28f83bdd9d72f1bdaa2fdda075af0abc.ppt
- Количество слайдов: 45

微生物遺傳與生物技術 (Microbial Genetics and Biotechnology) 金門大學 食品科學系 何國傑 教授

Bacterial gene expression and its regulation
I. Terminology 1. Gene: DNA region that carry the information for synthesis of RNA or RNA and protein. 2. Codon: each 3 nucleotides on DNA or RNA. 3. Genetic code: The assignment of each of the possible codons to amino acids. 4. The first step in gene expression is to transcribe (or copy) an RNA from one strand of DNA. 5. Types of RNA: There are many different types of RNA in cells. The major three type are m. RNA (messenger RNA), r. RNA (ribosomal RNA) and t. RNA (transfer RNA). (1) m. RNA – The RNA carries the gene’s messenger (or information) for protein. (2) r. RNA – RNA component of a ribosome. (3) t. RNA – The RNA carries an amino acid to a codon on m. RNA.
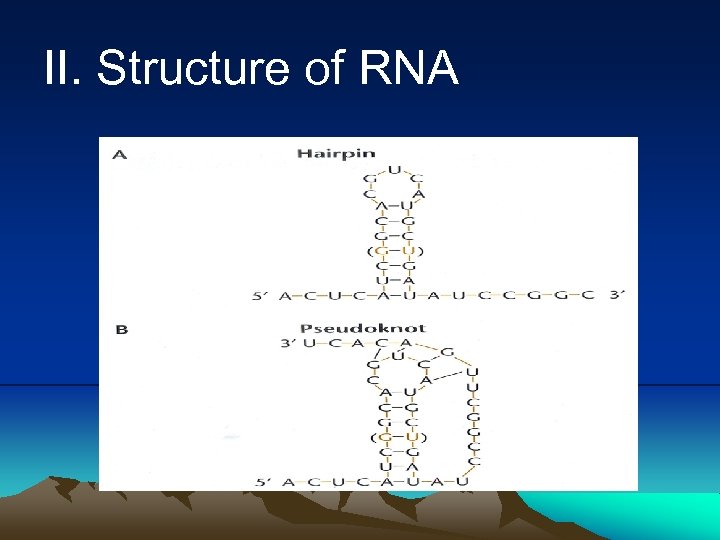
II. Structure of RNA 1. RNA is similar to DNA in that it is composed of a chain of nucleotides 2. Nucleotides of RNA contain the sugar ribose instead of deoxyribose. The second carbon of ribose is attached to a hydroxyl group rather than hydrogen in deoxyribose. 3. RNA has uracil instead of thymine found in DNA. 4. RNA is usually single stranded. However, a secondary structure can form by pairing between the bases in some regions of molecule may cause it to fold up on itself to form double-stranded region. 5. The rule for double-stranded RNA is slightly different from pairing rule for DNA. In RNA G can pair with U as well as C. GU does not share hydrogen bond, it does not contribute to the stability of the double-stranded RNA.
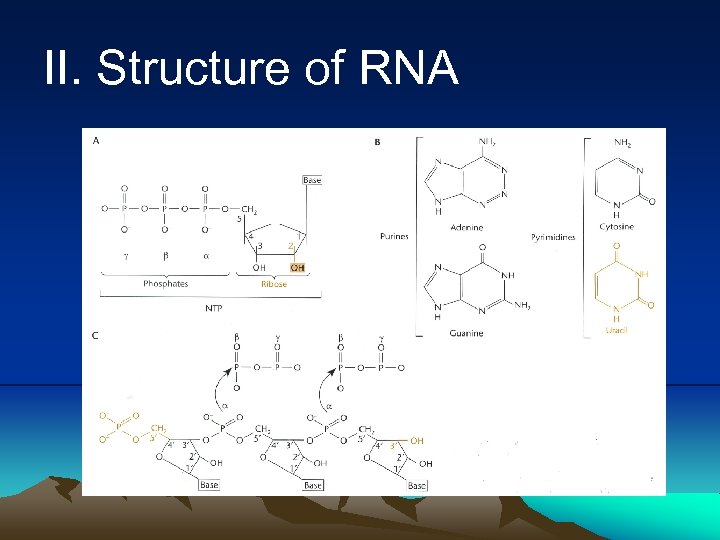
II. Structure of RNA
II. Structure of RNA 6. RNA can form tertiary structure when the unpaired region in a hairpin pairs with another region of the same RNA molecule to form a knot, a pseudoknot. 7. RNA processing and modification (1) In RNA processing, the covalent bonds can be broken and the smaller pieces of RNA can be religated into new recombination. One of the most extreme cases of RNA processing, called RNA editing, the nucleotides can be excised or added to m. RNA after it has been from DNA. (2) RNA modification involved altering the bases or sugar of RNA. For example, the methylation of bases and sugars.
II. Structure of RNA
II. Structure of RNA Ψ: pseudouracil
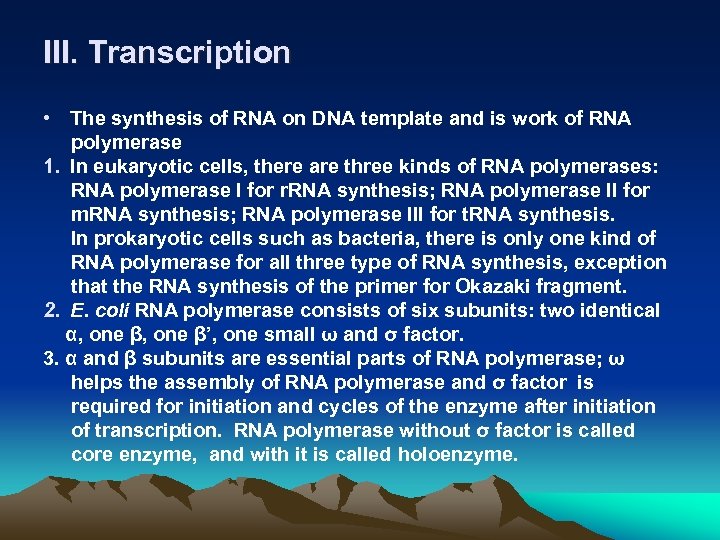
III. Transcription • The synthesis of RNA on DNA template and is work of RNA polymerase 1. In eukaryotic cells, there are three kinds of RNA polymerases: RNA polymerase I for r. RNA synthesis; RNA polymerase II for m. RNA synthesis; RNA polymerase III for t. RNA synthesis. In prokaryotic cells such as bacteria, there is only one kind of RNA polymerase for all three type of RNA synthesis, exception that the RNA synthesis of the primer for Okazaki fragment. 2. E. coli RNA polymerase consists of six subunits: two identical α, one β’, one small ω and σ factor. 3. α and β subunits are essential parts of RNA polymerase; ω helps the assembly of RNA polymerase and σ factor is required for initiation and cycles of the enzyme after initiation of transcription. RNA polymerase without σ factor is called core enzyme, and with it is called holoenzyme.
