩﺭﺍﺳﺔ ﺍﻟﻌﻀﻮ ﺍﻟﻤﺴﺘﻬﺪﻑ ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

Toxicity Data ) Repeated (Maximum tolerated dose- MTD )1( ﺗﻮﻟﻲ ﺍﻟﺘﺠﺎﺭﺏ ﻋﻠﻰ ﻧﻮﻋﻴﻦ ﻣﻦ ﺍﻟﺜﺪﻳﺎﺕ ﻗﺎﺭﺿﺔ )ﻓﺄﺮﺍﻥ ﻭﻣﺎﻳﺲ( ﻏﻴﺮ ﻗﺎﺭﺿﺔ )ﺃﺮﺍﻧﺐ( )2( ﺩﺭﺍﺳﺔ ﺍﻟﺘﺄﺜﻴﺮ ﻋﻠﻰ ﺍﻟﻤﺪﻯ ﺍﻟﻄﻮﻳﻞ: )3( ﺗﺤﻠﻴﻞ ﺗﺮﻛﻴﺰ ﺍﻟﺪﻭﺍﺀ ﻓﻲ ﺍﻟﺒﻼﺯﻣﺎ ﻭﻗﻴﻢ ﺍﻟﺪﺭﺍﺳﺔ ﺍﻟﺤﺮﻛﻴﺔ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ. )4( ﺩﺭﺍﺳﺔ ﺗﺄﺜﻴﺮ ﺍﻟﺪﻭﺍﺀ ﻋﻠﻰ ﺃﻨﺸﻄﺔ ﺩﻭﺍﻝ ﺍﻷﻌﻀﺎﺀ ﺍﻟﺤﻴﻮﻳﺔ ﺑﺎﻟﻜﺎﺋﻦ ﺍﻟﺤﻲ ﺗﺤﺖ ﺍﻟﺪﺭﺍﺳﺔ: )1( ﺍﻷﻮﻋﻴﺔ ﺍﻟﻘﻠﺒﻴﺔ Cardiovascular )2( ﺍﻟﺠﻬﺎﺯ ﺍﻟﺘﻨﻔﺴﻲ )3( ﺍﻟﺠﻬﺎﺯ ﺍﻟﻌﺼﺒﻲ ﺍﻟﻤﺮﻛﺰﻱ )5( ﺩﺭﺍﺳﺔ ﺗﺄﺜﻴﺮ ﺍﻟﺪﻭﺍﺀ ﻋﻠﻰ ﺑﻘﻴﺔ ﺣﻴﻮﺍﻧﺎﺕ ﺍﻟﺘﺠﺎﺭﺏ ﺑﻌﺪ ﺍﻻﻧﺘﻬﺎﺀ ﻣﻦ ﺇﺟﺮﺍﺀ ﺍﻻﺧﺘﺒﺎﺭﺍﺕ ﻋﻠﻴﻬﺎ. ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

Duration of Toxicity Studies ﻣﺪﺓ ﺍﺳﺘﻤﺮﺍﺭ ﺩﺭﺍﺳﺎﺕ ﺍﻟﺴﻤﻴﺔ ﺟﺪﻭﻝ 5/81 p ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

Number / Sex of Animals ﻋﺪﺩ ﻭﺟﻨﺲ ﺣﻴﻮﺍﻧﺎﺕ ﺍﻟﺘﺠﺎﺭﺏ )1( ﻳﻔﻀﻞ ﺍﺳﺘﻌﻤﺎﻝ ﻋﺪﺩ ﻣﻨﺎﺳﺐ ﻣﻦ ﺣﻴﻮﺍﻧﺎﺕ ﺍﻟﺘﺠﺎﺭﺏ ﻟـ: )1( ﻟﻠﺘﻮﺻﻞ ﻟﻨﺘﺎﺋﺞ ﺍﻟﺘﺄﺜﻴﺮﺍﺕ ﺍﻟﺴﺎﻣﺔ ﺑﺸﻜﻞ ﺩﻗﻴﻘﺔ ﻭﺍﻟﺤﺼﻮﻝ ﻋﻠﻰ ﻧﺘﺎﺋﺞ ﺻﺤﻴﺤﺔ ﻭﻣﺘﻜﺎﻣﻠﺔ. )2( ﻋﻨﺪ ﺍﻟﻀﺮﻭﺭﺓ ﻳﻤﻜﻦ ﺍﻟﺘﻀﺤﻴﺔ ﺑﺒﻌﺾ ﺣﻴﻮﺍﻧﺎﺕ ﺍﻟﺘﺠﺎﺭﺏ ﻣﻦ ﻓﺘﺮﺓ ﻷﺨﺮﻯ. )2( ﺗﻔﺎﺩﻱ ﺍﻟﻤﻐﺎﻻﺓ ﺍﻟﻤﺎﻟﻴﺔ ﻭﺍﻟﺒﺸﺮﻳﺔ ﻭﺍﻻﻋﺘﺒﺎﺭﺍﺕ ﺍﻟﻌﻤﻠﻴﺔ. )3( ﺗﺰﻭﻳﺪ ﻣﺪﺓ ﻭﺗﺘﺎﺑﻊ ﺍﻻﺧﺘﺒﺎﺭﺍﺕ ﻋﻠﻰ ﺣﻴﻮﺍﻧﺎﺕ ﺍﻟﺘﺠﺎﺭﺏ ﻣﻦ ﺍﻻﻣﻮﺭ ﺍﻟﻬﺎﻣﺔ ﻭﺧﺎﺻﺔ ﺑﺎﻟﻨﺴﺒﺔ ﻻﺧﺘﺒﺎﺭﺍﺕ ﺍﻟﺤﻴﻮﺍﻧﺎﺕ ﺍﻷﺴﺎﺳﻴﺔ ﺗﺤﺖ ﺍﻟﺪﺭﺍﺳﺔ. )4( ﻛﻼ ﺍﻟﺠﻨﺴﻴﻦ ﻳﺤﺐ ﺇﺟﺮﺍﺀ ﺍﻟﺘﺠﺎﺭﺏ ﻋﻠﻴﻬﻤﺎ ﺇﻻ ﻓﻲ ﺣﺎﻟﺔ ﺗﺤﺪﻳﺪ ﻧﻮﻋﻴﺔ ﺍﻟﺪﺭﺍﺳﺔ ﻋﻠﻰ ﺃﺤﺪﻫﻤﺎ ﻓﻘﻂ. )5( ﻓﻲ ﺣﺎﻟﺔ ﺍﻟﺪﺭﺍﺳﺔ ﻋﻠﻰ ﻓﺄﺮﺍﻥ ﺍﻟﻤﺎﻳﺲ ﻳﻔﻀﻞ ﺃﻦ ﺗﻜﻮﻥ ﻛﻞ ﻣﺠﻤﻮﻋﺔ ﺗﺤﺘﻮﻱ ﻋﻠﻰ 8 ﺇﻟﻰ 01 ﻣﺎﻳﺴﺎﺕ. ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ
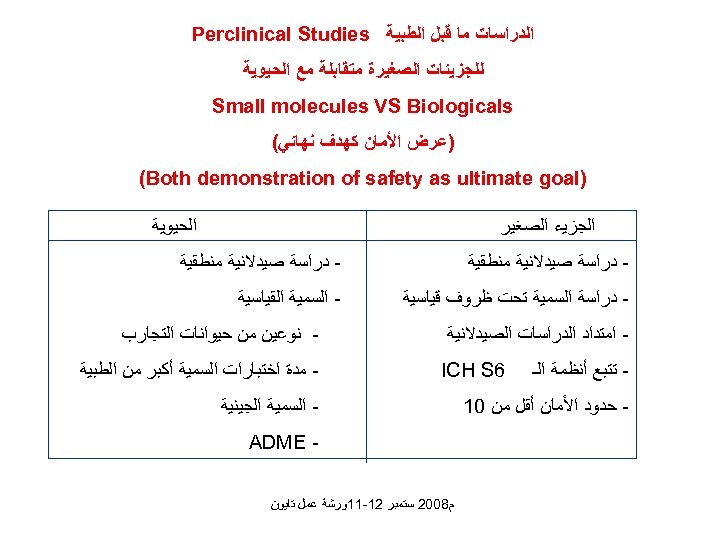
ﺍﻟﺪﺭﺍﺳﺎﺕ ﻣﺎ ﻗﺒﻞ ﺍﻟﻄﺒﻴﺔ Perclinical Studies ﻟﻠﺠﺰﻳﺌﺎﺕ ﺍﻟﺼﻐﻴﺮﺓ ﻣﺘﻘﺎﺑﻠﺔ ﻣﻊ ﺍﻟﺤﻴﻮﻳﺔ Small molecules VS Biologicals )ﻋﺮﺽ ﺍﻷﻤﺎﻥ ﻛﻬﺪﻑ ﻧﻬﺎﺋﻲ( ) (Both demonstration of safety as ultimate goal ﺍﻟﺤﻴﻮﻳﺔ ﺍﻟﺠﺰﻳﺀ ﺍﻟﺼﻐﻴﺮ - ﺩﺭﺍﺳﺔ ﺻﻴﺪﻻﻧﻴﺔ ﻣﻨﻄﻘﻴﺔ - ﺩﺭﺍﺳﺔ ﺍﻟﺴﻤﻴﺔ ﺗﺤﺖ ﻇﺮﻭﻑ ﻗﻴﺎﺳﻴﺔ - ﺍﻟﺴﻤﻴﺔ ﺍﻟﻘﻴﺎﺳﻴﺔ ﺍﻣﺘﺪﺍﺩ ﺍﻟﺪﺭﺍﺳﺎﺕ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ - ﺗﺘﺒﻊ ﺃﻨﻈﻤﺔ ﺍﻟـ 6 ICH S - ﺣﺪﻭﺩ ﺍﻷﻤﺎﻥ ﺃﻘﻞ ﻣﻦ 01 ﻧﻮﻋﻴﻦ ﻣﻦ ﺣﻴﻮﺍﻧﺎﺕ ﺍﻟﺘﺠﺎﺭﺏ ﻣﺪﺓ ﺍﺧﺘﺒﺎﺭﺍﺕ ﺍﻟﺴﻤﻴﺔ ﺃﻜﺒﺮ ﻣﻦ ﺍﻟﻄﺒﻴﺔ ﺍﻟﺴﻤﻴﺔ ﺍﻟﺠﻴﻨﻴﺔ - ADME ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

Preclinical Development Choice of animal species )1( ﺗﻌﺘﻤﺪ ﻋﻤﻠﻴﺔ ﺍﺧﺘﻴﺎﺭ ﺃﻨﻮﺍﻉ ﺣﻴﻮﺍﻧﺎﺕ ﺍﻟﺘﺠﺎﺭﺏ ﻋﻠﻰ ﺃﻔﻀﻞ ﻧﺘﺎﺋﺞ ﻳﺴﺘﻔﺎﺩ ﻣﻨﻬﺎ ﻓﻲ ﺍﻻﺧﺘﺒﺎﺭﺍﺕ ﺍﻟﺴﺮﻳﺮﻳﺔ ﻋﻠﻰ ﺍﻟﻌﻮﺍﻣﻞ ﺍﻟﺘﺎﻟﻴﺔ: )1( ﺍﺧﺘﻼﻑ ﺍﻟﻘﻨﺎﺓ ﺍﻟﻬﻀﻤﻴﺔ. )2( ﺍﻟﻨﺸﺎﻁ ﺍﻻﻧﺰﻳﻤﻲ )3( ﻧﻈﺎﻡ ﺗﺪﻭﻳﺮ ﺍﻟﺪﻭﺍﺀ )4( ﺍﻋﺘﺒﺎﺭﺍﺕ ﺃﺨﺮﻯ ﺗﺠﻌﻞ ﺍﺧﺘﻴﺎﺭ ﺍﻟﻨﻤﺎﺫﺝ ﺗﺘﻮﺍﺀﻡ ﻣﻊ ﺍﻟﺘﺮﻛﻴﺒﺔ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ ﻟﻠﺠﺮﻋﺎﺕ ﺍﻟﻌﻼﺟﻴﺔ ) (Dosage form ﻭﺟﻬﺔ ﺍﻟﻔﺎﻋﻠﻴﺔ ) (site of activity ﻭﺳﻤﻴﺔ ﺍﻷﻴﻀﻴﺔ (noxious ) metabolites )2( ﻋﻠﻰ ﺳﺒﻴﻞ ﺍﻟﻤﺜﺎﻝ: ﻓﺄﺮﺍﻥ ﺷﺒﻴﻬﺔ ﺑﺸﻜﻞ ﺍﻟﻜﻠﺐ canines ﻻ ﺗﻌﺘﺒﺮ ﻧﻤﺎﺫﺝ ﺟﻴﺪﺓ ﻓﻲ ﺣﺎﻟﺔ ﺍﻟﺘﻌﺎﻃﻲ ﻋﻦ ﻃﺮﻳﻖ ﺍﻟﻔﻢ ﻟﻌﺪﺓ ﺃﺴﺒﺎﺏ ﺧﻠﻘﻴﺔ. ﻭﻣﺜﺎﻝ آﺨﺮ ﻻ ﺗﺼﻠﺢ ﺍﻟﻔﺄﺮﺍﻥ ﺍﻟﻘﺎﺭﺿﺔ ﻻﺧﺘﺒﺎﺭﺍﺕ ﺃﺪﻭﻳﺔ ﺍﻟﻤﻀﺎﺩﺍﺕ ﺍﻟﺤﻴﻮﻳﺔ ﻟﻌﺪﺓ ﺃﺴﺒﺎﺏ. )3( ﻗﺪ ﻳﺴﻠﻚ ﺗﺄﺜﻴﺮ ﺍﻟﺪﻭﺍﺀ ﻧﻔﺲ ﺃﻮ ﺗﺨﺘﻠﻒ ﺍﻟﺘﻔﺎﻋﻼﺕ ﺍﻷﻴﻀﻴﺔ ﻓﻲ ﻧﻮﻋﻴﻦ ﻣﺨﺘﻠﻔﻴﻦ ﻣﻦ ﺣﻴﻮﺍﻧﺎﺕ ﺍﻟﺘﺠﺎﺭﺏ ﻭﻓﻲ ﻛﻼ ﺍﻟﺤﺎﻟﺘﻴﻦ ﺗﺆﺜﺮ ﻋﻠﻰ ﻓﻌﺎﻟﻴﺔ Afficacy ﻭﺳﻤﻴﺔ ﺍﻟﺪﻭﺍﺀ toxicology ﺩﺍﺧﻞ ﺟﺴﻢ ﺍﻻﻧﺴﺎﻥ. ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ
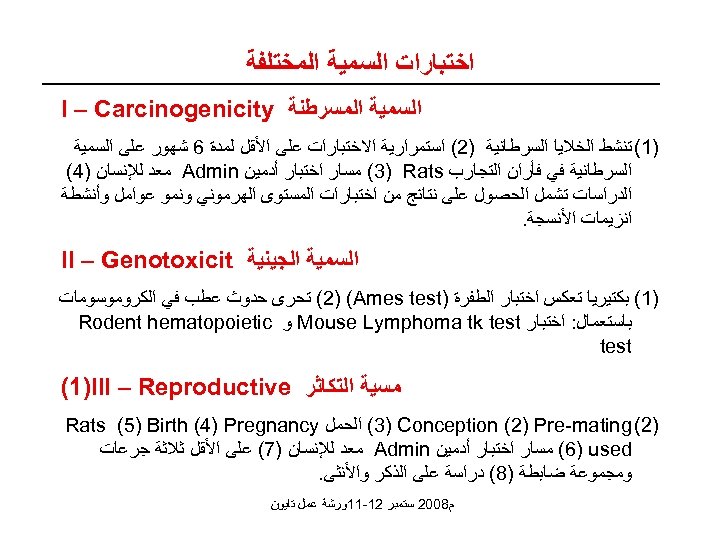
ﺍﺧﺘﺒﺎﺭﺍﺕ ﺍﻟﺴﻤﻴﺔ ﺍﻟﻤﺨﺘﻠﻔﺔ ﺍﻟﺴﻤﻴﺔ ﺍﻟﻤﺴﺮﻃﻨﺔ I – Carcinogenicity )1( ﺗﻨﺸﻂ ﺍﻟﺨﻼﻳﺎ ﺍﻟﺴﺮﻃﺎﻧﻴﺔ )2( ﺍﺳﺘﻤﺮﺍﺭﻳﺔ ﺍﻻﺧﺘﺒﺎﺭﺍﺕ ﻋﻠﻰ ﺍﻷﻘﻞ ﻟﻤﺪﺓ 6 ﺷﻬﻮﺭ ﻋﻠﻰ ﺍﻟﺴﻤﻴﺔ ﺍﻟﺴﺮﻃﺎﻧﻴﺔ ﻓﻲ ﻓﺄﺮﺍﻥ ﺍﻟﺘﺠﺎﺭﺏ (3) Rats ﻣﺴﺎﺭ ﺍﺧﺘﺒﺎﺭ ﺃﺪﻣﻴﻦ Admin ﻣﻌﺪ ﻟﻺﻧﺴﺎﻥ )4( ﺍﻟﺪﺭﺍﺳﺎﺕ ﺗﺸﻤﻞ ﺍﻟﺤﺼﻮﻝ ﻋﻠﻰ ﻧﺘﺎﺋﺞ ﻣﻦ ﺍﺧﺘﺒﺎﺭﺍﺕ ﺍﻟﻤﺴﺘﻮﻯ ﺍﻟﻬﺮﻣﻮﻧﻲ ﻭﻧﻤﻮ ﻋﻮﺍﻣﻞ ﻭﺃﻨﺸﻄﺔ ﺍﻧﺰﻳﻤﺎﺕ ﺍﻷﻨﺴﺠﺔ. ﺍﻟﺴﻤﻴﺔ ﺍﻟﺠﻴﻨﻴﺔ II – Genotoxicit )1( ﺑﻜﺘﻴﺮﻳﺎ ﺗﻌﻜﺲ ﺍﺧﺘﺒﺎﺭ ﺍﻟﻄﻔﺮﺓ ) (2) (Ames test ﺗﺤﺮﻯ ﺣﺪﻭﺙ ﻋﻄﺐ ﻓﻲ ﺍﻟﻜﺮﻭﻣﻮﺳﻮﻣﺎﺕ ﺑﺎﺳﺘﻌﻤﺎﻝ: ﺍﺧﺘﺒﺎﺭ Mouse Lymphoma tk test ﻭ Rodent hematopoietic test ﻣﺴﻴﺔ ﺍﻟﺘﻜﺎﺛﺮ (1)III – Reproductive )2( (3) Conception (2) Pre-mating ﺍﻟﺤﻤﻞ Rats (5) Birth (4) Pregnancy (6) used ﻣﺴﺎﺭ ﺍﺧﺘﺒﺎﺭ ﺃﺪﻣﻴﻦ Admin ﻣﻌﺪ ﻟﻺﻧﺴﺎﻥ )7( ﻋﻠﻰ ﺍﻷﻘﻞ ﺛﻼﺛﺔ ﺟﺮﻋﺎﺕ ﻭﻣﺠﻤﻮﻋﺔ ﺿﺎﺑﻄﺔ )8( ﺩﺭﺍﺳﺔ ﻋﻠﻰ ﺍﻟﺬﻛﺮ ﻭﺍﻷﻨﺜﻰ. ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

