e590364acc8742f313a72258f1f88445.ppt
- Количество слайдов: 16

혈액응고 검사의 정도관리 혈액학계 윤미경 2003. 3. 6
![Check Points [sources of error] Preanalytical variables specimen Testing variables instrument, Reagent, Control material Check Points [sources of error] Preanalytical variables specimen Testing variables instrument, Reagent, Control material](https://present5.com/presentation/e590364acc8742f313a72258f1f88445/image-2.jpg)
Check Points [sources of error] Preanalytical variables specimen Testing variables instrument, Reagent, Control material standard material, Methodology QC program Patient reports/interpretation
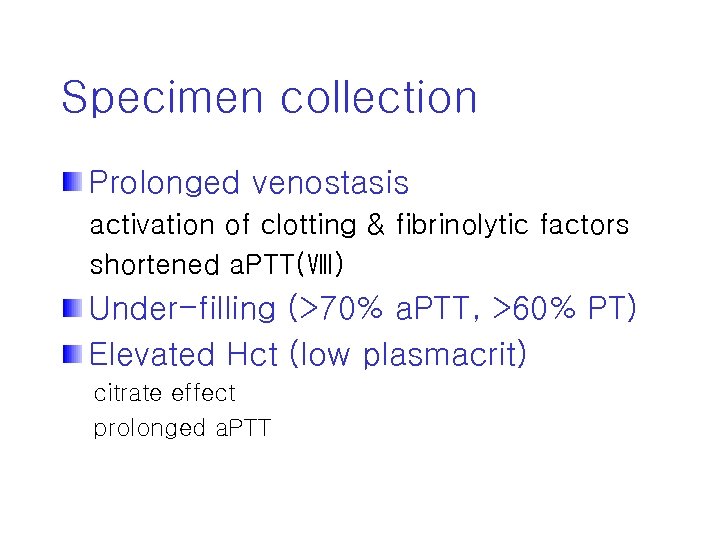
Specimen collection Prolonged venostasis activation of clotting & fibrinolytic factors shortened a. PTT(Ⅷ) Under-filling (>70% a. PTT, >60% PT) Elevated Hct (low plasmacrit) citrate effect prolonged a. PTT

Specimen collection Insufficient mixing micro-clots or fibrin strand (plasma) clotting factor consumption Excessive mixing hemolysis, clumps(preactivated) clotting factor activation
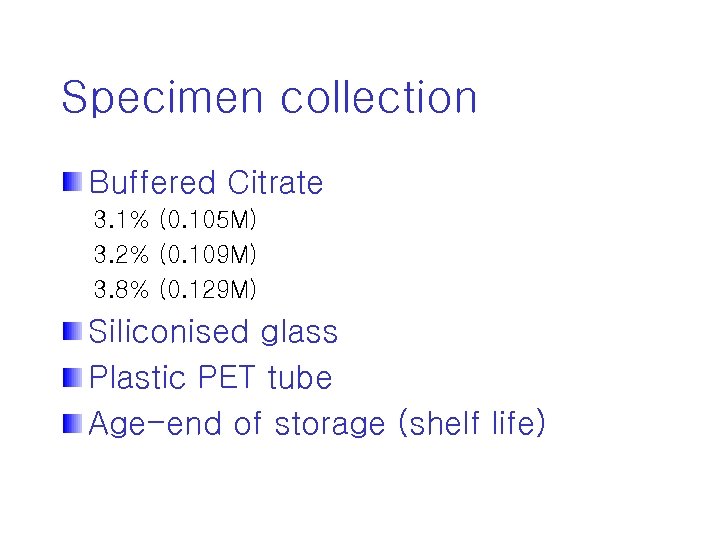
Specimen collection Buffered Citrate 3. 1% (0. 105 M) 3. 2% (0. 109 M) 3. 8% (0. 129 M) Siliconised glass Plastic PET tube Age-end of storage (shelf life)