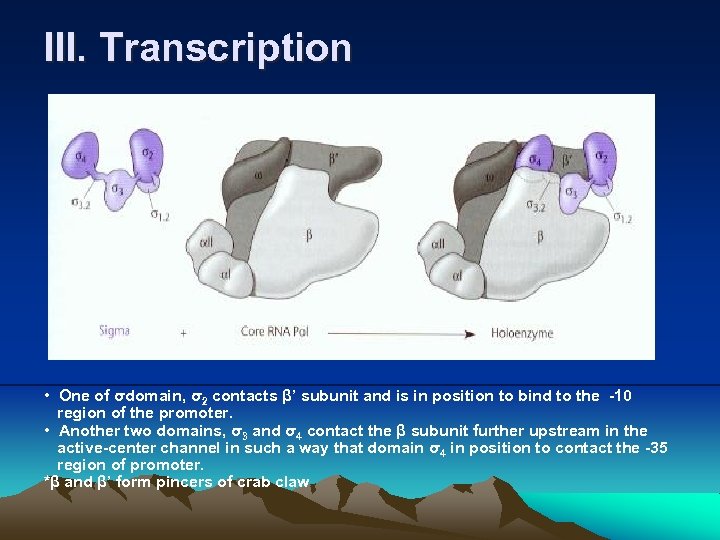
III. Transcription • One of σdomain, σ2 contacts β’ subunit and is in position to bind to the -10 region of the promoter. • Another two domains, σ3 and σ4 contact the β subunit further upstream in the active-center channel in such a way that domain σ4 in position to contact the -35 region of promoter. *β and β’ form pincers of crab claw
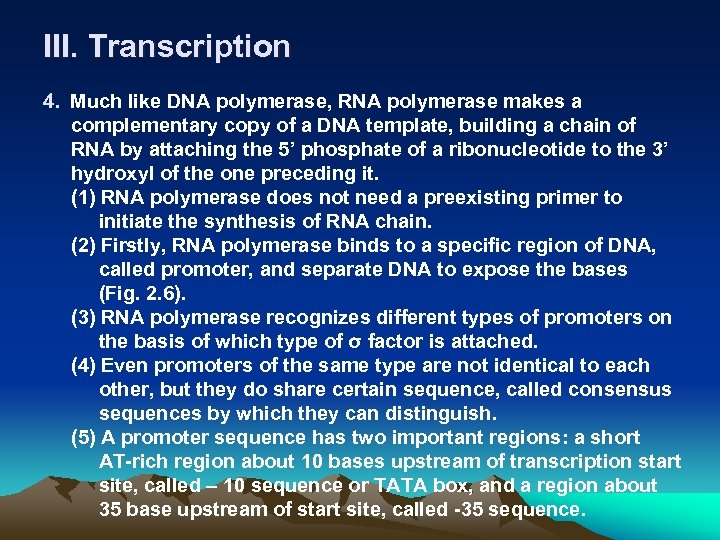
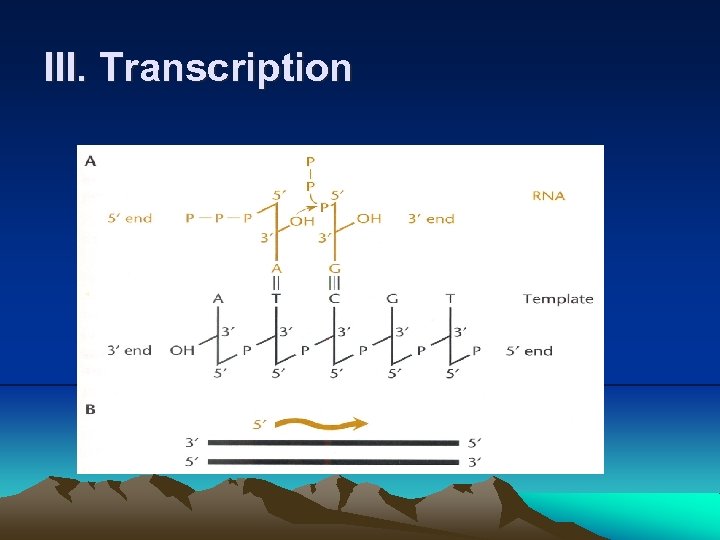
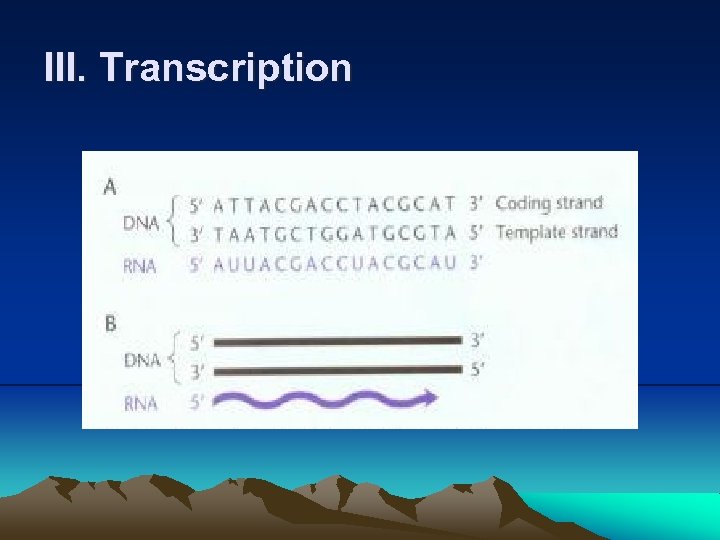
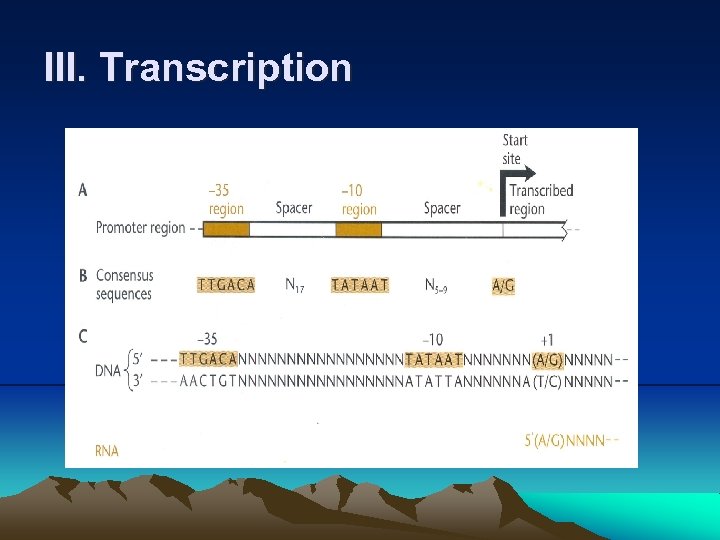
III. Transcription 4. Much like DNA polymerase, RNA polymerase makes a complementary copy of a DNA template, building a chain of RNA by attaching the 5’ phosphate of a ribonucleotide to the 3’ hydroxyl of the one preceding it. (1) RNA polymerase does not need a preexisting primer to initiate the synthesis of RNA chain. (2) Firstly, RNA polymerase binds to a specific region of DNA, called promoter, and separate DNA to expose the bases (Fig. 2. 6). (3) RNA polymerase recognizes different types of promoters on the basis of which type of σ factor is attached. (4) Even promoters of the same type are not identical to each other, but they do share certain sequence, called consensus sequences by which they can distinguish. (5) A promoter sequence has two important regions: a short AT-rich region about 10 bases upstream of transcription start site, called – 10 sequence or TATA box, and a region about 35 base upstream of start site, called -35 sequence.
