d19533f940234244ec456b8fddc91019.ppt
- Количество слайдов: 99

口腔生物學特論 Oral Cancer: Mechanism & Prevention 口腔癌之機轉與預防 陳玉昆教授: 高雄醫學大學 口腔病理科 07 -3121101~2755 yukkwa@kmu. edu. tw

參考書目 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. Gibbs WW. Untangling the roots of cancer. Sci Am 2003; 289: 56 -65. What you need to know about cancer. Sci Am 1996 ; 289: 28 -119. Hannen EJM, Riediger D. The quantification of angiogenesis in relation to metastasis in oral cancer: a review. Int. J Oral Maxillofac Surg 2004; 33: 2 -7. Shieh et al. Role of angiogenic and non-angiogenic mechanisms in oral squamous cell carcinoma: correlation with histologic differentiation and tumor progression. J Oral Pathol Med 2004; 33: 601 -6. Sharma DC. Betel quid and areca nut are carcinogenic without tobacco. Lancet Oncol 2003; 4: 587. Sharma DC. Indian betel quid more carcinogenic than anticipated. Lancet Oncol 2001; 2: 464. Braakhuis BJM et al. A genetic progression model of oral cancer: current evidence and clinical implications. J Oral Pathol Med 2004; 33: 317 -22. Braakhuis BJM et al. A Genetic explanation of slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res 2003; 63: 1727 -30. Loktionov A. Common gene polymorphisms, cancer progression and prognosis. Cancer Letters 2004; 208 : 1 -33. Desmaze C et al. Telomere-driven genomic instability in cancer cells. Cancer Letters 2003; 194: 173 -82. Hiyama E & Hiyama K. Telomerase as tumor marker. Cancer Letters 2003; 194: 221 -33. Kaohsiung Medical University, Oral Pathology Department Huang AH et al. Isolation and characterization of normal hamster buccal pouch stem/stromal cells – a potential oral cancer stem/stem-like cell model. Oral Oncol 2009; 45: e 189 -e 195. Umezawa & Gorham. Dueling models in head and neck tumor formation. Lab Investig 2010; 90: 1546 -8. Spillane JB, Henderson MA. Cancer stem cells: a review. ANZ J Surg 2007; 77: 464 -8. Zhou ZT, Jiang WW. Cancer stem cell model in oral squamous cell carcinoma. Curr Stem Cell Res Ther 2008; 3: 17– 20. Harper LJ et al. Stem cell patterns in cell lines derived from head and neck squamous cell carcinoma. J Oral Pathol Med 2007; 36: 594 -603. Lim YC et al. Cancer stem cell traits in squamospheres derived from primary head and neck squamous cell carcinomas. Oral Oncol 2011; 47: 83 -91.