Specimen collection (경험) Patient samples with heparin therapy : No prolongations of a. PTT Repeat with old collection tube(BD) Repeat with new collection tube(BD) Expiry date of evacuated tubes Rubber stopper-neutralize heparin
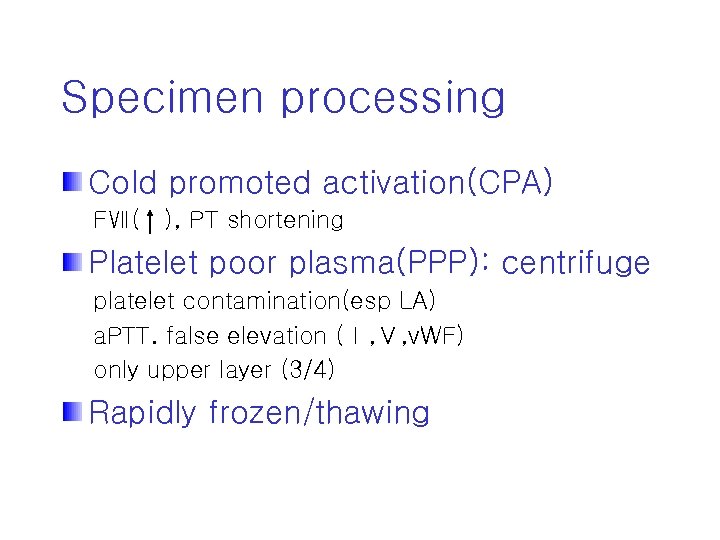
Specimen processing Cold promoted activation(CPA) FⅦ( ), PT shortening Platelet poor plasma(PPP): centrifuge platelet contamination(esp LA) a. PTT. false elevation (Ⅰ, Ⅴ, v. WF) only upper layer (3/4) Rapidly frozen/thawing
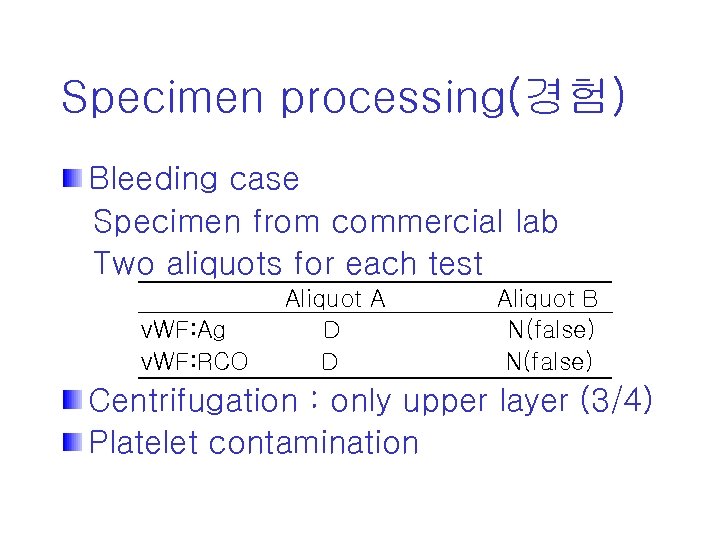
Specimen processing(경험) Bleeding case Specimen from commercial lab Two aliquots for each test v. WF: Ag v. WF: RCO Aliquot A D D Aliquot B N(false) Centrifugation : only upper layer (3/4) Platelet contamination
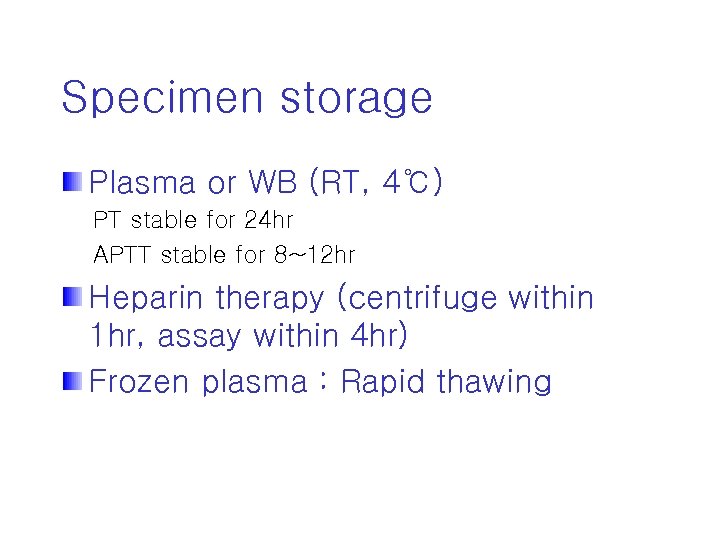
Specimen storage Plasma or WB (RT, 4℃) PT stable for 24 hr APTT stable for 8~12 hr Heparin therapy (centrifuge within 1 hr, assay within 4 hr) Frozen plasma : Rapid thawing
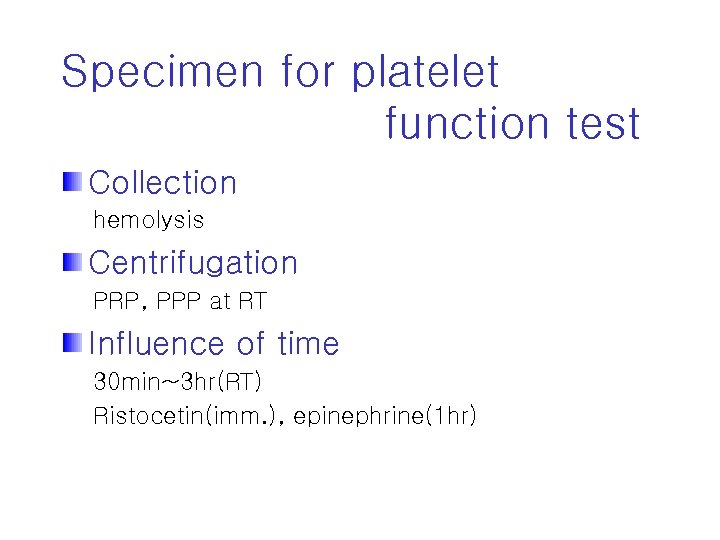
Specimen for platelet function test Collection hemolysis Centrifugation PRP, PPP at RT Influence of time 30 min~3 hr(RT) Ristocetin(imm. ), epinephrine(1 hr)