III. Transcription
III. Transcription
III. Transcription
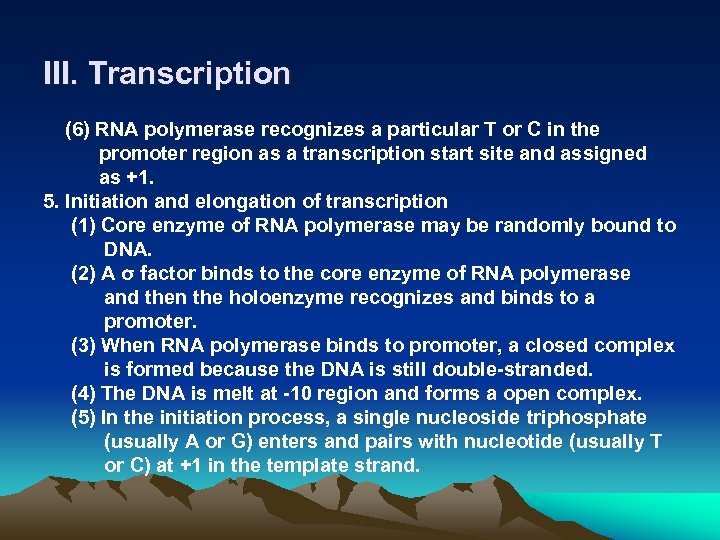
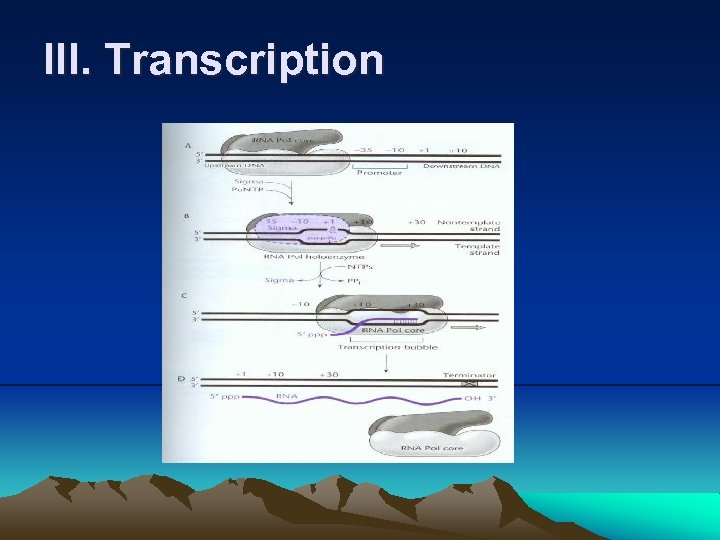
III. Transcription (6) RNA polymerase recognizes a particular T or C in the promoter region as a transcription start site and assigned as +1. 5. Initiation and elongation of transcription (1) Core enzyme of RNA polymerase may be randomly bound to DNA. (2) A σ factor binds to the core enzyme of RNA polymerase and then the holoenzyme recognizes and binds to a promoter. (3) When RNA polymerase binds to promoter, a closed complex is formed because the DNA is still double-stranded. (4) The DNA is melt at -10 region and forms a open complex. (5) In the initiation process, a single nucleoside triphosphate (usually A or G) enters and pairs with nucleotide (usually T or C) at +1 in the template strand.
III. Transcription
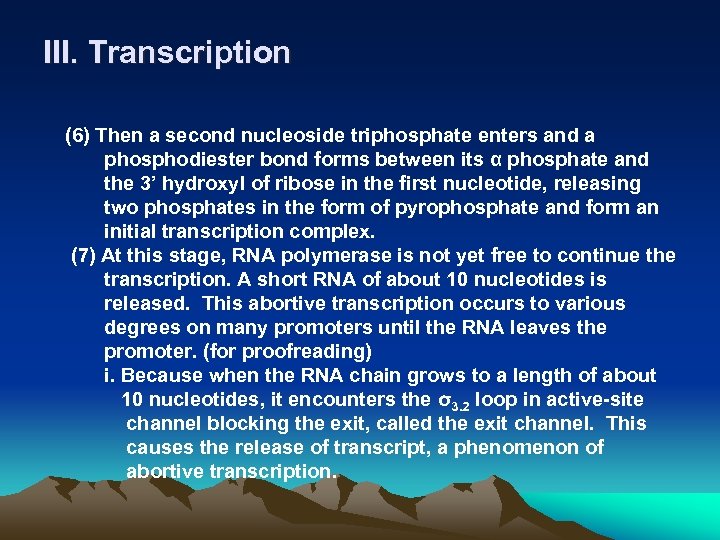
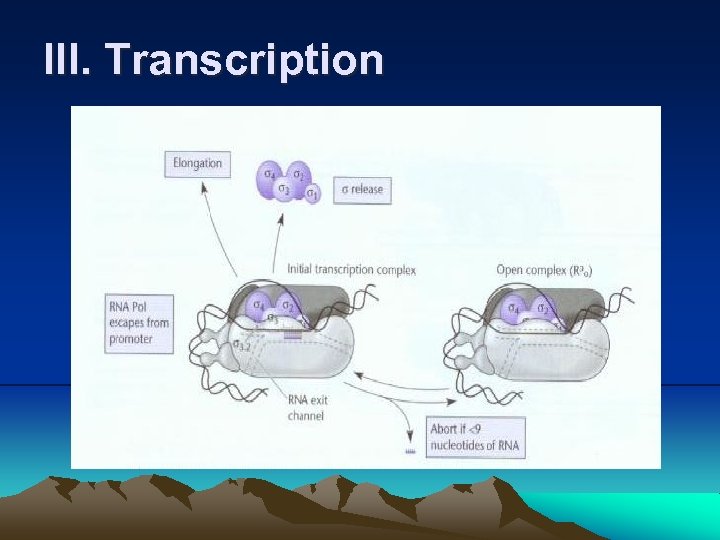
III. Transcription (6) Then a second nucleoside triphosphate enters and a phosphodiester bond forms between its α phosphate and the 3’ hydroxyl of ribose in the first nucleotide, releasing two phosphates in the form of pyrophosphate and form an initial transcription complex. (7) At this stage, RNA polymerase is not yet free to continue the transcription. A short RNA of about 10 nucleotides is released. This abortive transcription occurs to various degrees on many promoters until the RNA leaves the promoter. (for proofreading) i. Because when the RNA chain grows to a length of about 10 nucleotides, it encounters the σ3. 2 loop in active-site channel blocking the exit, called the exit channel. This causes the release of transcript, a phenomenon of abortive transcription.