ﻣﻌﻮﻗﺎﺕ ﺍﺳﺘﻌﻤﺎﻝ ﻓﺄﺮﺍﻥ Rats ﻛﻨﻤﺎﺫﺝ ﻟﺤﻴﻮﺍﻧﺎﺕ ﺍﻟﺘﺠﺎﺭﺏ ) Animal Species (RATS )1(ﻟﻢ ﺗﺸﺎﻫﺪ ﺗﻔﺎﻋﻼﺕ ﺃﻴﻀﻴﺔ ﻟﻠـ ”ﺩﻭﺍﺀ ﺍﻟﻘﺒﻠﻲ“ ” “Prodrug )2( ﻟﻢ ﻧﺸﺎﻫﺪ ﻋﻠﻰ ﻓﺄﺮﺍﻥ ﺍﻟﺘﺠﺎﺭﺏ ﻧﻤﻂ ﺗﺄﺜﻴﺮﻱ ﻋﻠﻰ ﺍﻷﻨﺴﺠﺔ ﻭ/ﺃﻮ ﺍﻷﻌﻀﺎﺀ. )3( ﻗﻴﻤﺔ ﺍﻟﺴﻤﻴﺔ ﺍﻟﻔﻄﺮﻳﺔ Innate toxicity ﻋﺎﻟﻴﺔ ﻳﺸﻜﻞ ﻏﻴﺮ ﻭﺍﻗﻌﻲ. )4(ﺃﺴﺒﺎﺏ ﻋﻠﻤﻴﺔ ﺃﺨﺮﻯ. ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ
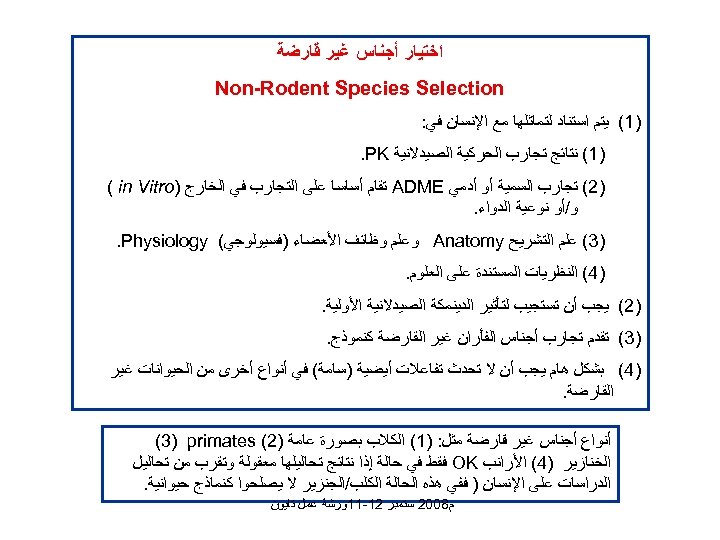
ﺍﺧﺘﻴﺎﺭ ﺃﺠﻨﺎﺱ ﻏﻴﺮ ﻗﺎﺭﺿﺔ Non-Rodent Species Selection )1( ﻳﺘﻢ ﺍﺳﺘﻨﺎﺩ ﻟﺘﻤﺎﺛﻠﻬﺎ ﻣﻊ ﺍﻹﻧﺴﺎﻥ ﻓﻲ: )1( ﻧﺘﺎﺋﺞ ﺗﺠﺎﺭﺏ ﺍﻟﺤﺮﻛﻴﺔ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ . PK )2( ﺗﺠﺎﺭﺏ ﺍﻟﺴﻤﻴﺔ ﺃﻮ ﺃﺪﻣﻲ ADME ﺗﻘﺎﻡ ﺃﺴﺎﺳﺎ ﻋﻠﻰ ﺍﻟﺘﺠﺎﺭﺏ ﻓﻲ ﺍﻟﺨﺎﺭﺝ ) ( in Vitro ﻭ/ﺃﻮ ﻧﻮﻋﻴﺔ ﺍﻟﺪﻭﺍﺀ. )3( ﻋﻠﻢ ﺍﻟﺘﺸﺮﻳﺢ Anatomy ﻭﻋﻠﻢ ﻭﻇﺎﺋﻒ ﺍﻷﻌﻀﺎﺀ )ﻓﺴﻴﻮﻟﻮﺟﻲ( . Physiology )4( ﺍﻟﻨﻈﺮﻳﺎﺕ ﺍﻟﻤﺴﺘﻨﺪﺓ ﻋﻠﻰ ﺍﻟﻌﻠﻮﻡ. )2( ﻳﺠﺐ ﺃﻦ ﺗﺴﺘﺠﻴﺐ ﻟﺘﺄﺜﻴﺮ ﺍﻟﺪﻳﻨﻤﻜﺔ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ ﺍﻷﻮﻟﻴﺔ. )3( ﺗﻘﺪﻡ ﺗﺠﺎﺭﺏ ﺃﺠﻨﺎﺱ ﺍﻟﻔﺄﺮﺍﻥ ﻏﻴﺮ ﺍﻟﻘﺎﺭﺿﺔ ﻛﻨﻤﻮﺫﺝ. )4( ﺑﺸﻜﻞ ﻫﺎﻡ ﻳﺠﺐ ﺃﻦ ﻻ ﺗﺤﺪﺙ ﺗﻔﺎﻋﻼﺕ ﺃﻴﻀﻴﺔ )ﺳﺎﻣﺔ( ﻓﻲ ﺃﻨﻮﺍﻉ ﺃﺨﺮﻯ ﻣﻦ ﺍﻟﺤﻴﻮﺍﻧﺎﺕ ﻏﻴﺮ ﺍﻟﻘﺎﺭﺿﺔ. ﺃﻨﻮﺍﻉ ﺃﺠﻨﺎﺱ ﻏﻴﺮ ﻗﺎﺭﺿﺔ ﻣﺜﻞ: )1( ﺍﻟﻜﻼﺏ ﺑﺼﻮﺭﺓ ﻋﺎﻣﺔ )2( (3) primates ﺍﻟﺨﻨﺎﺯﻳﺮ )4( ﺍﻷﺮﺍﻧﺐ OK ﻓﻘﻂ ﻓﻲ ﺣﺎﻟﺔ ﺇﺫﺍ ﻧﺘﺎﺋﺞ ﺗﺤﺎﻟﻴﻠﻬﺎ ﻣﻌﻘﻮﻟﺔ ﻭﺗﻘﺮﺏ ﻣﻦ ﺗﺤﺎﻟﻴﻞ ﺍﻟﺪﺭﺍﺳﺎﺕ ﻋﻠﻰ ﺍﻹﻧﺴﺎﻥ ) ﻓﻔﻲ ﻫﺬﻩ ﺍﻟﺤﺎﻟﺔ ﺍﻟﻜﻠﺐ/ﺍﻟﺠﻨﺰﻳﺮ ﻻ ﻳﺼﻠﺤﻮﺍ ﻛﻨﻤﺎﺫﺝ ﺣﻴﻮﺍﻧﻴﺔ. ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

ﺃﺜﺮ ﺍﻟﻤﻮﺍﻓﻘﺔ ﺍﻟﺪﻭﺍﺋﻴﺔ ﻋﻠﻰ ﻣﺼﻴﺮ ﺍﻷﺪﻭﻳﺔ ﺍﻟﻤﻜﺘﺸﻔﺔ )1( 1 ﻣﻦ ﻛﻞ 01 ﺃﺪﻭﻳﺔ ﻣﻄﺒﻖ ﻋﻠﻴﻬﺎ ﻣﻴﺜﺎﻕ IND ﻳﺤﺼﻞ ﻋﻠﻰ ﻣﻮﺍﻓﻘﺔ ﺣﺼﻮﻟﻪ ﻋﻠﻰ ﺗﺮﺧﻴﺺ FDA )2( %05 ﺗﺮﺳﺐ ﻧﺘﻴﺠﺔ ﺍﻟﻔﻌﺎﻟﻴﺔ efficacy )3( %33 ﺗﺮﺳﺐ ﻧﺘﻴﺠﺔ ﺍﻟﺴﻤﻴﺔ Toxicity )1( ﻧﺘﺎﺋﺞ ﺍﻟﺪﺭﺍﺳﺎﺕ ﻋﻠﻰ ﺣﻴﻮﺍﻧﺎﺕ ﺍﻟﺪﺭﺍﺳﺔ ﻏﻴﺮ ﻣﻔﻬﻮﻣﺔ ﺗﻤﺎﻣ ، ﻭﻋﻠﻴﻪ ﻣﻦ ﺍﻟﺼﻌﺐ ﺍﻟﺘﻮﺻﻞ ﻟﻠﺠﺮﻋﺔ ﺍﻻﻣﻨﺔ ﻋﻠﻰ ﺍﻹﻧﺴﺎﻥ ، ﻭﻛﺬﻟﻚ ﻛﻤﻴﺔ ﺟﻬﺪ ﺍﻟﺴﻤﻴﺔ Potential toxicity ﻓﻲ ﺍﻹﻧﺴﺎﻥ ﻣﻦ ﺍﻟﺼﻌﺐ ﺍﻟﺘﻮﺻﻞ ﻟﻪ. )2( ﻧﺘﺎﺋﺞ ﺍﻟﺪﺭﺍﺳﺎﺕ ﻋﻠﻰ ﺣﻴﻮﺍﻧﺎﺕ ﺍﻟﺪﺭﺍﺳﺔ ﻣﻔﻬﻮﻣﺔ ﺗﻤﺎﻣ ، ﻭﻋﻠﻴﻪ ﻳﻤﻜﻦ ﺍﻟﺘﻮﺻﻞ ﻟﻠﺠﺮﻋﺔ ﺍﻻﻣﻨﺔ ﻋﻠﻰ ﺍﻹﻧﺴﺎﻥ. ﻭﻛﺬﻟﻚ ﺣﺴﺎﺏ ﻣﻌﺪﻝ ﻛﻤﻴﺔ ﺟﻬﺪ ﺍﻟﺴﻤﻴﺔ Potential toxicity ﻓﻲ ﺍﻹﻧﺴﺎﻥ ﺗﻜﻮﻥ ﻣﻘﺒﻮﻟﺔ. )3( ﻋﺪ ﺍﻟﺘﻮﺻﻞ ﻟﻘﻴﻤﺔ ﺻﺤﻴﺤﺔ ﻟﻠﺪﻟﻴﻞ ﺍﻟﻌﻼﺟﻲ Therapeutic Index )4( ﺩﺭﺍﺳﺎﺕ ﺍﻟﺴﻤﻴﺔ ﻋﻠﻰ ﺣﻴﻮﺍﻧﺎﺕ ﺍﻟﺘﺠﺎﺭﺏ ﻟﻢ ﺗﺘﻨﺒﺄ ﺑﻜﻤﻲ ﺍﻟﺴﻤﻴﺔ ﻓﻲ ﺍﻹﻧﺴﺎﻥ. ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

ﺍﻟﺪﺭﺍﺳﺎﺕ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ Pharmacology ﻣﻌﻠﻮﻣﺎﺕ ﺃﺴﺎﺳﻴﺔ ﻓﻲ ﻋﻠﻢ ﺍﻟﺼﻴﺪﻟﺔ: )1( ﺃﻐﻠﺐ ﺍﻟﺪﺭﺍﺳﺎﺕ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ ﺗﺠﺮﻯ ﻓﻲ ﺍﻟﺨﺎﺭﺝ ﻟﻌﻠﻰ ﺍﻷﻨﺴﺠﺔ ﻓﻲ ﺍﻟﻤﺰﺍﺭﻉ ﺍﻟﺨﻠﻮﻳﺔ ﻭﻓﻲ ﺍﻟﺪﺍﺧﻞ ﻋﻠﻰ ﻧﻤﺎﺫﺝ ﻣﻦ ﻓﺄﺮﺍﻥ ﺍﻟﺘﺠﺎﺭﺏ. )2( ﺍﻟﺒﺤﺚ ﻋﻦ ﺍﻟﺠﻬﺪ ﻏﻴﺮ ﺍﻟﻤﺮﻏﻮﻑ ﻓﻲ ﺍﻟﺘﺄﺜﻴﺮﺍﺕ ﺍﻟﺪﻳﻨﻤﻜﺔ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ ﻋﻠﻰ ﻧﺸﺎﻁ ﻭﻇﺎﺋﻒ ﺍﻷﻌﻀﺎﺀ ﻭﻋﻼﻗﺘﻬﺎ ﺍﻟﻤﺠﺎﻝ ﺍﻟﻌﻼﺟﻲ ﻭﺃﻜﺜﺮ. )3( ICH S 7 A )4( ﻳﻔﻀﻞ ﺍﺳﺘﺨﺪﺍﻡ ﻃﺮﻕ ﺍﻟﺘﺤﺎﻟﻴﻞ ﺍﻟﺤﺪﻳﺜﺔ ﻭﺗﻘﻨﻴﺔ ﻋﺎﻟﻴﺔ. )5( ﻧﻘﻄﺔ ﻧﻬﺎﻳﺔ ﺍﻟﺪﺭﺍﺳﺎﺕ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ ﻫﻲ ﻣﻌﺮﻓﺔ ﺟﻤﻴﻊ ﻧﺘﺎﺋﺞ ﺍﻟﺴﻤﻴﺔ ﻭﺍﻟﺤﺮﻛﻴﺔ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ. )6( ﺗﻘﻮﻡ ﺃﺴﺎﺳﺎ ﺍﻟﺪﺭﺍﺳﺎﺕ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ ﻋﻠﻰ ﺍﻟﺨﻮﺍﺹ ﻣﻨﻔﺮﺩ ﻋﻠﻰ ﺍﻟﻤﺎﺩﺓ ﺍﻟﻤﺮﺍﺩ ﺍﺳﺘﻌﻤﺎﻟﻬﺎ ﻓﻲ ﺍﻻﺧﺘﺒﺎﺭﺍﺕ ﺍﻟﺴﺮﻳﺮﻳﺔ ﻟﺘﻜﻮﻥ ﻣﻌﺪﺓ ﻟﻜﻲ ﺗﺴﺘﺨﺪﻡ ﻛﺪﻭﺍﺀ. )7( AEs ؟؟؟؟؟؟؟؟؟؟؟؟؟ ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