Instrument (ACL, Futura) Methodology semiautomated (electro-mechanical) automated (photo-optical) Reagent systems Economic factors Test volume Computer interface Technologist acceptance

Instrument (경험) DIC (activated clotting factor) Short a. PTT from ACL(IL) coagulyzer Clot formation : shorter than lag phase No change in light transmission Artifactual prolongation mimicking coagulopathy Visual confirmation (clot)
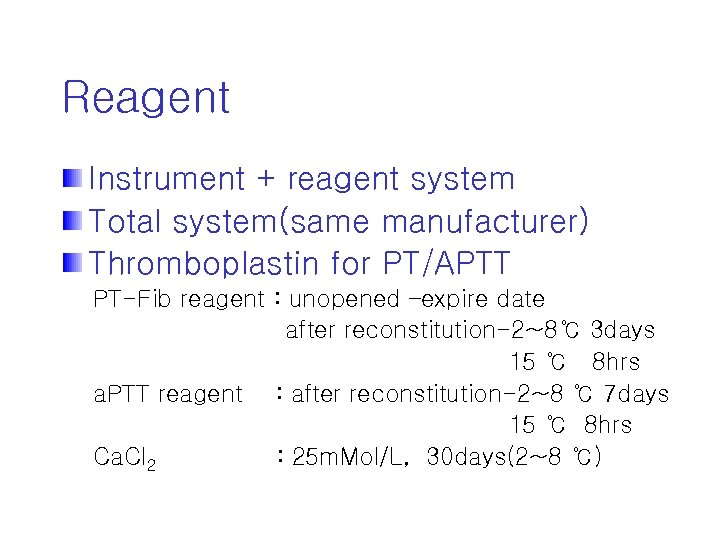
Reagent Instrument + reagent system Total system(same manufacturer) Thromboplastin for PT/APTT PT-Fib reagent : unopened –expire date after reconstitution-2~8℃ 3 days 15 ℃ 8 hrs a. PTT reagent : after reconstitution-2~8 ℃ 7 days 15 ℃ 8 hrs Ca. Cl 2 : 25 m. Mol/L, 30 days(2~8 ℃)
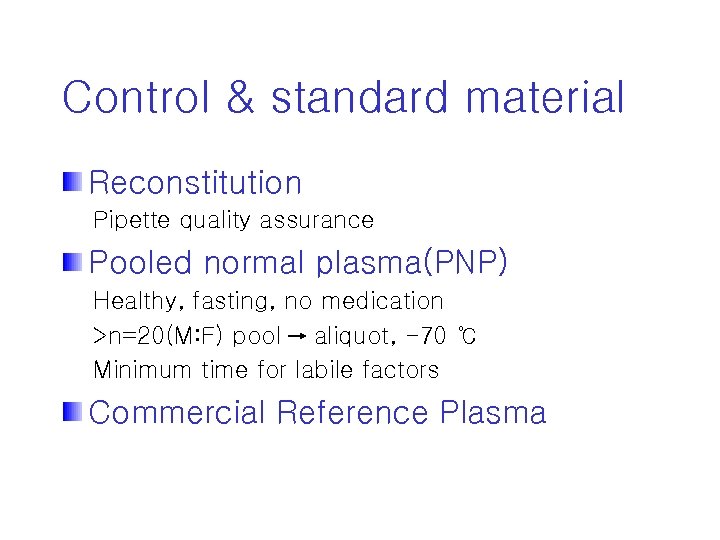
Control & standard material Reconstitution Pipette quality assurance Pooled normal plasma(PNP) Healthy, fasting, no medication >n=20(M: F) pool aliquot, -70 ℃ Minimum time for labile factors Commercial Reference Plasma

QC Program Reporting & Results Delta check Repeat if abnormal (not routine)

Conclusion Awareness interpretative medical knowledge possibility-open coexist or superimposed acquired > congenital Customize to each patient age, sex, clinical conditions 치료(약제, 마취제, 수혈) Future : near patient testing(POCT)
e590364acc8742f313a72258f1f88445.ppt