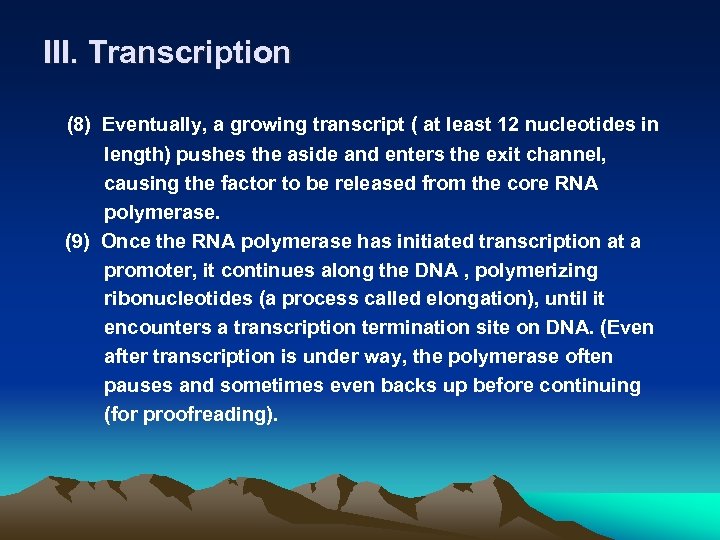
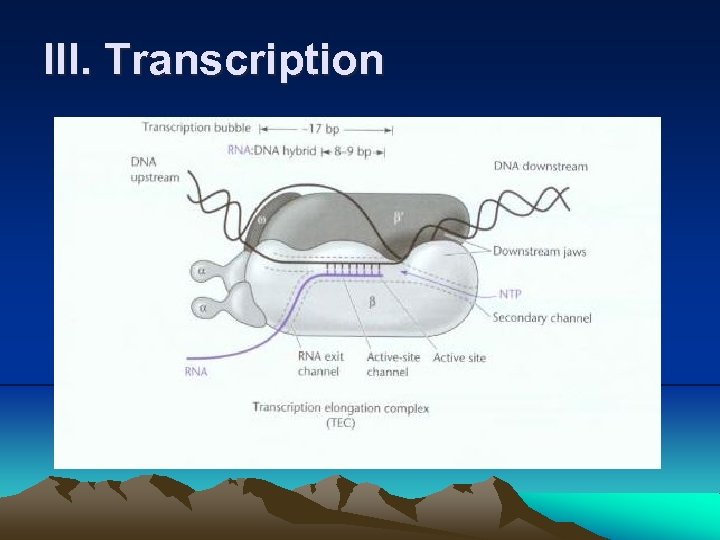
III. Transcription (8) Eventually, a growing transcript ( at least 12 nucleotides in length) pushes the aside and enters the exit channel, causing the factor to be released from the core RNA polymerase. (9) Once the RNA polymerase has initiated transcription at a promoter, it continues along the DNA , polymerizing ribonucleotides (a process called elongation), until it encounters a transcription termination site on DNA. (Even after transcription is under way, the polymerase often pauses and sometimes even backs up before continuing (for proofreading).
III. Transcription
III. Transcription
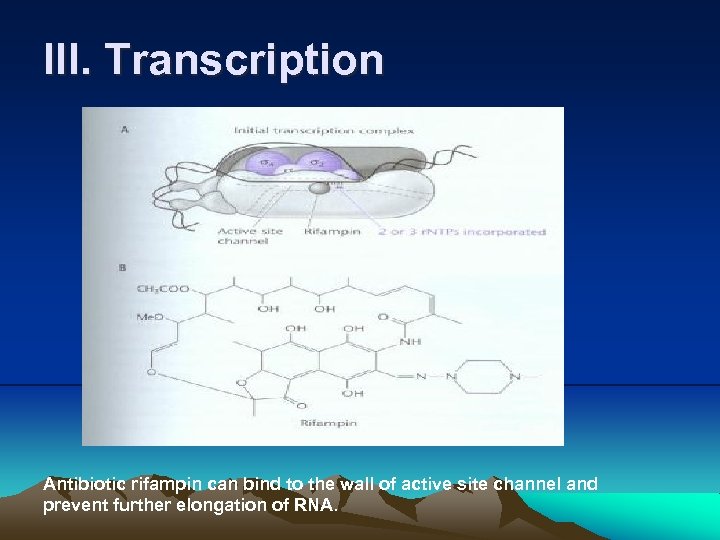
III. Transcription Antibiotic rifampin can bind to the wall of active site channel and prevent further elongation of RNA.
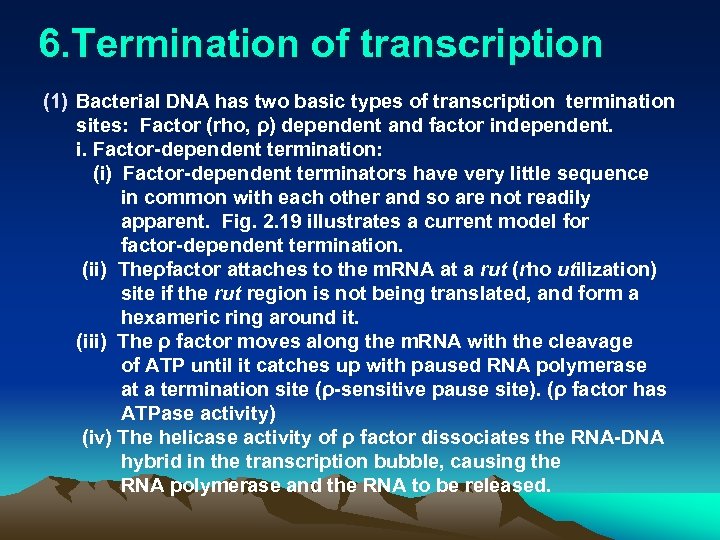
6. Termination of transcription (1) Bacterial DNA has two basic types of transcription termination sites: Factor (rho, ρ) dependent and factor independent. i. Factor-dependent termination: (i) Factor-dependent terminators have very little sequence in common with each other and so are not readily apparent. Fig. 2. 19 illustrates a current model for factor-dependent termination. (ii) Theρfactor attaches to the m. RNA at a rut (rho utilization) site if the rut region is not being translated, and form a hexameric ring around it. (iii) The ρ factor moves along the m. RNA with the cleavage of ATP until it catches up with paused RNA polymerase at a termination site (ρ-sensitive pause site). (ρ factor has ATPase activity) (iv) The helicase activity of ρ factor dissociates the RNA-DNA hybrid in the transcription bubble, causing the RNA polymerase and the RNA to be released.