ﺍﻟﺪﺭﺍﺳﺎﺕ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ Pharmacology ﻋﻠﻰ ﺍﻟﺠﻬﺎﺯ ﺍﻟﻌﺼﺒﻲ ﺍﻟﻤﺮﻛﺰﻱ : CNS )1( ﺩﺭﺍﺳﺔ ﻧﺸﺎﻁ ﺍﻟﺨﻼﻳﺎ ﺍﻟﻌﺼﺒﻴﺔ ﺍﻟﻤﺘﺤﺮﻛﺔ Motor activity )2( ﺩﺭﺍﺳﺔ ﺍﻟﺘﻐﻴﺮﺍﺕ ﺍﻟﺴﻠﻮﻛﻴﺔ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ. )3( ﺩﺭﺍﺳﺔ ﺗﻨﺎﺳﻘﻴﺔ. )4( ﺍﺳﺘﺠﺎﺑﺔ ﺭﺩ ﺍﻟﻔﻌﻞ ﺍﻟﻼﺇﺭﺍﺩﻱ ﻟﻤﺮﺍﻛﺰ ﺍﻻﺣﺴﺎﺱ ﻭﺍﻟﺨﻼﻳﺎ ﺍﻟﻌﺼﺒﻴﺔ ﺍﻟﻤﺘﺤﺮﻛﺔ Sensory/motor )5( ﺗﺘﺒﻊ ﻭﺗﻘﻴﻴﻢ ﺩﺭﺟﺔ ﺣﺮﺍﺭﺓ ﺍﻟﺠﺴﻢ ﺑﺎﺳﺘﻤﺮﺍﺭ. )6( ﺍﻟﺘﺠﺎﺭﺏ ﺍﻟﻌﻤﻠﻴﺔ ﻟﺘﺘﺒﻊ ﺃﺜﺮ ﺍﻟﺪﻭﺍﺀ ﻋﻠﻰ ﺍﻟﺠﻬﺎﺯ ﺍﻟﻌﺼﺒﻲ ﺍﻟﻤﺮﻛﺰﻱ ﻫﻲ: )7( ﺩﺭﺍﺳﺔ ﺍﻟﺴﻠﻮﻛﻴﺎﺕ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ. )8( ﺩﺭﺍﺳﺔ ﺍﺭﺗﺒﺎﻁ ﺍﻟﺪﻭﺍﺀ )ﻛﻤﺨﻠﺐ( ﺑﺸﻜﻞ ﺧﺎﺹ. Binding to receptors )9( ﺩﺭﺍﺳﺔ ﺍﻟﺘﺄﺜﻴﺮ ﻋﻠﻰ ﺍﻟﺒﺼﺮ ﻭﺍﻟﺴﻤﻊ Visual/Auditory )01( ﺇﺟﺮﺍﺀ ﺍﻟﺘﺠﺎﺭﺏ ﻭﻇﺎﺋﻒ ﺍﻷﻌﻀﺎﺀ ﻛﻬﺮﺑﺎﺋﻴ. Electrophysiology Exm )11( ﺩﺭﺍﺳﺔ ﺍﻟﻜﻴﻤﻴﺎﺀ ﺍﻟﻌﺼﺒﻴﺔ. )21( ﺗﺘﺒﻊ ﻭﺗﻘﻴﻴﻢ ﺩﺭﺟﺔ ﺣﺮﺍﺭﺓ ﺍﻟﺠﺴﻢ ﺑﺎﺳﺘﻤﺮﺍﺭ. )31( ﺍﺳﺘﺠﺎﺑﺔ ﺍﻷﻌﻀﺎﺀ ﻟﻠـ ﺃﺠﻮﻧﻴﺴﺖ agonists ﺃﻮ ﻣﻀﺎﺩ ﺍﻷﺠﻮﻧﻴﺴﺖ Antagonists )41( ﺩﺭﺍﺳﺔ ﻣﺪﻯ ﺗﻨﺸﻴﻂ ﺍﻟﺠﻬﺎﺯ ﺍﻟﻌﺼﺒﻲ ﺍﻟﻼﺇﺭﺍﺩﻱ autonomic ﻭﻗﻴﺎﺱ ﻣﺪﻯ ﺍﺳﺘﺠﺎﺑﺔ ﺍﻷﻮﻋﻴﺔ ﺍﻟﻘﻠﺒﻴﺔ )51( ﺍﺧﺘﺒﺎﺭ ﺍﻟﺮﺩ ﺍﻟﻔﻌﻠﻲ ﺍﻟﻌﺼﺒﻲ Baroreflex ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ )61( ﻣﺘﻐﻴﺮﺍﺕ ﻣﻌﺪﻝ ﻧﺒﻀﺎﺕ ﺍﻟﻘﻠﺐ.

ﺍﻟﺪﺭﺍﺳﺎﺕ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ Pharmacology ﻋﻠﻰ ﺍﺟﻬﺎﺯ ﺍﻷﻮﻋﻴﺔ ﺍﻟﻘﻠﺒﻴﺔ: )1( )2( )3( )4( )5( ﻗﻴﺎﺱ ﺿﻐﻂ ﺍﻟﺪﻡ ﻣﻌﺪﻝ ﻧﺒﻀﺎﺕ ﺍﻟﻘﻠﺐ ﺗﻘﻴﻴﻢ ﺍﻟـ ECG ﻣﻌﺮﻓﺔ ﺍﻻﺳﺘﻘﻄﺎﺏ ﻭﺍﻟﻮﺟﻪ ﺍﻟﻄﺒﻴﻌﻴﺔ Polarization & conductance abnormalities ﺍﻹﻃﺎﻟﺔ ﻓﻲ ﺇﻋﺪﺍﺩ ﻛﻞ ﻣﻦ QT ﻭ QTc ﻋﻠﻰ ﻓﺘﺮﺍﺕ ﺗﻌﻄﻲ ﻧﺘﻴﺠﺔ: )1( ﺳﺤﺐ ﺍﻟﺪﻭﺍﺀ ﻣﻦ ﺍﻷﺴﻮﺍﻕ. )2( ﺗﺄﺨﺮ ﺍﻟﺤﺼﻮﻝ ﻋﻠﻰ ﺗﺮﺧﻴﺺ ﺑﻴﻊ ﺍﻟﺪﻭﺍﺀ. )3( ﻳﺴﺘﺒﻌﺪ ﻟﺤﺎﻟﺔ ﺍﻟﻤﺴﺘﻮﻯ ﺍﻟﺜﺎﻧﻲ. ﺍﻟﺘﺠﺎﺭﺏ ﺍﻟﻌﻤﻠﻴﺔ ﻟﺘﺘﺒﻊ ﺃﺜﺮ ﺍﻟﺪﻭﺍﺀ ﻋﻠﻰ ﺟﻬﺎﺯ ﺍﻻﻭﻋﻴﺔ ﺍﻟﻘﻠﺒﻴﺔ ﻫﻲ: )1( )2( )3( )4( ﺩﺭﺍﺳﺎﺕ ﻋﻠﻰ ﺍﻟﻘﻠﺐ ﻭﺍﻷﻮﻋﻴﺔ Cardiac output ﺍﻟﺒﻄﻴﻦ ﺍﻟﻴﻤﻴﻦ ﻭﺍﻷﻴﺴﺮ ﻓﻲ ﺍﻟﻘﻠﺐ Ventricular contractility ﻣﻘﺎﻭﻣﺔ ﺍﻻﺗﺴﺎﻉ ﺍﻟﻮﻋﺎﺋﻲ Vascular resistance ﺗﺄﺜﻴﺮ ؟؟؟؟؟؟ Effect of endogenous and/or exogenous substances ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

ﺍﻟﺪﺭﺍﺳﺎﺕ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ Pharmacology ﻋﻠﻰ ﺍﻟﺠﻬﺎﺯ ﺍﻟﺘﻨﻔﺴﻲ: )1( ﻗﻴﺎﺱ ﻣﻌﺪﻝ ﺍﻟﺘﻨﻔﺲ )2( ﻗﻴﺎﺳﺎﺕ ﺃﺨﺮﻯ ﻣﺜﻞ ﺣﺠﻢ tidal ﺃﻮ ﻗﻴﺎﺱ ﺗﺸﺒﻊ ﻫﻴﻤﻮﺟﻠﻮﺑﻴﻦ ﺍﻟﺪﻡ ﺑﺎﻷﻜﺴﺠﻴﻦ. )3( ﺍﻟﻜﺸﻒ ﺍﻟﻄﺒﻲ ﻻ ﻳﻮﺍﺋﻢ ﺗﻘﻴﻴﻢ ﻭﻇﻴﻔﺔ ﺟﻬﺎﺯ ﺍﻟﺘﻨﻔﺲ. ﺍﻟﺘﺠﺎﺭﺏ ﺍﻟﻌﻤﻠﻴﺔ ﻟﺘﺘﺒﻊ ﺃﺜﺮ ﺍﻟﺪﻭﺍﺀ ﻋﻠﻰ ﺍﻟﺠﻬﺎﺯ ﺍﻟﺘﻨﻔﺴﻲ ﻫﻲ: )1( )2( )3( )4( )5( ﻗﻴﺎﺱ ﻣﻌﺪﻝ ﺍﻟﺘﻨﻔﺲ ﺍﻟﺮﺿﻮﺥ ﻷﻨﻈﻤﺔ ﺩﺭﺍﺳﺎﺕ ﺗﻘﻴﻴﻢ ﺍﻷﻤﺮﺍﺽ ﺍﻟﺮﺋﻮﻳﺔ Pulmonary compliances ﺗﺘﺒﻊ ﺍﻟﻀﻐﻂ ﺍﻟﺮﺋﻮﻱ ﺍﻟﺸﺮﻳﺎﻧﻲ Pulmonary arterial pressure ﻣﻌﺪﻝ ﺗﺮﻛﻴﺰ ﺍﻟﻐﺎﺯ ﻓﻲ ﺍﻟﺪﻡ ﻗﻴﻤﺔ p. H ﺍﻟﺪﻡ ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

ﺍﻟﺪﺭﺍﺳﺎﺕ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ Pharmacology ﺍﻟﺘﺠﺎﺭﺏ ﺍﻟﻌﻤﻠﻴﺔ ﻟﺘﺘﺒﻊ ﺃﺜﺮ ﺍﻟﺪﻭﺍﺀ ﻋﻠﻰ ﺍﻟﺠﻬﺎﺯ ﺍﻟﻜﻠﻮﻱ ﺍﻟﺒﻮﻟﻰ Renal/Urinary ﻫﻲ: )1( ﻗﻴﺎﺱ ﺣﺠﻢ ﺍﻟﺒﻮﻝ )2( ﻗﻴﺎﺱ ﺍﻟﺠﺎﺫﺑﻴﺔ ﺍﻟﻨﻮﻋﻴﺔ Specific Gravity )3( ؟؟؟؟؟؟؟ Osmolality )4( ﻗﻴﺎﺱ p. H ﺍﻟﺒﻮﻝ )5( ﺍﻻﺗﺰﺍﻥ ﺑﻴﻦ ﺍﻟﺴﻮﺍﺋﻞ ﻭﺍﻹﻟﻜﺘﺮﻭﻟﻴﺘﺎﺕ. )6( ﻗﻴﺎﺱ ﻗﻤﻴﺔ ﺍﻟﺒﺮﻭﺗﻴﻨﺎﺕ ﻓﻲ ﺍﻟﺒﻮﻝ )7( ؟؟؟؟؟؟ Cytology )8( ﻛﻴﻤﻴﺎﺀ ﺍﻟﺪﻡ )ﻧﻴﺘﺮﻭﺟﻴﻦ ﺩﻡ ﺍﻟﺒﻮﻝ – ، Blood Urea Nitrogen ﻛﺮﻳﺎﺗﻴﻨﻴﻦ ، Creatinine ﺑﺮﻭﺗﻴﻨﺎﺕ ﺍﻟﺒﻼﺯﻣﺎ (Plasma Proteins ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

ﺍﻟﺪﺭﺍﺳﺎﺕ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ Pharmacology ﺍﻟﺘﺠﺎﺭﺏ ﺍﻟﻌﻤﻠﻴﺔ ﻟﺘﺘﺒﻊ ﺃﺜﺮ ﺍﻟﺪﻭﺍﺀ ﻋﻠﻰ ﺍﻟﺠﻬﺎﺯ ﺍﻟﻬﻀﻤﻲ: )1( ﻗﻴﺎﺱ ﻣﻌﺪﻝ ﺇﻓﺮﺍﺯﺍﺕ ﺍﻟﻌﺼﺎﺭﺓ ﺍﻟﻤﻌﺪﻳﺔ Gastric secretion )2( ﻗﻴﺎﺱ ﺟﻬﺪ ﺍﻹﺻﺎﺑﺔ ﺍﻟﻤﻌﺪﻳﺔ Gastrointestinal injury potential )3( ﻗﻴﺎﺱ ﻣﻌﺪﻝ ﺇﻓﺮﺍﺯﺍﺕ ﺍﻟﻌﺼﺎﺭﺓ ﺍﻟﺼﻔﺮﺍﻭﻳﺔ Bile decretion )4( ﻗﻴﺎﺱ ﺍﻟﻮﻗﺖ ﺍﻟﻤﺮﻭﺭﻱ ﻓﻲ ﺍﻟﺪﺍﺧﻞ Transit time in vivo )5( ﺗﻘﻠﺼﺎﺕ ﺍﻹﻟﻴﺎﻝ ﻓﻲ ﺍﻟﺪﺍﺧﻞ ileal contraction in vivo )6( ﻗﻴﺎﺱ p. H ﺍﻟﻤﻌﺪﺓ ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ
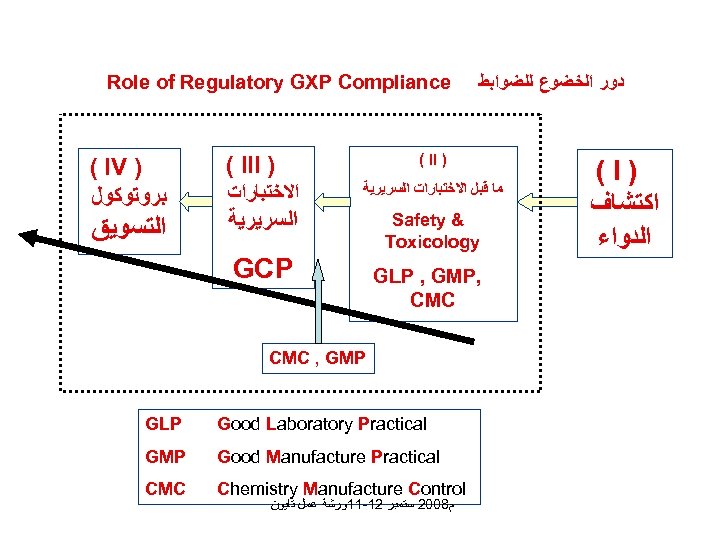
Role of Regulatory GXP Compliance ( IV ) ﺑﺮﻭﺗﻮﻛﻮﻝ ﺍﻟﺘﺴﻮﻳﻖ ﺩﻭﺭ ﺍﻟﺨﻀﻮﻉ ﻟﻠﻀﻮﺍﺑﻂ ( II ) ( III ) ﺍﻻﺧﺘﺒﺎﺭﺍﺕ ﻣﺎ ﻗﺒﻞ ﺍﻻﺧﺘﺒﺎﺭﺍﺕ ﺍﻟﺴﺮﻳﺮﻳﺔ Safety & Toxicology GCP GLP , GMP, CMC , GMP GLP Good Laboratory Practical GMP Good Manufacture Practical CMC Chemistry Manufacture Control ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ (I) ﺍﻛﺘﺸﺎﻑ ﺍﻟﺪﻭﺍﺀ

ﺍﻟﻤﺤﺎﺿﺮﺓ 6 Clinical Studies Phase I ﺃﻬﺪﺍﻑ ﺍﻟﻤﺤﺎﺿﺮﺓ ﻫﻮ ﻣﻌﺮﻓﺔ: ﺍﻟﺠﺮﻋﺔ ﺍﻟﻤﻌﺘﻤﺪﺓ ﻭﺍﻟﻨﻈﺎﻡ ﺍﻟﺤﻜﻮﻣﻲ ﺍﻟﺴﺎﺋﺪ ﻟﻠﺒﺪﺀ ﻓﻲ ﺍﻻﺧﺘﺒﺎﺭﺍﺕ ﺍﻟﺴﺮﻳﺮﻳﺔ ﻓﻲ ﺍﻻﻧﺴﺎﻥ ). (FIM ﺍﻟﺘﺼﻨﻴﻒ ﺍﻟﻤﻜﻴﺎﻟﻲ )ﺗﺤﺪﻳﺪ ﺍﻟﺠﺮﻋﺔ ﺍﻷﻤﻨﺔ( . Interspecies scaling ﺍﻟﻌﻮﺍﻣﻞ ﺍﻻﻣﻨﺔ. ﺃﻌﻠﻰ ﺟﺮﻋﺔ ﺑﺪﺀ ﻣﻌﺘﻤﺪﺓ . MRSD ﻻ ﻳﻼﺣﻆ ﺃﻲ ﻣﺴﺘﻮﻯ ﻣﻦ ﻣﺴﺘﻮﻳﺎﺕ ﺗﻐﻴﻴﺮ ﻏﻴﺮ ﻣﻼﺋﻤﺔ ﻣﻌﺎﻛﺴﺔ . NOAEL - ﺍﻟﺨﻀﻮﻉ ﻟﻸﻨﻈﻤﺔ ﺍﻟﻤﺴﺎﺭ . I ﺍﻟﻘﺎﺋﺪ ﻫﻮ ﺍﻟﺬﻱ ﻳﺤﺼﻞ ﻋﻠﻰ ﺗﺮﺧﻴﺺ ﻟﺪﻭﺍﺋﻪ ﺍﻟﻤﻜﺘﺸﻒ ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

ﺍﻟﺒﺮﻧﺎﻣﺞ ﺍﻟﻄﺒﻲ ﺗﻌﺮﻳﻒ ﻣﺎ ﺍﻟﻤﻘﺼﻮﺩ ﺑـ ﺍﻻﺧﺘﺒﺎﺭﺍﺕ ﺍﻟﻄﺒﻴﺔ Clinical Trial Definition ﺃﻲ ﺇﺟﺮﺍﺀﺍﺕ ﻣﻌﺪﺓ ﻟﻠﻜﺸﻒ ﺃﻮ ﻟﻠﻤﻌﺮﻓﺔ ﺍﻟﻄﺒﻴﺔ ﻭﻟﻠﺪﺭﺍﺳﺎﺕ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ ﻭ/ﺃﻮ ﻟﻠﺘﺄﺜﻴﺮﺍﺕ ﺍﻟﺪﻳﻨﻤﻜﺔ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ ﻟﻠﺘﺤﻘﻴﻖ ﻣﻦ ﺍﻟﺪﻭﺍﺀ ﻭ/ﺃﻮ ﻟﺪﺭﺍﺳﺔ ﺍﻻﻣﺘﺼﺎﺹ ﻭﺍﻟﺘﻮﺯﻳﻊ ﻭﺍﻟﺘﻔﺎﻋﻼﺕ ﺍﻷﻴﻀﻴﺔ ﻭﻟﻠﺘﺤﻘﻴﻖ ﻓﻲ ﻛﻴﻨﻮﻧﺔ ﺍﻟﺪﻭﺍﺀ ﺍﻟﻤﺼﺎﺣﺒﺔ ﻟﻠﻮﺻﻮﻝ ﻟﻤﺮﺣﻠﺔ ﺳﻼﻣﺔ ﻭﺃﻤﻦ ﺍﻟﺠﺮﻋﺔ ﺍﻟﻤﻌﺎﻟﺠﺔ ﻭ/ﺃﻮ ﻓﻌﺎﻟﻴﺘﻬﺎ . efficacy ﺧﻼﻝ ﺍﻟﻌﺸﺮﻳﻦ ﺳﻨﺔ ﺍﻟﻤﺎﺿﻴﺔ ﺃﺮﺗﻔﻊ ﻣﺘﻮﺳﻂ ﺍﻷﺪﻭﻳﺔ ﺍﻟﺘﻲ ﺗﺤﺖ ﺍﻻﺧﺘﺒﺎﺭﺍﺕ ﺍﻟﺴﺮﻳﺮﻳﺔ ﻣﻦ 03 ﺇﻟﻰ 07. ﺭﻗﻢ ﺍﻟﻤﺮﺿﻰ ﺍﻟﻤﺘﻄﻮﻋﻴﻦ ﻟﻠﺨﻀﻮﻉ ﻟﻼﺧﺘﺒﺎﺭﺍﺕ ﺍﻟﺴﺮﻳﺮﻳﺔ ﻗﺪ ﺃﺮﺗﻔﻊ ﻣﻦ 0051 ﺇﻟﻰ 0025 ﺣﺴﺐ ﺍﻷﻨﻈﻤﺔ ﺍﻟﻤﻮﺿﻮﻋﺔ ﻣﻦ ﻗﺒﻞ ﺍﻟﺠﻬﺎﺕ ﺍﻟﻤﺎﻧﺤﺔ ﻟﻠﺘﺮﺍﺧﻴﺺ ﺍﻟﺪﻭﺍﺋﻴﺔ. ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