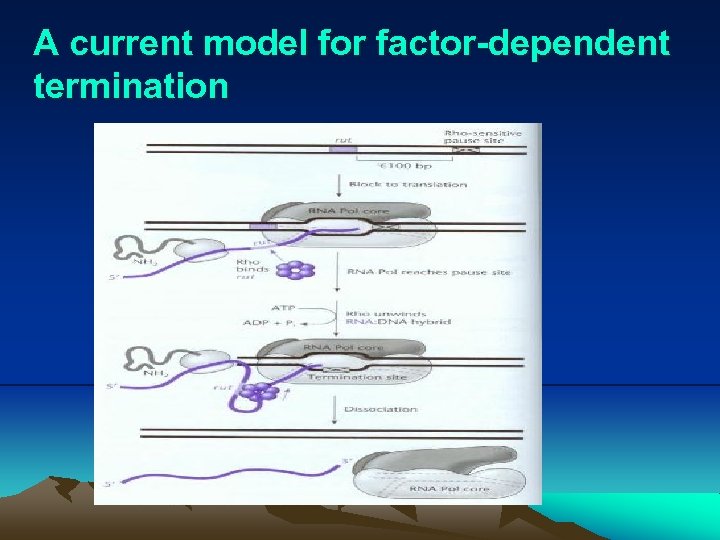
A current model for factor-dependent termination
6. Termination of transcription ii. Factor-independent termination: (i) Factor-independent terminators typically consist an inverted repeat (GC-rich) followed by a short string of A’s, usually 6 residues. (ii) Fig. 2. 19 illustrates a current model for factorindependent termination. (iii) The U-rich RNA causes RNA polymerase to pause, allowing a hairpin loop to form, dissociating RNA polymerase and RNA.
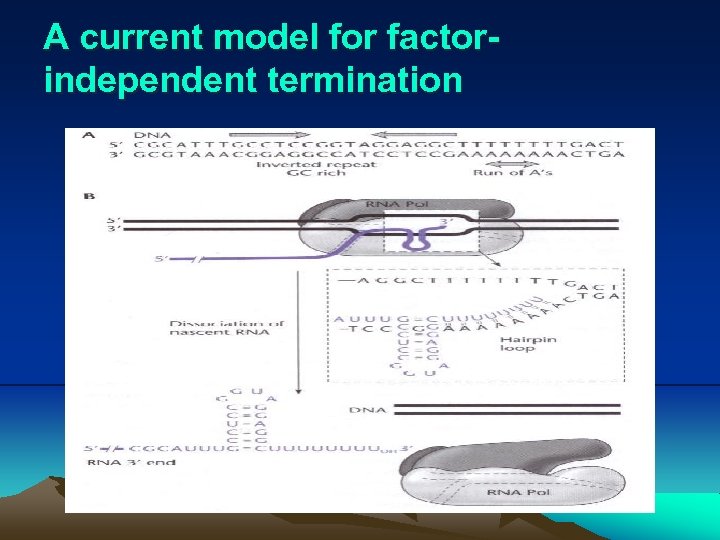
A current model for factorindependent termination
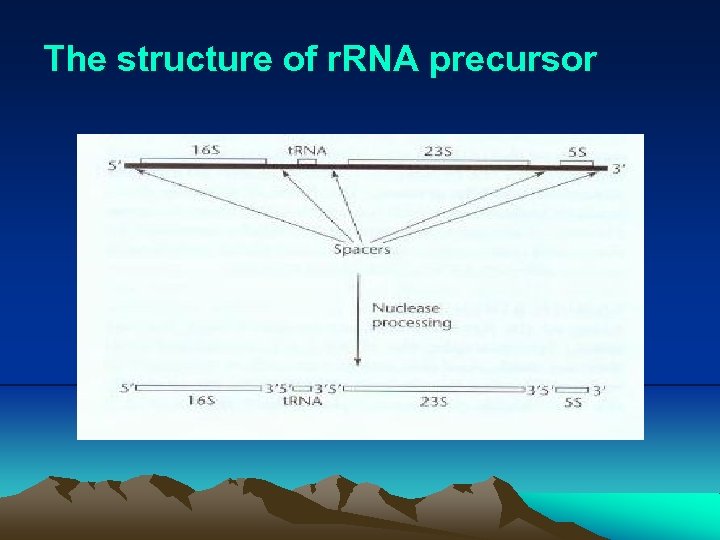
III. Transcription 7. r. RNA and t. RNA synthesis (1) Transcription of the genes for all the RNAs of the cell is basically the same. r. RNA and t. RNA are synthesis as a precursor, the individual r. RNA and t. RNA are cut from it. At some point during the processing, the RNAs are modified to make the mature r. RNAs and t. RNAs. (2) r. RNA i. The structural component of ribosome, where the proteins are synthesized. ii. Bacterial ribosome contains three types of r. RNA: 16 S, 23 S and 5 S. The 16 S and 23 S r. RNA are made in a precursor and then processed. (They are 18 S, 28 S and 5. 8 S in eukaryotic cells). iii. The r. RNAs are among the most highly evolutionarily conserved of all the cellular constituents. For this reason, they are always used as the candidate for molecular phylogeny analysis to clarify species.