ﺍﻻﻋﺘﺒﺎﺭﺍﺕ ﺍﻷﺨﻼﻗﻴﺔ Ethical Considerations ﺍﻟﻘﻴﻤﺔ ﺍﻻﺟﺘﻤﺎﻋﻴﺔ ﺍﻟﻌﺎﺋﺪﺓ ﻋﻠﻲ ﺍﻟﻤﺠﺘﻤﻊ ﻣﻦ ﺍﻛﺘﺸﺎﻑ ﺍﻷﺪﻭﻳﺔ. - ﺍﻟﺘﻘﻴﺪ ﺑﺎﻟﺘﺸﺮﻳﻊ ﺍﻷﺨﻼﻗﻲ ﺍﻟﻌﻠﻤﻲ. - Social value - Scientific validity ﺍﻛﺘﺸﺎﻑ ﺍﻟﺪﻭﺍﺀ ﻭﺍﺧﺘﺒﺎﺭﻩ ﺑﻄﺮﻕ ﻋﺎﺩﻟﺔ ﻣﺘﻘﻨﺔ ﻣﻊ ﺍﻟﻠﻮﺍﺋﺢ ﻭﺍﻷﻨﻈﻤﺔ. ﺍﻟﻤﻮﺍﻓﻘﺔ ﺍﻟﻤﺮﺿﻴﺔ ﺫﺍﺕ ﺍﻟﺨﺒﺮﺓ ﺍﻟﻌﺎﻟﻴﺔ ﻋﻠﻰ ﺗﻨﻔﻴﺬ ﺍﻻﺧﺘﺒﺎﺭﺍﺕ ﺍﻟﺴﺮﻳﺮﻳﺔ. - Fair subject selection - Informed consent ﺍﻟﺘﻮﺍﺯﻥ ﺑﻴﻦ ﻧﺴﺒﺔ ﺍﻟﻔﺎﺋﺪﺓ ﺇﻟﻲ ﺍﻟﺨﻄﻮﺭﺓ ﻹﻗﺮﺍﺭ ﺍﻻﺳﺘﺨﺪﺍﻡ ﺍﻻﺩﻣﻲ. - Favourable risk-benefit ratio ﺍﺣﺘﺮﺍﻡ ﺍﻟﻬﻮﻳﺔ ﺍﻟﻔﺮﺩﻳﺔ ﻋﻨﺪ ﺗﻄﺒﻴﻖ ﺃﻨﻈﻤﺔ ﺍﻻﺧﺘﺒﺎﺭﺍﺕ ﺍﻟﺴﺮﻳﺮﻳﺔ. - Respect for human subjects - Independent review ﺍﻟﻔﺮﻳﻖ ﺍﻻﺧﻼﻗﻲ ﺍﻟﻌﻠﻤﻲ ﺫﻭ ﺍﻟﺨﺒﺮﺓ ﻓﻲ ﺍﻟﺘﺄﻜﺪ ﻣﻦ ﻧﺘﺎﺋﺞ ﺍﺧﺘﺒﺎﺭﺍﺕ board/independent ethics ﺍﻟﺪﻭﺍﺀ / ﻟﺠﻨﺔ ﺍﻻﺧﻼﻕ ﺍﻟﻤﺴﺘﻘﻠﺔ. committee ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ
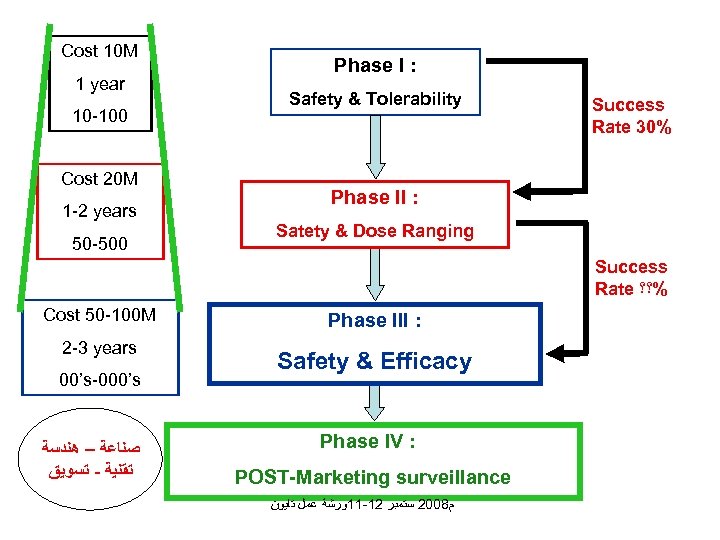
Cost 10 M 1 year 10 -100 Cost 20 M 1 -2 years 50 -500 Phase I : Safety & Tolerability Success Rate 30% Phase II : Satety & Dose Ranging Success Rate %؟؟ Cost 50 -100 M 2 -3 years 00’s-000’s ﺻﻨﺎﻋﺔ – ﻫﻨﺪﺳﺔ ﺗﻘﻨﻴﺔ - ﺗﺴﻮﻳﻖ Phase III : Safety & Efficacy Phase IV : POST-Marketing surveillance ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ
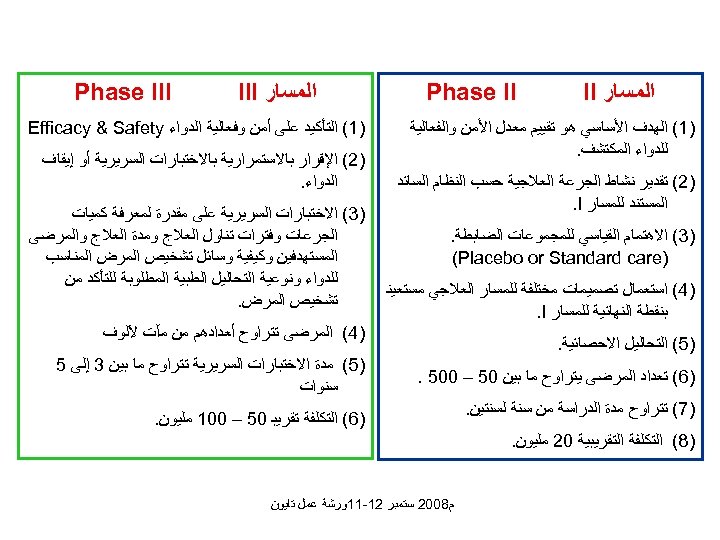
ﺍﻟﻤﺴﺎﺭ II Phase II )1( ﺍﻟﻬﺪﻑ ﺍﻷﺴﺎﺳﻲ ﻫﻮ ﺗﻘﻴﻴﻢ ﻣﻌﺪﻝ ﺍﻷﻤﻦ ﻭﺍﻟﻔﻌﺎﻟﻴﺔ ﻟﻠﺪﻭﺍﺀ ﺍﻟﻤﻜﺘﺸﻒ. )2( ﺗﻘﺪﻳﺮ ﻧﺸﺎﻁ ﺍﻟﺠﺮﻋﺔ ﺍﻟﻌﻼﺟﻴﺔ ﺣﺴﺐ ﺍﻟﻨﻈﺎﻡ ﺍﻟﺴﺎﺋﺪ ﺍﻟﻤﺴﺘﻨﺪ ﻟﻠﻤﺴﺎﺭ . I )3( ﺍﻻﻫﺘﻤﺎﻡ ﺍﻟﻘﻴﺎﺳﻲ ﻟﻠﻤﺠﻤﻮﻋﺎﺕ ﺍﻟﻀﺎﺑﻄﺔ. ) (Placebo or Standard care )4( ﺍﺳﺘﻌﻤﺎﻝ ﺗﺼﻤﻴﻤﺎﺕ ﻣﺨﺘﻠﻔﺔ ﻟﻠﻤﺴﺎﺭ ﺍﻟﻌﻼﺟﻲ ﻣﺴﺘﻌﻴﻨ ﺑﻨﻘﻄﺔ ﺍﻟﻨﻬﺎﺋﻴﺔ ﻟﻠﻤﺴﺎﺭ . I )1( ﺍﻟﺘﺄﻜﻴﺪ ﻋﻠﻰ ﺃﻤﻦ ﻭﻓﻌﺎﻟﻴﺔ ﺍﻟﺪﻭﺍﺀ Efficacy & Safety )2( ﺍﻹﻗﺮﺍﺭ ﺑﺎﻻﺳﺘﻤﺮﺍﺭﻳﺔ ﺑﺎﻻﺧﺘﺒﺎﺭﺍﺕ ﺍﻟﺴﺮﻳﺮﻳﺔ ﺃﻮ ﺇﻳﻘﺎﻑ ﺍﻟﺪﻭﺍﺀ. )3( ﺍﻻﺧﺘﺒﺎﺭﺍﺕ ﺍﻟﺴﺮﻳﺮﻳﺔ ﻋﻠﻰ ﻣﻘﺪﺭﺓ ﻟﻤﻌﺮﻓﺔ ﻛﻤﻴﺎﺕ ﺍﻟﺠﺮﻋﺎﺕ ﻭﻓﺘﺮﺍﺕ ﺗﻨﺎﻭﻝ ﺍﻟﻌﻼﺝ ﻭﻣﺪﺓ ﺍﻟﻌﻼﺝ ﻭﺍﻟﻤﺮﺿﻰ ﺍﻟﻤﺴﺘﻬﺪﻓﻴﻦ ﻭﻛﻴﻔﻴﺔ ﻭﺳﺎﺋﻞ ﺗﺸﺨﻴﺺ ﺍﻟﻤﺮﺽ ﺍﻟﻤﻨﺎﺳﺐ ﻟﻠﺪﻭﺍﺀ ﻭﻧﻮﻋﻴﺔ ﺍﻟﺘﺤﺎﻟﻴﻞ ﺍﻟﻄﺒﻴﺔ ﺍﻟﻤﻄﻠﻮﺑﺔ ﻟﻠﺘﺄﻜﺪ ﻣﻦ ﺗﺸﺨﻴﺺ ﺍﻟﻤﺮﺽ. )4( ﺍﻟﻤﺮﺿﻰ ﺗﺘﺮﺍﻭﺡ ﺃﻌﺪﺍﺩﻫﻢ ﻣﻦ ﻣآﺖ ﻵﻠﻮﻑ )5( ﺍﻟﺘﺤﺎﻟﻴﻞ ﺍﻻﺣﺼﺎﺋﻴﺔ. )6( ﺗﻌﺪﺍﺩ ﺍﻟﻤﺮﺿﻰ ﻳﺘﺮﺍﻭﺡ ﻣﺎ ﺑﻴﻦ 05 – 005. )7( ﺗﺘﺮﺍﻭﺡ ﻣﺪﺓ ﺍﻟﺪﺭﺍﺳﺔ ﻣﻦ ﺳﻨﺔ ﻟﺴﻨﺘﻴﻦ. ﺍﻟﻤﺴﺎﺭ III Phase III )5( ﻣﺪﺓ ﺍﻻﺧﺘﺒﺎﺭﺍﺕ ﺍﻟﺴﺮﻳﺮﻳﺔ ﺗﺘﺮﺍﻭﺡ ﻣﺎ ﺑﻴﻦ 3 ﺇﻟﻰ 5 ﺳﻨﻮﺍﺕ )6( ﺍﻟﺘﻜﻠﻔﺔ ﺗﻘﺮﻳﺒ 05 – 001 ﻣﻠﻴﻮﻥ. )8( ﺍﻟﺘﻜﻠﻔﺔ ﺍﻟﺘﻘﺮﻳﺒﻴﺔ 02 ﻣﻠﻴﻮﻥ. ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ
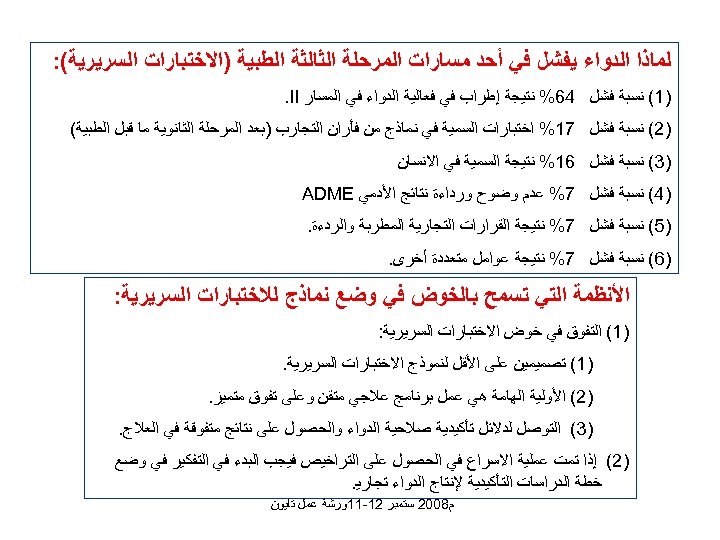
ﻟﻤﺎﺫﺍ ﺍﻟﺪﻭﺍﺀ ﻳﻔﺸﻞ ﻓﻲ ﺃﺤﺪ ﻣﺴﺎﺭﺍﺕ ﺍﻟﻤﺮﺣﻠﺔ ﺍﻟﺜﺎﻟﺜﺔ ﺍﻟﻄﺒﻴﺔ )ﺍﻻﺧﺘﺒﺎﺭﺍﺕ ﺍﻟﺴﺮﻳﺮﻳﺔ(: )1( ﻧﺴﺒﺔ ﻓﺸﻞ 46% ﻧﺘﻴﺠﺔ ﺇﻃﺮﺍﺏ ﻓﻲ ﻓﻌﺎﻟﻴﺔ ﺍﻟﺪﻭﺍﺀ ﻓﻲ ﺍﻟﻤﺴﺎﺭ . II )2( ﻧﺴﺒﺔ ﻓﺸﻞ 71% ﺍﺧﺘﺒﺎﺭﺍﺕ ﺍﻟﺴﻤﻴﺔ ﻓﻲ ﻧﻤﺎﺫﺝ ﻣﻦ ﻓﺄﺮﺍﻥ ﺍﻟﺘﺠﺎﺭﺏ )ﺑﻌﺪ ﺍﻟﻤﺮﺣﻠﺔ ﺍﻟﺜﺎﻧﻮﻳﺔ ﻣﺎ ﻗﺒﻞ ﺍﻟﻄﺒﻴﺔ( )3( ﻧﺴﺒﺔ ﻓﺸﻞ 61% ﻧﺘﻴﺠﺔ ﺍﻟﺴﻤﻴﺔ ﻓﻲ ﺍﻻﻧﺴﺎﻥ )4( ﻧﺴﺒﺔ ﻓﺸﻞ 7% ﻋﺪﻡ ﻭﺿﻮﺡ ﻭﺭﺩﺍﺀﺓ ﻧﺘﺎﺋﺞ ﺍﻷﺪﻣﻲ ADME )5( ﻧﺴﺒﺔ ﻓﺸﻞ 7% ﻧﺘﻴﺠﺔ ﺍﻟﻘﺮﺍﺭﺍﺕ ﺍﻟﺘﺠﺎﺭﻳﺔ ﺍﻟﻤﻄﺮﺑﺔ ﻭﺍﻟﺮﺩﺀﺓ. )6( ﻧﺴﺒﺔ ﻓﺸﻞ 7% ﻧﺘﻴﺠﺔ ﻋﻮﺍﻣﻞ ﻣﺘﻌﺪﺩﺓ ﺃﺨﺮﻯ. ﺍﻷﻨﻈﻤﺔ ﺍﻟﺘﻲ ﺗﺴﻤﺢ ﺑﺎﻟﺨﻮﺽ ﻓﻲ ﻭﺿﻊ ﻧﻤﺎﺫﺝ ﻟﻼﺧﺘﺒﺎﺭﺍﺕ ﺍﻟﺴﺮﻳﺮﻳﺔ: )1( ﺍﻟﺘﻔﻮﻕ ﻓﻲ ﺧﻮﺽ ﺍﻻﺧﺘﺒﺎﺭﺍﺕ ﺍﻟﺴﺮﻳﺮﻳﺔ: )1( ﺗﺼﻤﻴﻤﻴﻦ ﻋﻠﻰ ﺍﻷﻘﻞ ﻟﻨﻤﻮﺫﺝ ﺍﻻﺧﺘﺒﺎﺭﺍﺕ ﺍﻟﺴﺮﻳﺮﻳﺔ. )2( ﺍﻷﻮﻟﻴﺔ ﺍﻟﻬﺎﻣﺔ ﻫﻲ ﻋﻤﻞ ﺑﺮﻧﺎﻣﺞ ﻋﻼﺟﻲ ﻣﺘﻘﻦ ﻭﻋﻠﻰ ﺗﻔﻮﻕ ﻣﺘﻤﻴﺰ. )3( ﺍﻟﺘﻮﺻﻞ ﻟﺪﻻﺋﻞ ﺗﺄﻜﻴﺪﻳﺔ ﺻﻼﺣﻴﺔ ﺍﻟﺪﻭﺍﺀ ﻭﺍﻟﺤﺼﻮﻝ ﻋﻠﻰ ﻧﺘﺎﺋﺞ ﻣﺘﻔﻮﻗﺔ ﻓﻲ ﺍﻟﻌﻼﺝ. )2( ﺇﺫﺍ ﺗﻤﺖ ﻋﻤﻠﻴﺔ ﺍﻻﺳﺮﺍﻉ ﻓﻲ ﺍﻟﺤﺼﻮﻝ ﻋﻠﻰ ﺍﻟﺘﺮﺍﺧﻴﺺ ﻓﻴﺠﺐ ﺍﻟﺒﺪﺀ ﻓﻲ ﺍﻟﺘﻔﻜﻴﺮ ﻓﻲ ﻭﺿﻊ ﺧﻄﺔ ﺍﻟﺪﺭﺍﺳﺎﺕ ﺍﻟﺘﺄﻜﻴﺪﻳﺔ ﻹﻧﺘﺎﺝ ﺍﻟﺪﻭﺍﺀ ﺗﺠﺎﺭﻳ. ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ
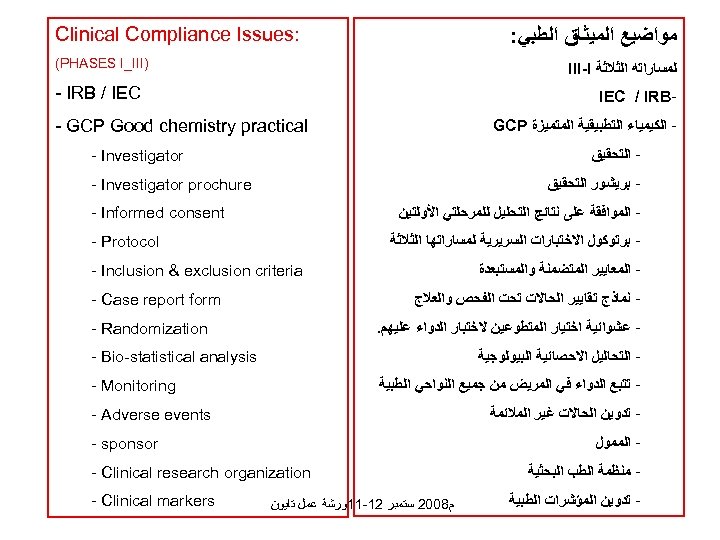
: Clinical Compliance Issues ﻣﻮﺍﺿﻴﻊ ﺍﻟﻤﻴﺜﺎﻕ ﺍﻟﻄﺒﻲ: ) (PHASES I_III ﻟﻤﺴﺎﺭﺍﺗﻪ ﺍﻟﺜﻼﺛﺔ III-I - IRB / IEC - IEC / IRB - GCP Good chemistry practical ﺍﻟﻜﻴﻤﻴﺎﺀ ﺍﻟﺘﻄﺒﻴﻘﻴﺔ ﺍﻟﻤﺘﻤﻴﺰﺓ GCP - ﺍﻟﺘﺤﻘﻴﻖ - Investigator - ﺑﺮﻳﺸﻮﺭ ﺍﻟﺘﺤﻘﻴﻖ - Investigator prochure - ﺍﻟﻤﻮﺍﻓﻘﺔ ﻋﻠﻰ ﻧﺘﺎﺋﺞ ﺍﻟﺘﺤﻠﻴﻞ ﻟﻠﻤﺮﺣﻠﺘﻲ ﺍﻷﻮﻟﺘﻴﻦ - Informed consent ﺑﺮﺗﻮﻛﻮﻝ ﺍﻻﺧﺘﺒﺎﺭﺍﺕ ﺍﻟﺴﺮﻳﺮﻳﺔ ﻟﻤﺴﺎﺭﺍﺗﻬﺎ ﺍﻟﺜﻼﺛﺔ - ﺍﻟﻤﻌﺎﻳﻴﺮ ﺍﻟﻤﺘﻀﻤﻨﺔ ﻭﺍﻟﻤﺴﺘﺒﻌﺪﺓ - Protocol - Inclusion & exclusion criteria - ﻧﻤﺎﺫﺝ ﺗﻘﺎﻳﻴﺮ ﺍﻟﺤﺎﻻﺕ ﺗﺤﺖ ﺍﻟﻔﺤﺺ ﻭﺍﻟﻌﻼﺝ - Case report form - ﻋﺸﻮﺍﺋﻴﺔ ﺍﺧﺘﻴﺎﺭ ﺍﻟﻤﺘﻄﻮﻋﻴﻦ ﻻﺧﺘﺒﺎﺭ ﺍﻟﺪﻭﺍﺀ ﻋﻠﻴﻬﻢ. - Randomization - ﺍﻟﺘﺤﺎﻟﻴﻞ ﺍﻻﺣﺼﺎﺋﻴﺔ ﺍﻟﺒﻴﻮﻟﻮﺟﻴﺔ - Bio-statistical analysis - ﺗﺘﺒﻊ ﺍﻟﺪﻭﺍﺀ ﻓﻲ ﺍﻟﻤﺮﻳﺾ ﻣﻦ ﺟﻤﻴﻊ ﺍﻟﻨﻮﺍﺣﻲ ﺍﻟﻄﺒﻴﺔ - Monitoring - ﺗﺪﻭﻳﻦ ﺍﻟﺤﺎﻻﺕ ﻏﻴﺮ ﺍﻟﻤﻼﺋﻤﺔ - Adverse events ﺍﻟﻤﻤﻮﻝ ﻣﻨﻈﻤﺔ ﺍﻟﻄﺐ ﺍﻟﺒﺤﺜﻴﺔ - ﺗﺪﻭﻳﻦ ﺍﻟﻤﺆﺸﺮﺍﺕ ﺍﻟﻄﺒﻴﺔ - sponsor - Clinical research organization ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ - Clinical markers
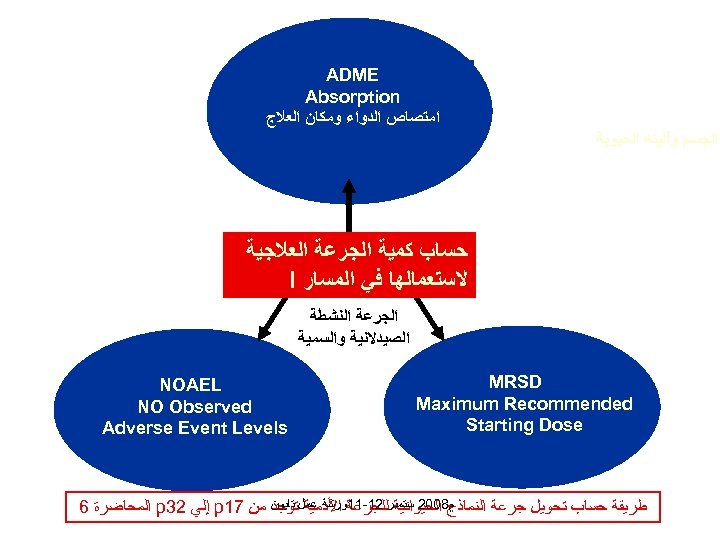
ADME Absorption ﺍﻣﺘﺼﺎﺹ ﺍﻟﺪﻭﺍﺀ ﻭﻣﻜﺎﻥ ﺍﻟﻌﻼﺝ ﺍﻟﺠﺴﻢ ﻭآﻠﻴﺘﻪ ﺍﻟﺤﻴﻮﻳﺔ ﺣﺴﺎﺏ ﻛﻤﻴﺔ ﺍﻟﺠﺮﻋﺔ ﺍﻟﻌﻼﺟﻴﺔ ﻻﺳﺘﻌﻤﺎﻟﻬﺎ ﻓﻲ ﺍﻟﻤﺴﺎﺭ I ﺍﻟﺠﺮﻋﺔ ﺍﻟﻨﺸﻄﺔ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ ﻭﺍﻟﺴﻤﻴﺔ MRSD Maximum Recommended Starting Dose NOAEL NO Observed Adverse Event Levels ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ ﻣﻦ 71 p ﺇﻟﻲ 23 p ﺍﻟﻤﺤﺎﺿﺮﺓ 6 ﻃﺮﻳﻘﺔ ﺣﺴﺎﺏ ﺗﺤﻮﻳﻞ ﺟﺮﻋﺔ ﺍﻟﻨﻤﺎﺫﺝ ﺍﻟﺤﻴﻮﺍﻧﻴﺔ ﻟﻠﺠﺮﻋﺔ ﺍﻷﺪﻣﻴﺔ ﺗﻮﺟﺪ

ﺍﻟﻤﺴﺎﺭ I PHASE I )1( ﻧﻘﻄﺔ ﺑﺪﺍﻳﺔ ﺍﻻﺧﺘﺒﺎﺭﺍﺕ ﺍﻟﺴﺮﻳﺮﻳﺔ. )2( ﺗﺤﺪﻳﺪ ﺍﻟﺠﺮﻋﺎﺕ ﺍﻟﻌﻼﺟﻴﺔ ﺍﺳﺘﻨﺎﺩ ﻟﻨﺘﺎﺋﺞ ﺍﻟﻤﺮﺣﻠﺔ ﺍﻟﺜﺎﻧﻮﻳﺔ ﻣﺎ ﻗﺒﻞ ﺍﻟﻄﺒﻴﺔ . MTD )3( ﺍﻟﻬﺪﻑ ﺍﻻﺳﺎﺳﻲ ﻫﻮ ﺗﻘﻴﻴﻢ ﺃﻤﻦ ﻭﺍﺣﺘﻤﺎﻟﻴﺔ ﺳﻼﻣﺔ ﺍﻟﺪﻭﺍﺀ ﻋﻨﺪ ﺩﺭﺍﺳﺘﻪ ﻋﻠﻰ ﺍﻻﻧﺴﺎﻥ. )4( ﺍﻟﻤﺮﺿﻰ ﺍﻟﻤﺘﻄﻮﻋﻴﻦ ﺃﻮ ﺍﻟﻤﺮﺿﻲ ﺍﻟﻤﺮﺍﺩ ﺍﻧﻘﺎﺽ ﺣﻴﺎﺗﻬﻢ ﻣﺜﻞ ﻣﺮﺿﻰ ﺍﻟﺴﺮﻃﺎﻥ. )5( ﺗﻌﺪﺍﺩ ﺍﻟﻤﺮﺿﻰ ﻳﺘﺮﺍﻭﺡ ﻣﺎ ﺑﻴﻦ 01 ﺇﻟﻰ ﻣﺎﺋﺔ. )6( ﻣﺪﺓ ﺍﻟﺪﺭﺍﺳﺔ ﻣﺎ ﺑﻴﻦ 7 ﺇﻟﻲ 21 ﺷﻬﺮ )7( ﺍﻟﺘﻜﻠﻔﺔ ﻗﺪ ﺗﺼﻞ ﺇﻟﻲ 01 ﻣﻼﻳﻴﻦ. ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

PHASE I Clinical Trials ﺍﻟﻤﺴﺎﺭ I ﺍﻻﺧﺘﺒﺎﺭﺍﺕ ﺍﻟﻄﺒﻴﺔ )ﻻﺧﺘﺒﺎﺭ ﺍﻷﺪﻭﻳﺔ ﺍﻟﺴﺮﻃﺎﻧﻴﺔ( 1( ﺗﻘﺪﻳﺮ ﻛﻞ ﻣﻦ : ﺍﻷﻤﻦ – ﺍﻟﺴﻤﻴﺔ – ﻣﺪﻩ ﺍﻟﺠﺮﻋﺎﺕ ﺍﻟﻤﺘﻮﺍﺋﻤﺔ ﻣﻊ ﻣﺴﺎﺭ ﺩﺭﺍﺳﺎﺕ MTD 2( ﺍﻟﻘﻮﺍﻋﺪ ﺍﻟﻌﺎﻣﺔ ﺍﻟﻤﻄﺒﻘﺔ ﻋﻠﻰ ﻣﺮﺿﻰ ﺍﻟﺴﺮﻃﺎﻥ: 1( ﻋﺪﺩ ﺍﻟﻤﺮﺿﻰ ﺗﺤﺖ ﺍﻻﺧﺘﺒﺎﺭﺍﺕ ﺍﻟﺴﺮﻳﺮﻳﺔ. 2( ﺑﺤﻈﺮ ﺗﺘﺒﻊ ﻣﺴﻴﺮﺓ ﺍﻟﻤﻌﺎﻟﺠﺔ ﺍﻟﺴﻤﻴﺔ. 3( ﺍﻟﺪﺭﺍﺳﺎﺕ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ ﻭ ﺍﻟﺤﺮﻛﻴﺔ ﺍﻟﺼﻴﺪﻻﻧﻴﺔ. 3( ﻃﺮﻳﻘﺔ ﺣﺴﺎﺏ ﺍﻟﺠﺮﻋﺎﺕ ﺍﻟﺤﺎﺩﺓ ﻭﺍﻟﺴﺎﻣﺔ ﻟﻌﻼﺝ ﺍﻟﺴﺮﻃﺎﻥ ﻣﻮﺿﺤﺔ ﺑﺎﻟﺼﻔﺤﺔ 53 p ﺍﻟﻤﺤﺎﺿﺮﺓ 6 : 4) Novel dose – escalation schemes : May use surrogate endopoints as ” 5) “Proof of principle 6) BUT NOT accepted as 09 ” 7)“Proof of efficacy ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

PHASE I Clinical Trials ﺍﻟﻤﺴﺎﺭ I ﺍﻻﺧﺘﺒﺎﺭﺍﺕ ﺍﻟﻄﺒﻴﺔ )ﻻﺧﺘﺒﺎﺭ ﺍﻷﺪﻭﻳﺔ ﻏﻴﺮ ﺍﻟﺴﺮﻃﺎﻧﻴﺔ( ﺗﻘﺪﻳﺮ ﻛﻞ ﻣﻦ : 1( ﺍﻷﻤﻦ – ﺍﻟﺴﻤﻴﺔ. 2( ﺍﺳﺘﻌﻤﺎﻝ ﻣﺪﻯ ﺍﻟﺠﺮﻋﺎﺕ ﺍﻟﻤﺘﻮﺍﺋﻤﺔ ﻣﻊ ﺍﻟﺪﺭﺍﺳﺎﺕ ﻣﺎ ﻗﺒﻞ ﺍﻟﻤﺮﺿﻴﺔ. 3( ﻻ ﺗﺴﺘﺨﺪﻡ ﺃﻌﻠﻰ ﺟﺮﻋﺔ ﻣﺤﺘﻤﻠﺔ MTD ﻣﻄﻠﻘ. 4( ﺍﻟﻘﻮﺍﻋﺪ ﺍﻟﻌﺎﻣﺔ ﺍﻟﻤﻄﺒﻘﺔ ﻋﻠﻰ ﺍﻟﻤﺮﺿﻰ ﺍﻟﻤﺘﻄﻮﻋﻴﻦ ﺑﺸﻜﻞ ﻃﺒﻴﻌﻲ. 5( ﻃﺮﻳﻘﺔ ﺣﺴﺎﺏ ﺍﻟﺠﺮﻋﺎﺕ ﺍﻟﺤﺎﺩﺓ ﻭﺍﻟﺴﺎﻣﺔ ﻟﻌﻼﺝ ﺍﻟﺴﺮﻃﺎﻥ ﻣﻮﺿﺤﺔ ﺑﺎﻟﺼﻔﺤﺔ 53 p ﺍﻟﻤﺤﺎﺿﺮﺓ 6. 6) One Tenth the Observed Adverse Effect Level (NOAEL) in most : appropriate species on a mg/m 2 basis ” Most appropriate = “Most Sensitive 19 ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ
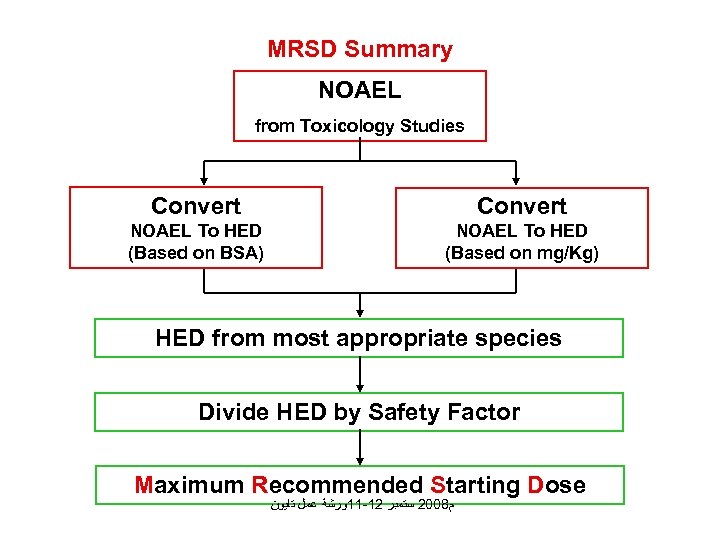
MRSD Summary NOAEL from Toxicology Studies Convert NOAEL To HED (Based on BSA) NOAEL To HED (Based on mg/Kg) HED from most appropriate species Divide HED by Safety Factor Maximum Recommended Starting Dose ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

ﺍﻟﻤﺤﺎﺿﺮﺓ 7 Regulatory Affairs & Compliance ﺍﻟﺮﺿﻮﺥ ﻭﺍﻟﺸﺆﻮﻥ ﺍﻟﺘﻨﻈﻴﻤﺔ ﺗﻬﺪﻑ ﺍﻟﻤﺤﺎﺿﺮﺓ ﻟﺘﻮﺿﻴﺢ: )1( ﺍﻟﻤﺴﺎﺭﺍﺕ ﻭﺍﻻﺳﺘﺮﺍﺗﻴﺠﻴﺎﺕ ﺍﻟﺘﻨﻈﻴﻤﻴﺔ. )2( ﺍﻟﺸﺆﻮﻥ ﺍﻟﺘﻨﻈﻴﻤﻴﺔ ﺍﻟﺤﺴﺎﺳﺔ ﻟﻠﻤﻴﻠﻴﺴﺘﻮﻥ Milestones ﻭﻧﻘﺎﻁ ﺍﻟﻘﺮﺍﺭﺍﺕ ﻟﻼﺳﺘﻤﺮﺍﺭﻳﺔ ﺍﻭ ﺍﺳﺘﺒﻌﺎﺩ ﺍﻟﺪﻭﺍﺀ )3( ﺍﺳﺘﺮﺍﺗﻴﺠﻴﺎﺕ ﻣﺎ ﻗﺒﻞ . IND )4( ﺗﺮﺗﻴﺐ ﻣﻠﻒ ﺍﻟـ IND ﺛﻢ ﺇﺭﺳﺎﻟﻪ. )5( ﺍﻟﻤﻴﺜﺎﻕ ﺍﻟﺘﻨﻈﻴﻤﻲ )6( ﺧﻄﻮﺭﺓ ﺍﻟﺘﺤﺎﻟﻴﻞ ﺍﺳﺘﺮﺍﺗﻴﺠﻴﺎﺕ ﺗﺴﻜﻴﻦ ﺍﻵﻼﻡ ﻣﺜﻼ. )7( ﺍﻟﺤﺬﺭ ﺍﻟﺼﻴﺪﻻﻧﻲ Pharmacovigilance 39 ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

The Label (Claim) is the Driver Compliance Issues ﺇﺻﺪﺍﺭﺍﺕ ﺍﻟﺮﺿﻮﺥ: )1( ﺧﻄﻂ ﺍﻷﻌﻤﺎﻝ ﺍﻟﺘﺠﺎﺭﻳﺔ )2( ﺍﻟﻤﻨﺘﺠﺎﺕ )3( ﺍﻻﺳﺘﺮﺍﺗﻴﺠﻴﺎﺕ )4( ﺍﻻﺣﺘﻴﺎﻝ )ﺍﻟﺨﺪﺍﻉ( )5( ؟؟؟؟ GXP )6( ﺍﻻﺧﻼﻕ )7( ﺍﻟﻤﻠﻜﻴﺔ ﺍﻟﻔﻜﺮﻳﺔ IP ﻭﺍﻟﻤﺴﺆﻮﻝ ﻋﻦ ﺭﻓﻊ ﺍﻟﺪﻋﻮﻯ ﺍﻟﻘﻀﺎﺋﻴﺔ Litigation )8( ﺍﻟﺤﺬﺭ ﺍﻟﺼﻴﺪﻻﻧﻲ 49 ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ
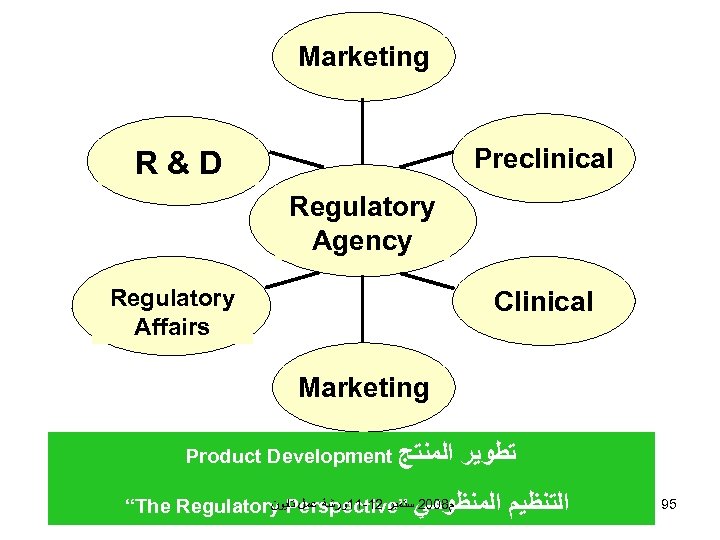
Marketing Preclinical R&D Regulatory Agency Regulatory Affairs Clinical Marketing Product Development ﺍﻟﻤﻨﺘﺞ ﺗﻄﻮﻳﺮ ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ “The Regulatory Perspective” ﺍﻟﻤﻨﻈﻮﻣﻲ ﺍﻟﺘﻨﻈﻴﻢ 95

Major Regulatory Agencies ﺻﻔﺤﺔ 7 & 6 p ﻣﺤﺎﺿﺮﺓ 7 69 ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

ﺍﻟﺴﻠﻄﺎﺕ ﺍﻷﺨﺮﻯ ﺍﻟﺘﻲ ﺗﺮﺍﻗﺐ ﻭﺗﺸﺮﻑ ﻋﻠﻰ ﺗﺼﻨﻴﻊ ﻭﺗﺴﻮﻳﻖ ﻭﺗﻮﺯﻳﻊ ﺍﻷﺪﻭﻳﺔ ﻫﻲ: ﺻﻔﺤﺔ 8 ﺍﻟﻤﺤﺎﺿﺮﺓ 7 79 ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

ﻣﺼﺎﺩﺭ ﺍﻟﺤﺼﻮﻝ ﻋﻠﻰ ﻣﻌﻠﻮﻣﺎﺕ ﻟﻮﺍﺋﺢ ﻭﺃﻨﻈﻤﺔ ﺍﻛﺘﺸﺎﻑ ﺍﻟﺪﻭﺍﺀ ﻭﺗﺮﺧﻴﺼﺎﺗﻪ ﻫﻲ: )1( ﺷﺒﻜﺔ ﺍﻹﻧﺘﺮﻧﻴﺖ )1( ﺷﺮﻛﺎﺕ ﻟﻮﺍﺋﺢ ﻭﺃﻨﻈﻤﺔ ﺗﺮﺧﻴﺺ ﺍﻟﺪﻭﺍﺀ )2( FOI )2( ﺍﻟﻤﺴﺢ ﺍﻻﺩﺑﻲ ﻓﻲ ﺍﻟﻤﺠﺎﻻﺕ ﻭﺍﻟﻤﺮﺍﺟﻊ )3( ﺗﻘﺎﺭﻳﺮ ﺍﻟﺨﺒﺮﺍﺀ Expert reports )4( ﺍﻹﻋﻼﻧﺎﺕ )5( ﺍﻟﻤﺴﺢ ﺍﻟﺤﺎﺳﻮﺑﻲ 89 ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