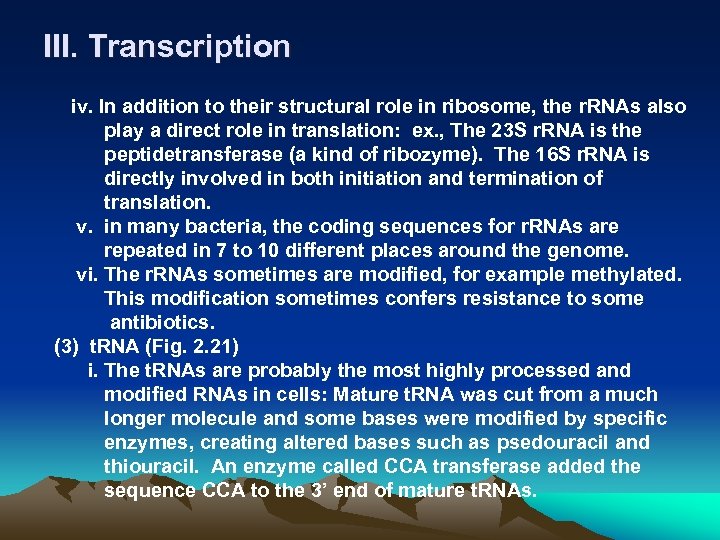
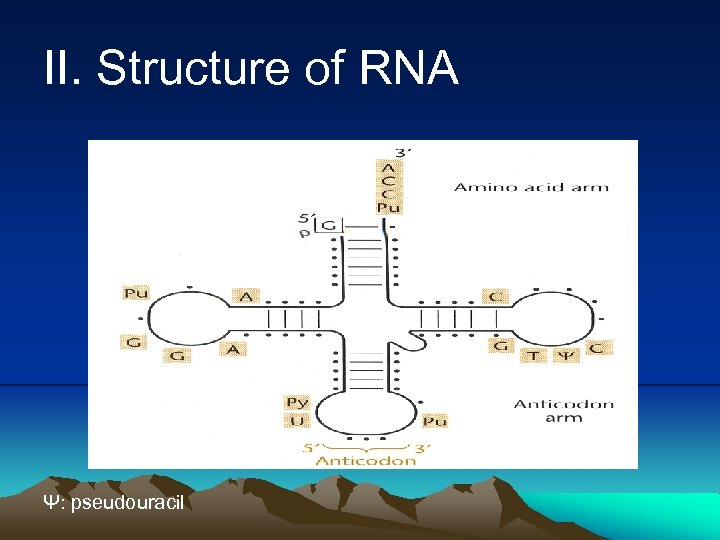
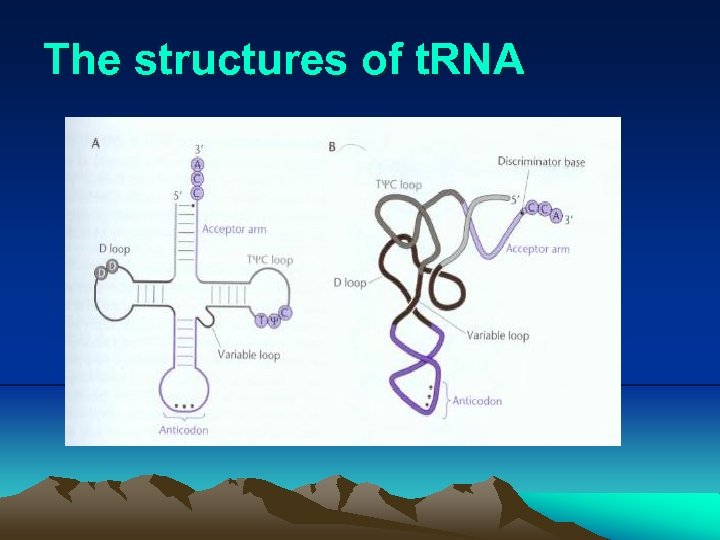
III. Transcription iv. In addition to their structural role in ribosome, the r. RNAs also play a direct role in translation: ex. , The 23 S r. RNA is the peptidetransferase (a kind of ribozyme). The 16 S r. RNA is directly involved in both initiation and termination of translation. v. in many bacteria, the coding sequences for r. RNAs are repeated in 7 to 10 different places around the genome. vi. The r. RNAs sometimes are modified, for example methylated. This modification sometimes confers resistance to some antibiotics. (3) t. RNA (Fig. 2. 21) i. The t. RNAs are probably the most highly processed and modified RNAs in cells: Mature t. RNA was cut from a much longer molecule and some bases were modified by specific enzymes, creating altered bases such as psedouracil and thiouracil. An enzyme called CCA transferase added the sequence CCA to the 3’ end of mature t. RNAs.
The structure of r. RNA precursor
The structures of t. RNA
IV. Translation @ Translate the sequence of nucleotides in m. RNA into the sequence of amino acids in protein, occurring on ribosome. 1. Ribosome consists of one copy each of the 16 S, 23 S, and 5 S r. RNAs as well as over 50 different proteins. 2. The complete ribosome, called 70 S ribosome, consists of two subunits, the 30 S subunit and 50 S subunit. The 30 S subunit contains 16 S r. RNA and 21 different proteins. The 50 S subunit contains 23 S r. RNA and 31 different proteins. 3. Reading frame (1) Each three nucleotide-sequence, or codon, in the m. RNA encodes a specific amino acid, and the assignment of the codons is known as the genetic code. (2) Translation begins at an initiator codon and ends at a terminator codon, establishing a reading frame of translation.
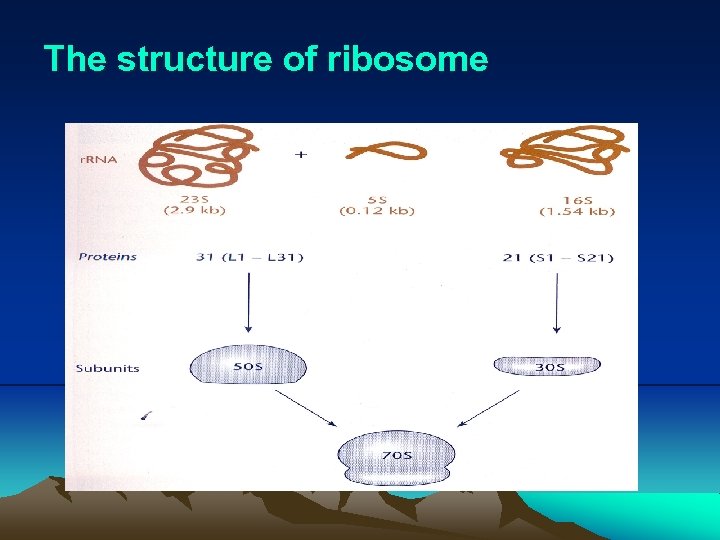
The structure of ribosome

IV. Translation (3) Before translation can begin, a specific amino acid is attached to nucleotide A of 3’ CCA sequence of each t. RNA by its cognate aminoacyl-t. RNA synthetase. (4) During translation, the ribosome moves three nucleotides at a time along the m. RNA in the 5’- to 3’- direction, allowing aat. RNAs to pair with larger m. RNA through codon-anticodon pairing (Fig. 2. 25, 2. 26). i. Actually, only two bases is sufficient to direct the codonanticodon interaction. In other word, codon-anticodon paring is a wobble or degenerated. This pattern of redundancy is due to the pairing between first base in anticodon on the t. RNA and last (third) base in the codon is less stringent. As a consequence of wobble, the same codon can have more than one t. RNAs. These t. RNAs are called cognate t. RNA, which carry the same amino acid (Table 2. 1).