ﻗﻮﺍﻋﺪ ﺍﻟﻤﻮﺍﻓﻘﺔ ﺍﻟﺪﻭﺍﺋﻴﺔ Basis for Drug Approval )1( ﻋﺮﺽ ﻓﻌﺎﻟﻴﺔ Afficacy ﺑﺠﺎﻧﺐ ﺃﻤﺎﻥ ﺍﻟﺪﻭﺍﺀ ﺍﻟﻤﻼﺋﻢ ﻭﺗﺤﻜﻢ ﺟﻴﺪ ﻷﺪﺍﺀ ﺍﻟﺪﺭﺍﺳﺎﺕ. )2( ﺍﻟﻘﺪﺭﺓ ﻟﻠﺘﻮﺻﻞ ﻟﻤﻨﺘﺞ ﺩﻭﺍﺋﻲ ﻣﺼﻨﻒ ﻭﻣﻮﺿﻮﻋﺔ ﺍﺳﺘﺮﺍﺗﻴﺠﻴﺔ ﺍﺳﺘﻌﻤﺎﻟﺔ ﻟﻌﻼﺝ ﺷﺮﻳﺤﺔ ﻣﻦ ﺍﻟﻤﺮﺿﻰ. )3( ﺗﻘﺪﻳﻢ ﺍﻟﺒﺮﺍﻫﻴﻦ ﺍﻟﻤﻼﺋﻤﺔ ﻟﺪﻗﺔ ﺃﻤﻦ ﺍﻟﺪﻭﺍﺀ ﻭﻣﺪﻯ ﻓﻌﺎﻟﻴﺔ ﺍﺳﺘﻌﻤﺎﻟﻪ. )4( FDA ﻣﻨﻈﻤﺔ ﺍﻟﺪﻭﺍﺀ ﻭﺍﻟﻐﺬﺍﺀ ﺗﻘﺪﻡ ﺗﺮﺍﺧﻴﺺ ﻟﺘﺴﻮﻳﻖ ﺍﻷﺪﻭﻳﺔ ﺍﻟﺘﻲ: ﺗﺘﻮﺍﺀﻡ ﻣﻊ ﺍﻟﺘﺼﻤﻴﻤﺎﺕ ﺍﻟﻘﻴﺎﺳﻴﺔ ﻭﻧﻘﺎﻭﺓ ﺍﻟﻤﻨﺘﺞ ﻭﻗﺪﺭﺓ ﻓﻌﺎﻟﻴﺔ ﺍﻟﺪﻭﺍﺀ ﻓﻲ ﺍﻟﻤﻌﺎﻟﺠﺔ. 262 43 USC section - ) 21 CFR 600. 3(s 99 ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

Regulatory Agency Role ﻗﻮﺍﻋﺪ ﺍﻟﻮﻛﺎﻟﺔ ﺍﻟﺘﻨﻈﻴﻤﻴﺔ - Approval ﺍﻟﻤﻮﺍﻓﻘﺔ - Quality ﺍﻟﺠﻮﺩﺓﻪ - Safety ﺍﻷﻤﻦ - Effecacy ﺍﻟﻔﻌﺎﻟﻴﺔ - Timely availability ﺍﻟﻮﻗﺖ ﺍﻟﻤﺘﺎﺡ - GMP compliance - Pharmacovigilance GMP ﺍﻟﺮﺿﻮﺥ ﻟـ ﺍﻟﺤﺬﺭ ﺍﻟﺼﻴﺪﻻﻧﻲ - Risk management approach ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ ﻣﻨﺎﻫﺰﺓ ﻣﺠﺎﺯﻓﺔ ﺍﻟﺘﻌﺎﻣﻞ 100

Compliance Issues 1) Regulatory - GMP - Product - Safety 2) Business 3) Intellectual - Patents - Trademarks - Copyright ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ 101

Regulatory Project Management (1) Assessment of the regulatory information to develop and/or refine regulatory strategy. (2) Develop regulatory project plan budgets, schedules, regulatory milestones/go/no go decision points, filing strategies and timing, target contactdates with FDA/ES, GXP inspection preparation. (3) Strategies for meeting, ethics, submissions, approval and maintenance. (4) Compliance issues associated with business plans, product and strategies. ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

Regulatory Project Management (1) Resources (2) $, personnel, time, facility/expertise (3) Budgets (4) Schedules (5) Tasks & Subtasks (6) Responsibilities (7) Regulatory/ Miestones (8) GO/ no go decision points ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ
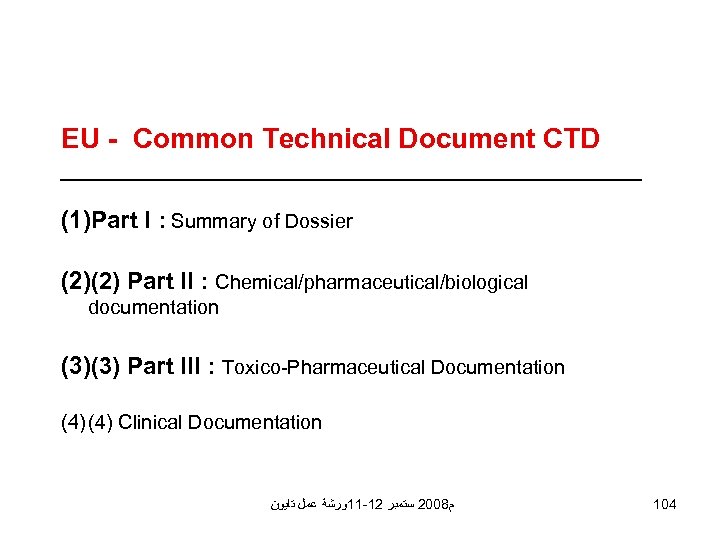
EU - Common Technical Document CTD (1)Part I : Summary of Dossier (2)(2) Part II : Chemical/pharmaceutical/biological documentation (3)(3) Part III : Toxico-Pharmaceutical Documentation (4) Clinical Documentation ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ 104
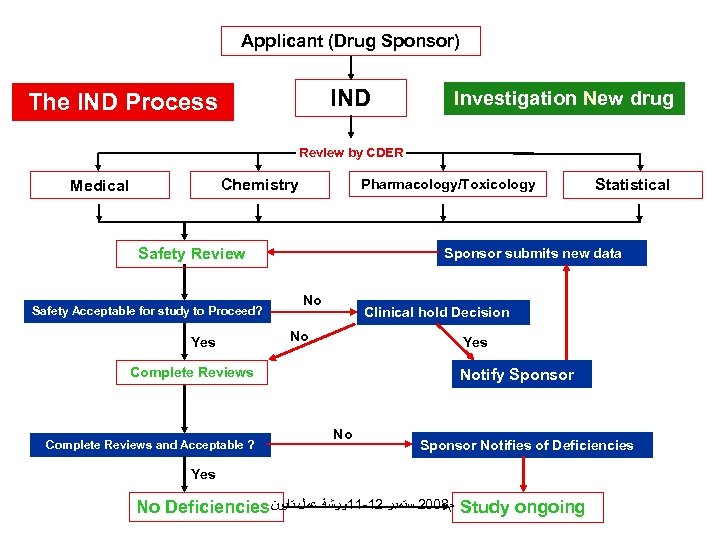
Applicant (Drug Sponsor) IND The IND Process Investigation New drug Review by CDER Chemistry Medical Pharmacology/Toxicology Safety Review Safety Acceptable for study to Proceed? Yes Sponsor submits new data No Clinical hold Decision No Yes Complete Reviews and Acceptable ? Statistical Notify Sponsor Notifies of Deficiencies Yes No Deficiencies ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ Study ongoing

Regulatory Milestones (1) Filing strategies and timing (2) Target contact dates with FDA/EU (3) GXP inspection preparation (4) Dossier submission (5) Application fees ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ
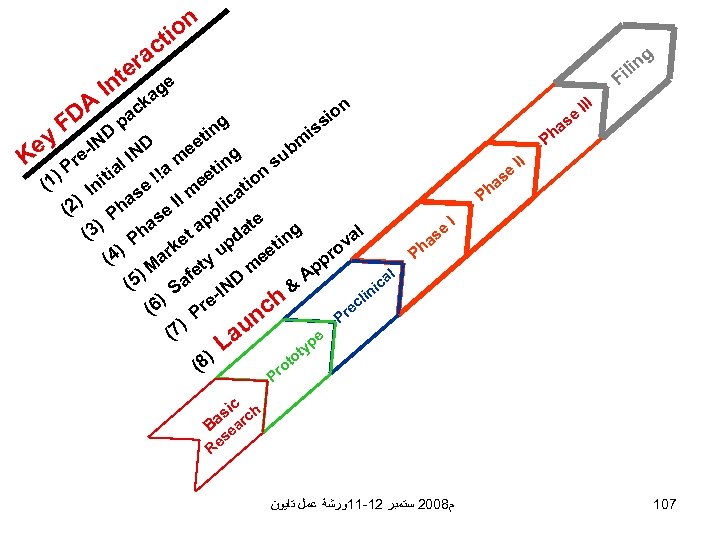
on ti ac r ey K DA F I te n D IN e- g n ili F e ag k ac p D IN g tin ee n io s m ub s g tin n i ee io (1 e t In I m lica ) as I (2 e Ph e pp ) as a at g 3 h ( al d P et in v k up eet ro 4) ar ( y M pp m et ) A al D af (5 ic & S IN in ) h ecl 6 e ( Pr Pr nc ) (7 e au L yp t to 8) ( ro Pr ) l tia !!a m e s ha is III P e as II Ph P e as h I P c si rch a B ea s Re ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ 107

Regulatory Strategies Pre-IND Meeting (1) Role in development strategy (2) Process: (1) Manufacturing details of API (2) Preclinical (3) CMC (4) MOA (5) GLP (6) Toxicology (3) Conduct ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ
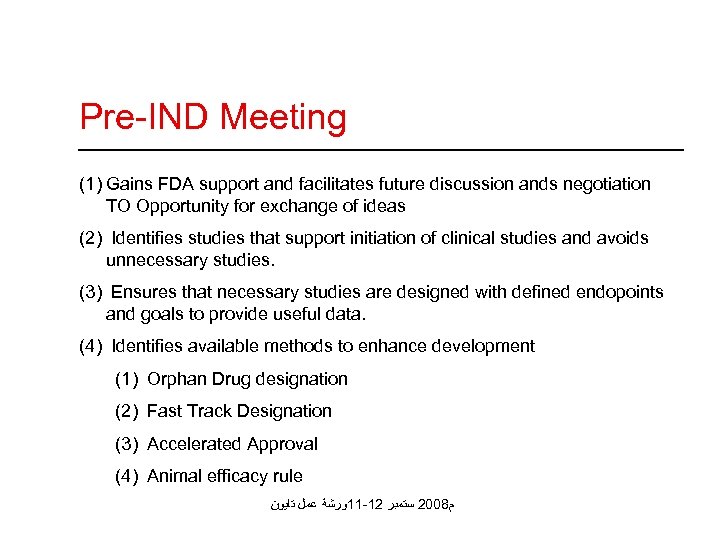
Pre-IND Meeting (1) Gains FDA support and facilitates future discussion ands negotiation TO Opportunity for exchange of ideas (2) Identifies studies that support initiation of clinical studies and avoids unnecessary studies. (3) Ensures that necessary studies are designed with defined endopoints and goals to provide useful data. (4) Identifies available methods to enhance development (1) Orphan Drug designation (2) Fast Track Designation (3) Accelerated Approval (4) Animal efficacy rule ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

Pre-IND Meeting Are Particularly Valuable if (1) Product for treatment of serious or life-threatening disease (2) Novel indication (3) No current guidance documents (4) Sponsor is new to drug development (5) Sponsor has quitions of FDA (6) Pharmacological, safety or toxicology causes for concern (7) Drug is new chemical entity ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ 110

Pre-IND Meeting The Process (1) Meeting objective (2) Proposed agenda (3) List of specific questions (arrange by discipline) (4) List of requested FDA participants (5) Quantitative composition of the drug/biologyic (6) Propsed indication (7) Dosing regimen (8) Proposed meeting date (6 -8 weeks in future) (9) Timing of submission package ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

Pre-IND Meeting Information Package Purpose (1) Background information on development concept (2) Information on the API (3) Initial preclinical and clinical strategy (4) Clear and concise development strategy (5) Allow FDA opportunity to comment ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

Pre-IND Meeting Information Package Content Time of animal studies and models (gets FDA “buy in”) (1) Overall summary (2) Primary and secondary pharmacology (3) Bio-distribution (ADME) (1) Starting dose and dose regimen (4) Preliminary toxicology – rationale (2) Safety monitoring based on target organ toxicities (5) Rationale for dose scheme (3) Pre-clinical (6) Clinical protocol synopsis for IND trial (7) Development plan (4) Mechanism of action (if known) (5) Mechanism of (8) CMC data for API and dosage – assays, breakdown/inactivation any issues deviating from the standard: (1) e. g. sterility issues for products applied to open wounds (6) Relevance of animal species (7) Immunogenicity (2) compliance to general monographs (8) Species specificity (3) impunity profiles (9) Potential adverse effects ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ 113

Pre-IND Meeting Information Package Content (6) Sources of toxicology data (1) Significant findings (animal deaths) a- GLP studies (2) GLP issues b- In-house & contract studies (3) Need for special evaluations eg. c- Published data (peer histopathology that requires tissue samples reviewed journals) (4) Long term studies eg. d- Cross-reference to similar Reproduction toxicity, approved, well-studied mutagenicity, carcinogenicity. (5) Excipients characterized. products e- Citation of approved additives (GRAS status) ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ 114

Pre-IND Meeting Information Package common Deficiencies (1)Inadequate CMC: (1)Insuficient pre-clinical support (2) Duration of studies (3) Dose selection of studies (4) Choice of species (2) Unacceptable clinical trial design (3) Non compliance with GXP’s (4) Lack of dose definition ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ 115

Pre-IND Meeting What happens in the meeting ? (1)Duration about 60 minutes (2)No formal sponsor presentation (3) Techinical/scientific personnel attend (avoid the CEO, lawyers, board members etc) (4) Discussion of submitted sponsor questions-FDA will not respond to items not in the briefing package (5) Sponsor commitment to FDA suggestions not mandatory ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ 116

IND – Form 1571 (1) Table of contents (2) Introductory Statement (3) General investigational plan (4) Investigator’s brochure (1) Study (2) Investigator data (3) Facilities data (4) IRB data (5) Chemistry, Manufacturing Control data (6) Pharmacology and Toxicology data (7) Previous human experience (8) Additional information ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ 117
Pre-IND Meeting Further Strategies (1)Consider a pre-IND if very early compound and/or with novel mechanism of action (2)Gain FDA support which can facilitate future discussion and negotiation New toolkits “The medical product development process has not kept with basic scientific innovation of drug discovery resulting in a disconnect between discovery and development” ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ 118
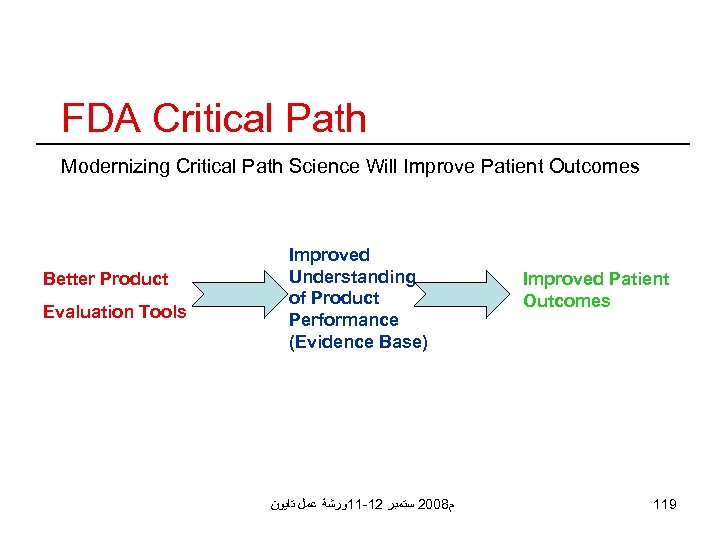
FDA Critical Path Modernizing Critical Path Science Will Improve Patient Outcomes Better Product Evaluation Tools Improved Understanding of Product Performance (Evidence Base) ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ Improved Patient Outcomes 119

Improving Development Sciences will Improve Satey: FDA View (1) eg. Markers that predict which patients are likely to respond positively to a product, the use of new safety biomarkers can translate rapidly from the experimental setting to the clinic. (2) Patients with a high probabillity of an adverse effect can be identified and their exposure avoided. (3) Inaddition, safety biomarkers could be used to monitor patients for emergence of toxicity during treatment, so that therapy can be stopped before harm has ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ occurred. 120

How to quicken pace (1) New aggressive collaborative effort to create new generation of performance standards and predictive tools for drug development (2) Build on knowledge with (1) Bio-information (2) Genomics (3) Imaging technologies (4) Materials science (3) Better science (4) Identify poor candidates as early as possible Better markers/models for : (1) Safety (2) Eficacy (3) Physicochemistry (4) Bioinformation (5) Better evaluation tools ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ 121

Regulatory Risks (1) Product Recall (2) Product complaints (3) AE’s & ADR’s (4) Product Failure in market (1) Quality (2) Safety (3) efficacy (5) TGA application failure ( Cat 1, Cat 3, notifications etc) (6) Breaches of “The Act”, regulations and schedules> ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ 122

GMP Audit Deficiencies (1) Consumer Safety issue (Identity, Purity, Strength, Safety) (2) Related to potential harm to the patient (3) Major: (4) May impact safety “related or indirect” (5) Non compliance with basic GMP principles (6) Non compliance with Registration details (7) Minor/Other: (8) General Housekeeping (9) Unlikly to impact product quality ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ 123

Pharmacovigilance (Drug Safety) What is Pharmacovigilance ? (1) The science and activities relating to the detection, assessment understanding, preventing of adverse effects or any other medicine related problem. (WHO) (EU Guide 9 A) (2) The purpose of the Vigilance system ( )ﻧﻈﺎﻡ ﺍﻟﺤﺬﺭ is to improve the health and safety of patients, users and others by reducing the likeihood of the same type of adverse incident being repeated in different places at different times. (3) (MEDDEV 2. 12 -1 rev 4) ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ 124
Pharmacovigilance Some definitions Adverse Event (AE); (ICH E 2 A) (1) “Any untoward medical occurrence in a patient or clinical investigation subject administered a pharmaceutical product and which does not necessarily have a causal relationship with this treatment” (2) “Any unfavorable and unintended sign (including an abnormal laboratory finding, for example), symptom, or disease temporally associated with the use of any dose of a medicinal product, whether or not considered related to the medication” Adverse Reaction (AR); (ICH E 2 A) “All noxious and unintended responses to a medicinal product related to any dose should be considered adverse drug reactions. This means that causal relationship between a medicinal product and an AE is at least a reasonable possibility, ie the relationship cannot be ruled out” ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ 125

Pharmacovigilance Serious Adverse Event (SAE) AND Serious Adverse Reaction (SAR); (ICH E 2 A) A serious adverse event (experience) or serious adverse reaction is any untoward medical occurrence that at any dose: Results in death Is life-threatening ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ 126

Pharmacovigilance is important ! (1) Clinical Trials can only go so far in proving safety and efficacy. (1) Relatively small numbers vs general population exposed (1) Ethnic, Racial factors eg. USA – European, Afro Americans, Hispanics, vs Australia (1) Other factors: Age, gender, health status, pregnancy …. etc. ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ 127

Why is important ! (1) many new drugs have only been tested in relatively small numbers of people before they come to market. (2) Phase I: 20 -80 people. Establish metabolism and tolerability. (3) Phase II: 100 -300 people with disease. Further safety data, preliminary evidence of efficacy effects, (4) Phase III: 1000 -3000 people with disease, further evidence of efficacy, monitors side effects, comparison with standard treatments. (5) Phase IV: Post Market – long term safety, optimal use, risks and benefits, different populations. ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ 128

Quality Problems May include: (1) Contamination, glass, insects, other foreign bodies. (2) Packaging problems eg. Sterile products, leaking caps. (3) Degraded product eg. Speckled tablets (4) Ineffective products, devices not performing correctly (5) Wrong counts, missed tablets in blisters (6) Injectables – wrong fill, wrong consistency, broken ampoules Principles of Quality Risk Management The evaluation of the risk to quality should ultimately link back to the protection of the patient ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ 129

Some definitions to keep in mind (ICH Q 9 – Draft Guidance – Quality Risk Management) • Definition of Risk: Combination of the probability of occurrence of harm and the severity of that harm (ISO/IEC Guide 51). • Severity : Measure of the possible consequences of a hazard. • Harm : Damage to health, including the damage that can occur from loss of product quality or availability. • ICH Q 9 : Statements “It is commonly understood that risk is defined as the combination of the probability of occurrence of harm and the severity of that harm” ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ 130

8 ﺍﻟﻤﺤﺎﺿﺮﺓ Emerging Trends as Development Drivers ﺍﻻﺗﺠﺎﻫﺎﺕ ﺍﻟﻤﻨﺒﺜﻘﺔ ﻣﺜﻞ ﻗﺎﺋﺪ ﺍﻟﺘﻄﻮﻳﺮ Summary: (1) Future perspectives (2) Emerging technology in drug discovery (3) Pro-drugs (4) Molecular targeting (5) Protein kinase inhibitors (6) Novel drug delivery systems (7) Novel clinical trial designs for Phase I and Phase II studies for targeted therapies (8) Changing face of patents in pharmaceutical development. ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ 131

ﻣﻨﻈﻮﻣﻴﺔ ﻣﺴﺘﻘﺒﻠﻴﺔ Future Perspective ﺃﻤﺮﺍﺽ ﻛﺒﺎﺭ ﺍﻟﺴﻦ ﻭﺍﻟﺸﻴﺨﻮﺧﺔ Old Age Diseases & Aging ﺍﻟﺘﻮﺗﺮ ﺍﻟﻌﺼﺒﻲ (1) Hypertension ﺍﻟﺴﻜﺘﺔ ﺍﻟﺪﻣﺎﻏﻴﺔ ﺃﻮ ﺍﻟﻘﻠﺒﻴﺔ (2) Stroke (3) Alzheimer’s disease ﻣﺮﺽ ﻓﻘﺪﺍﻥ ﺍﻟﺬﺍﻛﺮﺓ ﺍﻟﺒﻮﺍﻝ ﺍﻟﺴﻜﺮﻱ ﻫﺸﺎﺷﺔ ﺍﻟﻌﻈﺎﻡ 231 (4) Diabetes (5) Osteoporosis ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

Future Perspectives (Life style Drugs) ( ﻣﻨﻈﻮﺭ ﻣﺴﺘﻘﺒﻠﻲ )ﺃﺪﻭﻳﺔ ﻃﺮﺍﺯ ﺍﻟﺤﻴﺎﺓ - ﺃﺴﻠﻮﺏ ﺍﻟﺤﻴﺎﺓ - ﺍﻟﻤﺴﺘﻘﺒﻠﻲ (1) Obesity treatment (Xenical) (2) Aging: enhance muscular tone and youthful vitality (Hormones) (3) Aging: anti-wrinkles (Botox – Vitamin A) (4) Memory enhancement ( Tacrine – Donepezil) (5) Sexual dysfunction (Viagra) (6) Smoking cessation (Zyban) (7) Hair loss therapy (Finasteride) ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ 133

Emerging Technologies - In Drug Discovery ﺍﻧﺒﺜﺎﻕ ﺃﻮ ﻇﻬﻮﺭ ﺍﻟﺘﻘﻨﻴﺎﺕ ﺍﻟﺤﺪﻳﺜﺔ (1) Development of cell and molecular biology in drug targeting. (2) Recombinant DNA technology. (3) Genomics. (4) Proteomics. (5) Bio 0 and chemical informatics. (6) Laboratory equipment and automation (HTS). ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ 134

Future Perspectives (Life style Drugs) ( ﻣﻨﻈﻮﺭ ﻣﺴﺘﻘﺒﻠﻲ )ﺃﺪﻭﻳﺔ ﻃﺮﺍﺯ ﺍﻟﺤﻴﺎﺓ - ﺃﺴﻠﻮﺏ ﺍﻟﺤﻴﺎﺓ - ﺍﻟﻤﺴﺘﻘﺒﻠﻲ (1) Obesity treatment (Xenical) (2) Aging: enhance muscular tone and youthful vitality (Hormones) (3) Aging: anti-wrinkles (Botox – Vitamin A) (4) Memory enhancement ( Tacrine – Donepezil) (5) Sexual dysfunction (Viagra) (6) Smoking cessation (Zyban) (7) Hair loss therapy (Finasteride) ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ 135

Individualized Medicine ﺍﻟﺘﺨﺼﻴﺺ ﺍﻟﻄﺒﻲ (1) Tailor made for individual and adapted to each person’s own genetic make-up. (2) Minimize and/or eliminate AE’s. (3) Blood sample analyzed study of single nucleotide polymorphisms and pharmacogenomics responsible gene treatment ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ 136
Emerging Technologies - RNA interference ﺍﻧﺒﺜﺎﻕ ﺃﻮ ﻇﻬﻮﺭ ﺍﻟﺘﻘﻨﻴﺎﺕ ﺍﻟﺤﺪﻳﺜﺔ (1) Ability to affect gene expression in a specific manner. (2) Surrogate for pharmacological knockdown of protein activity. (3) Application in: (1) Aid in target identification & validation. (2) Establishment of mechanism-based cellular models. (3) Proof in principle experiments. (4) HIV therapy (identification of viral budding). ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ 137

Emerging Technologies - Gene Therapy ﺍﻧﺒﺜﺎﻕ ﺃﻮ ﻇﻬﻮﺭ ﺍﻟﺘﻘﻨﻴﺎﺕ ﺍﻟﺤﺪﻳﺜﺔ (1) Supply healthy genes to replace those that are missing or flowed. (2) Success factors require (1) Transport vectors (2) Understanding of gene effect and function (3) Ethical questions (who decides? ) (4) Used for: (1) Sickle cell anaemia (2) Cystic fibrosis (3) Familial hyper-cholesterol-aemia (4) SCID “baby bubble” syndrome/ ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ 138

Pro-drugs - Bioprecursors ﺍﻷﺪﻭﻳﺔ ﺍﻟﻤﺮﺗﺒﻄﺔ Also in slide 56 Lecture 4 & In book page 44 / 4 Molecular modification of active principle • New compound whick is a substrate for metabolizing enzymes. • Active metabolite. Examples include: • Sulindac • Fenbufen • Acylovir • Losartan • Salicin & salicylic acid ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ 139
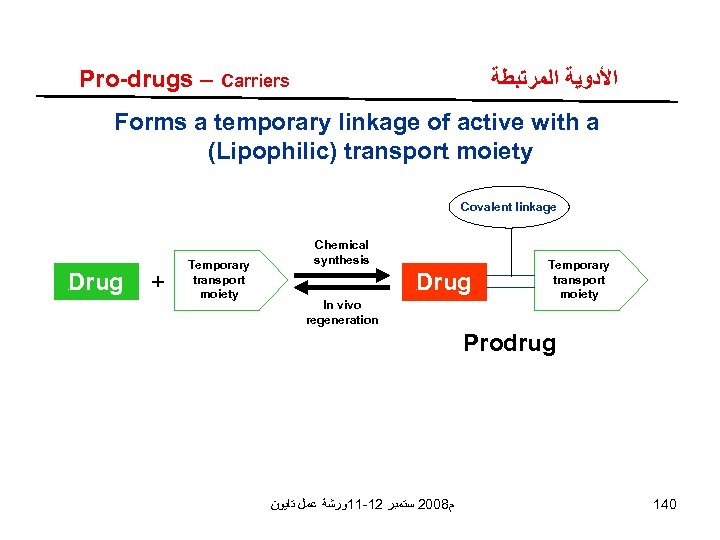
Pro-drugs – Carriers ﺍﻷﺪﻭﻳﺔ ﺍﻟﻤﺮﺗﺒﻄﺔ Forms a temporary linkage of active with a (Lipophilic) transport moiety Covalent linkage Drug + Temporary transport moiety Chemical synthesis Drug In vivo regeneration Temporary transport moiety Prodrug ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ 140

Pro-drugs - ﺍﻷﺪﻭﻳﺔ ﺍﻟﻤﺮﺗﺒﻄﺔ Carriers – criteria for design (1) Linkage between drug and carrier is usually covalent bond. (2) Produrg is inactive or less active than parent. (3) Linkage must be broken in vivo. (4) Prodrug and parent drug must be non toxic (5) Generation of active must have rapid kinetics. ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ 141
Changing Face of Patents ﺗﻐﻴﻴﺮ ﻭﺟﻬﺔ ﻧﻈﺮ ﺑﺮﺍﺀﺍﺕ ﺍﻻﺧﺘﺮﺍﻉ In Pharmaceutical development (1) Prozac (Eli Lilly), sales in first half 2001 (2) US 1. 3 B US 380 M within 12 month of ptent expiry and advent of generics (3) Patent strategies for patented drugs (1) New claims. (2) Reformulations (omeprazole subcoat). (3) Isolation of effective enantiomer (eg omeprazole and esoprazole). ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ 142

Lifecycle Management ﺍﺩﺍﺭﺓ ﺩﻭﺭﺓ ﺍﻟﺤﻴﺎﺓ Strategies (1) Branding (2) Product support (3) Trade relationships (4) Manufacturing cost advantage (5) Product improvement (6) Product line extensions ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ 143
Changing Face of Patents ﺗﻐﻴﻴﺮ ﻭﺟﻬﺔ ﻧﻈﺮ ﺑﺮﺍﺀﺍﺕ ﺍﻻﺧﺘﺮﺍﻉ In Generic Pharmaceutical development (1) Benefits of the latter three strategies are significantly improved if patent protection is obtained to monopolise the result of the innovator’s research and development efforts. (2) Data exclusivity provisions seldom offer useful protection of research conducted to provide the basis for product improvements. Such strategies would therefore be too risky in terms of the financial return on the investment if reliant on data exclusivity alone. (3) Patent monopoly is far more effective than simply preventing a third party from referencing data, as the data can be easily reproduced if found to be approvable. (4) Costs of challenging patents are very restrictive to many generic competitors. ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ 144

Patents For the majority of pharmaceutical products still under patent in major territories, there appears to be at least one patent-related obstacle laid down by the innovator that must either be challenged or circumvented to enable launch of an equivalent generic product. ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ 145

Changing Face of Patents ﺗﻐﻴﻴﺮ ﻭﺟﻬﺔ ﻧﻈﺮ ﺑﺮﺍﺀﺍﺕ ﺍﻻﺧﺘﺮﺍﻉ In Generic Pharmaceutical development (1) In the past, generics manufacturers have to wait until one patent expired – that protecting the active ingredient patent. In addition to the chemical compound per se, this patent would usually disclose at least one month of synthesis and any potential uses, as well as some formulations for its administration. (2) Innovator companies have taken advantage of the opportunity to patent almost any improvement to the basic information provided in the molecule patent, including: polymorphic forms, salts, hydrates, reagents, reaction, conditions, catalysis, purification methods, assay techniques, formulations, excipients, packaging, routes of administration, dosing regimen, and new medical use. ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ 146
Changing Face of Patents ﺗﻐﻴﻴﺮ ﻭﺟﻬﺔ ﻧﻈﺮ ﺑﺮﺍﺀﺍﺕ ﺍﻻﺧﺘﺮﺍﻉ In Generic Pharmaceutical development (3)Although the number of patent challenges mounnted by generic companies to remove these obstacles is on the increase, a more strategic approach is being adopted by generic firms themselves in filing for patents to protect their own research and development efforts aimed at circumventing the innovator patents. (4)Athough the lifecycle management strategy of protecting improvement to products. Their use and their manufacture results in patent protection for the innovator. (5)The problem is significantly worsended by the response of generic competitors, (6) To the extent that each and every one requires substantive patent information, management practices and expertise in dealing with patent strategy high volume, low quality of generic patent applications with broad claims ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ 147

Changing Face of Patents ﺗﻐﻴﻴﺮ ﻭﺟﻬﺔ ﻧﻈﺮ ﺑﺮﺍﺀﺍﺕ ﺍﻻﺧﺘﺮﺍﻉ In Generic Pharmaceutical development The more strategic generic companies are seeking comprehensive patent information as the first stage in the development process, and are doing so very early on in the innovator products life cycle. ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ 148

941 ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ

ﻃﺮﻕ ﺗﻨﺎﻭﻝ ﺍﻟﺪﻭﺍﺀ: )1( ﻋﻦ ﻃﺮﻳﻖ ﺍﻟﻮﺟﻪ ﺑﺼﺮﻱ Ocular Oral ﻓﻢ ﺃﻨﻒ ﺃﺬﻥ )2( ﻋﻦ ﻃﺮﻳﻖ ﻣﻨﺎﻃﻖ ﺍﻹﺧﺮﺍﺝ Vaginal ﻣﻬﺒﻞ Rectal ﻣﺴﺘﻘﻴﻢ ﻟﺒﻮﺱ )3( ﻋﻦ ﻃﺮﻳﻖ ﺍﻟﺠﻠﺪ ﺣﻘﻦ ﻓﻲ ﺍﻟﻮﺭﻳﺪ ﺩﻫﺎﻥ – ﻣﺮﻫﻢ ﻡ 8002 ﺳﺘﻤﺒﺮ 21 -11ﻭﺭﺷﺔ ﻋﻤﻞ ﺗﺎﻳﻮﻥ
a170e2b53b02e227787bb14d5d849203.ppt