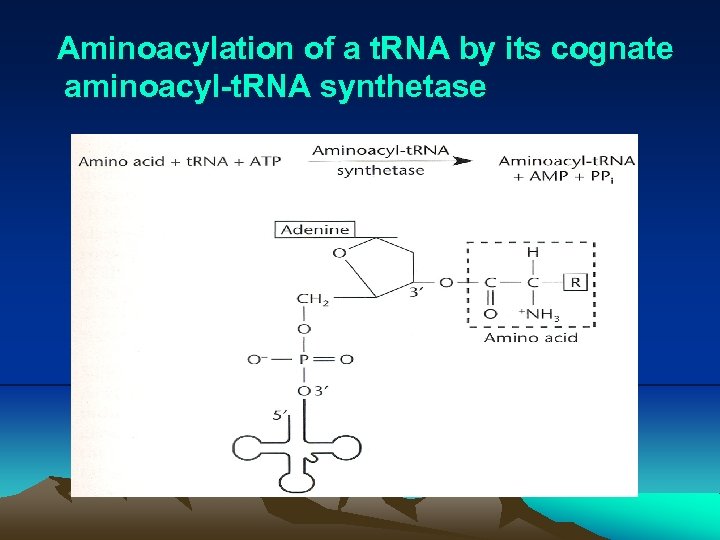
Aminoacylation of a t. RNA by its cognate aminoacyl-t. RNA synthetase
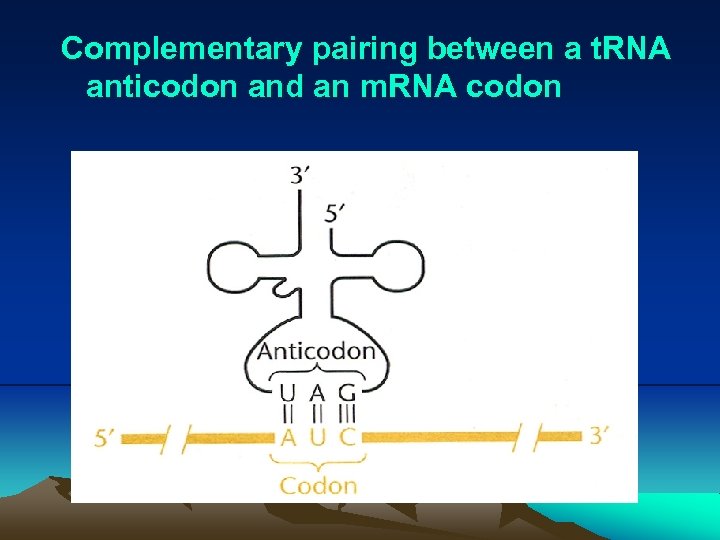
Complementary pairing between a t. RNA anticodon and an m. RNA codon
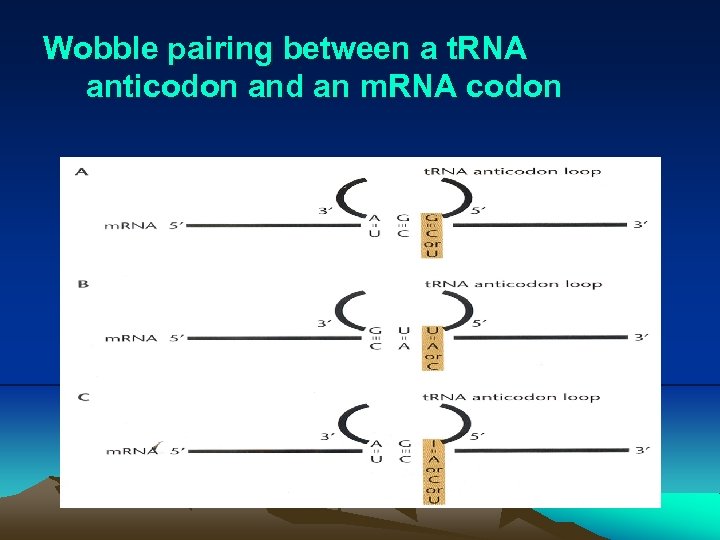
Wobble pairing between a t. RNA anticodon and an m. RNA codon
IV. Translation 4. Detail of translation (1) Translation initiation i. Initiation codon(s) – The initiation codon is usually AUG, but in bacteria sometimes are GUG, UUG or AUA. No matter which sequence, it specifies the amino acid methionine (Met). ii. m. RNA has sequence called translational initiation region (TIR), which contains an initiation codon and usually a short sequence (~4 bases), called ribosomal binding site (or Shine-Dalgarno sequence), upstream from the initiation codon. In fact, the 5’ end of the m. RNA may be some distance from TIR. This region is called the 5’ untranslated region (5’UTR) or leader sequence.
IV. Translation iii. Ribosomal binding site, also called Shine-Dalgarno (SD) sequence is complementary to short sequence located at 3’ of 16 S r. RNA iv. There are two sites in ribosome: A and P sites for aa-t. RNA to enter the ribosome and an E site for t. RNA to exist from ribosome. v. Fig. 2. 32 diagram the initiation of translation. (i) Initiation factor IF 3 binds the 30 S subunit to keep it dissociated from 50 S subunit during initiation. (ii) IF 1 binds to A site to block this site. (iii) The P site of 30 S subunit binds the m. RNA TIR site. (iv) The formyl-Met (f. Met) binds to initiation t. RNA, t. RNAf. Met. (v) The f. Met-t. RNAf. Met enters the P site on 30 S subunit in the presence of IF 2 -GTP.
IV. Translation (vi) IF 1 and IF 3 are released, the cleavage of GTP on IF 2 correctly positions the f. Met-t. RNAf. Met on the P site, and the 50 S subunit binds. (vii) The 70 S ribosome is ready to accept another aminoacylt. RNA at A site. (viii) Normally, polypeptides do not have a formyl group attached to their N terminus, which is removed by peptide deformylase after synthesized. In fact, they usually do not even have methionine as their N terminal amino acid, which is removed by methionine aminopeptidase.
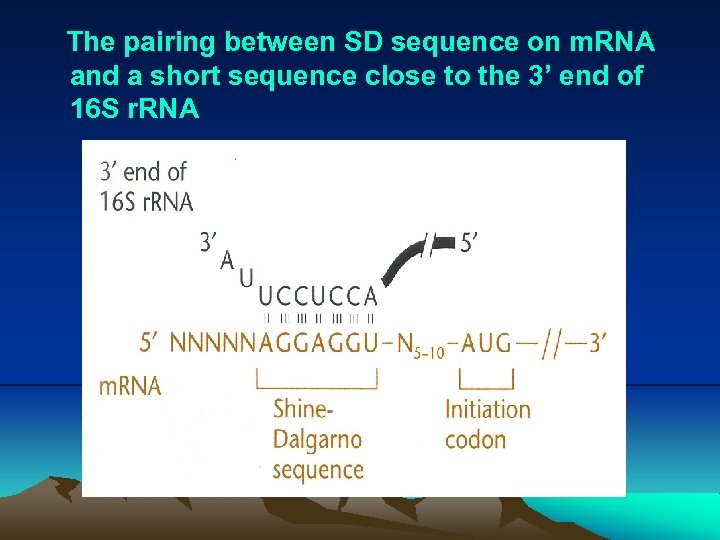
The pairing between SD sequence on m. RNA and a short sequence close to the 3’ end of 16 S r. RNA
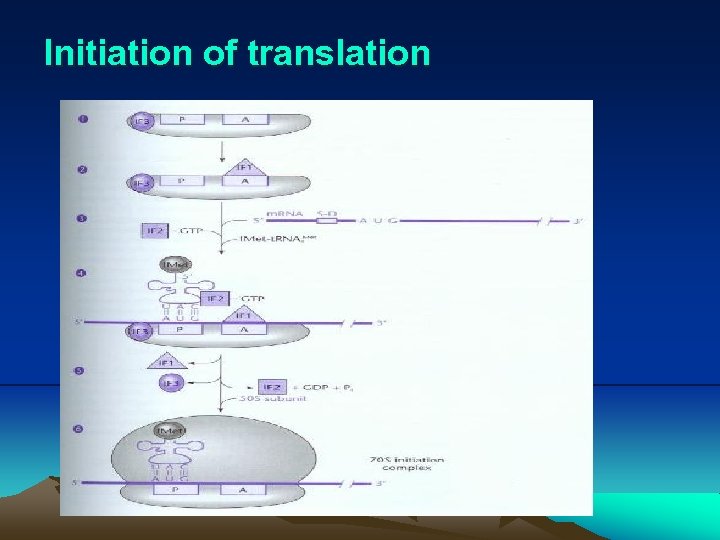
Initiation of translation
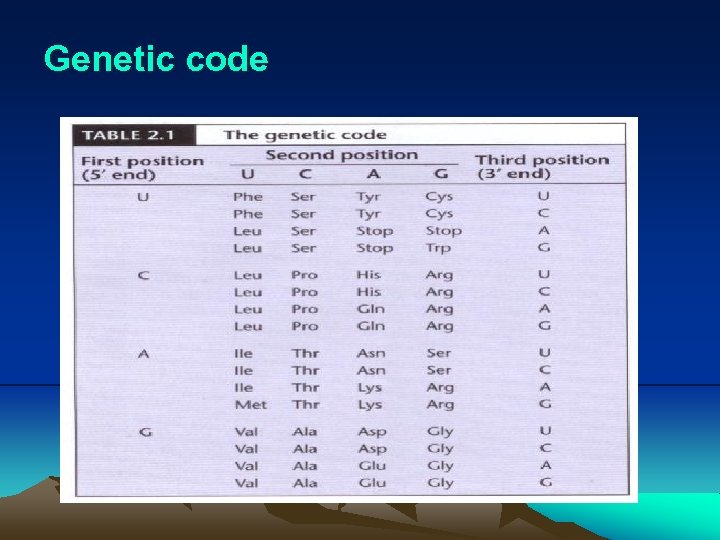
Genetic code
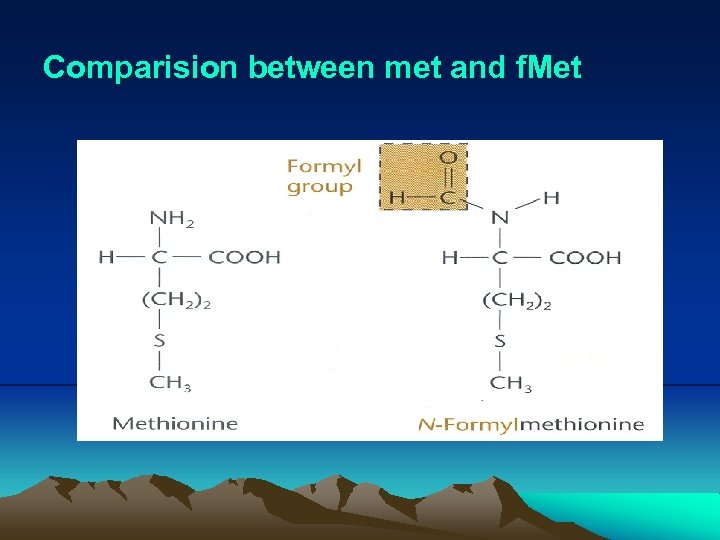
Comparision between met and f. Met
IV. Translation (2) Translation elongation (Fig. 2. 27) i. When 70 S ribosome forms, another aminoacyl-t. RNA can enter the A site. (i) Translation elongation factor Tu (EF-Tu) binds another aminoacyl-t. RNA and helps position it in A site by using the energy of GTP cleavage. (ii) The Tu-GDP is phosphorylated to Tu-GTP by Ts-GTP, another elongation factor. ii. The peptidyltransferase then joins this coming amino acid to the f. Met at P site. iii. The growing peptide is then move to P site by translation elongation factor G (EF-G), making room at A site for another aminoacyl-t. RNA and using the energy of GTP cleavage.
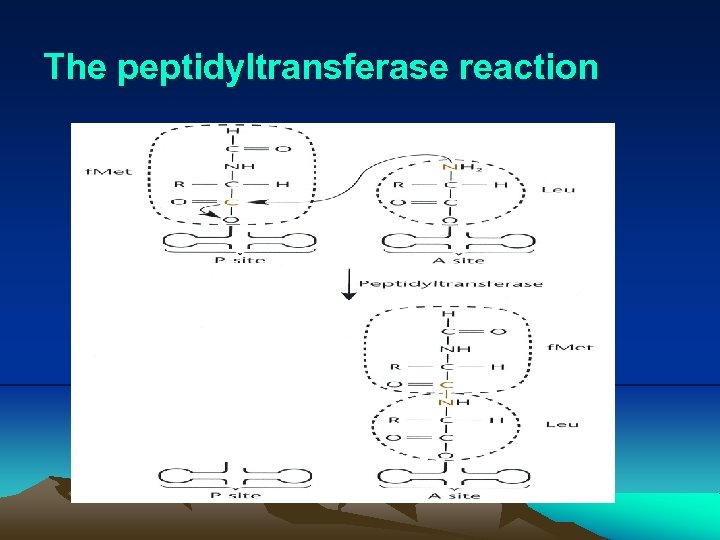
The peptidyltransferase reaction
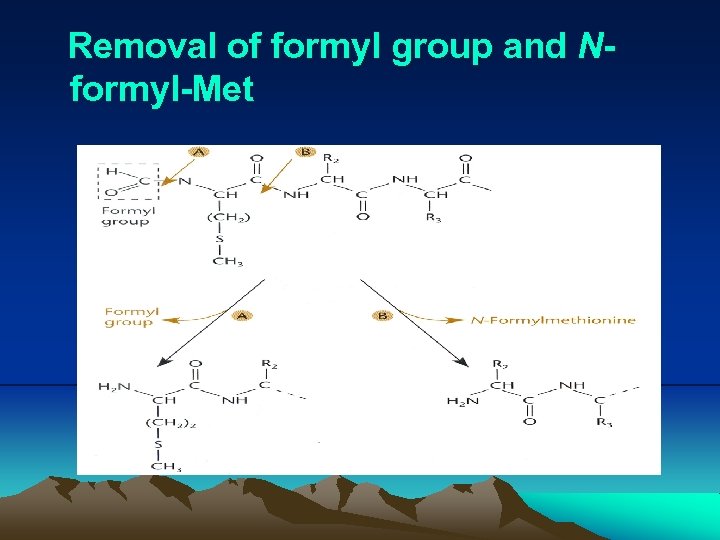
Removal of formyl group and Nformyl-Met
28f83bdd9d72f1bdaa2fdda075af0abc.ppt