學習目標 探索癌症之旅 Field cancerization 癌化的標準教條 3 5 4 癌細胞的六種超能力 四種癌化理論 6 2 Stages of carcinogenesis 癌症的預防 始點 1 How cancer arise 7 終點
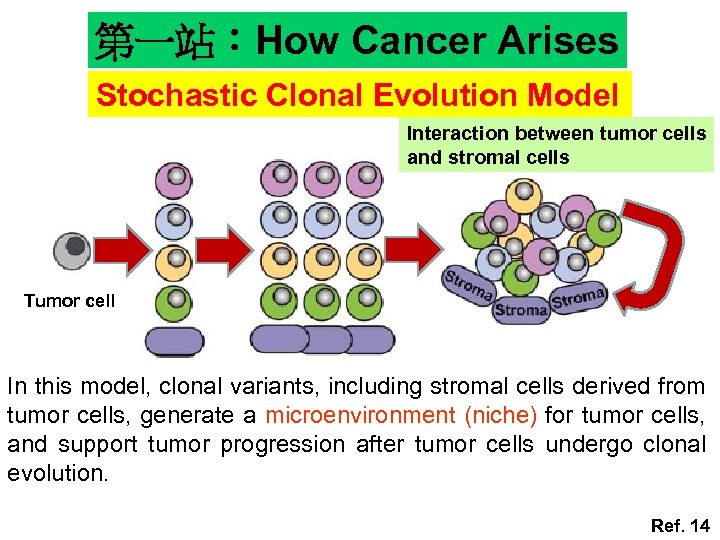
第一站:How Cancer Arises Stochastic Clonal Evolution Model Interaction between tumor cells and stromal cells Tumor cell In this model, clonal variants, including stromal cells derived from tumor cells, generate a microenvironment (niche) for tumor cells, and support tumor progression after tumor cells undergo clonal evolution. Ref. 14
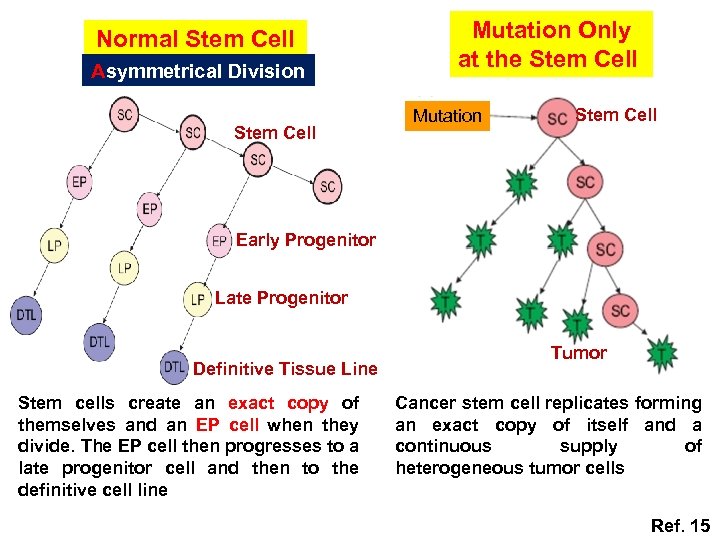
Normal Stem Cell Asymmetrical Division Stem Cell Mutation Only at the Stem Cell Mutation Stem Cell Early Progenitor Late Progenitor Definitive Tissue Line Stem cells create an exact copy of themselves and an EP cell when they divide. The EP cell then progresses to a late progenitor cell and then to the definitive cell line Tumor Cancer stem cell replicates forming an exact copy of itself and a continuous supply of heterogeneous tumor cells Ref. 15
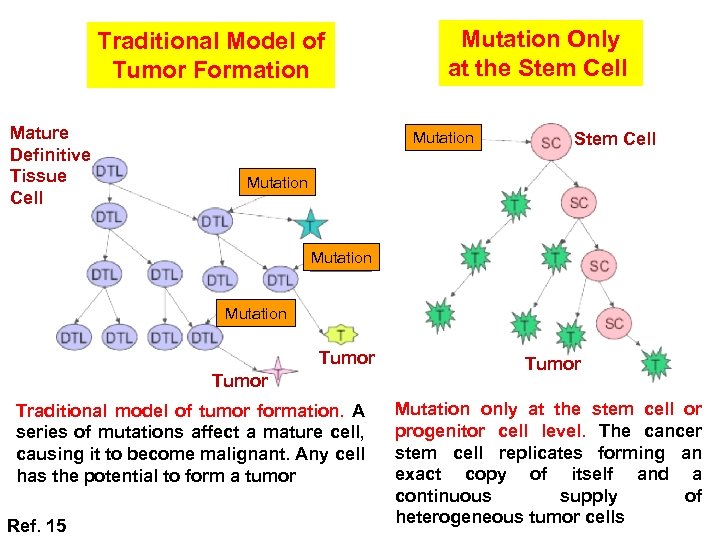
Traditional Model of Tumor Formation Mature Definitive Tissue Cell Mutation Only at the Stem Cell Mutation Tumor Traditional model of tumor formation. A series of mutations affect a mature cell, causing it to become malignant. Any cell has the potential to form a tumor Ref. 15 Tumor Mutation only at the stem cell or progenitor cell level. The cancer stem cell replicates forming an exact copy of itself and a continuous supply of heterogeneous tumor cells
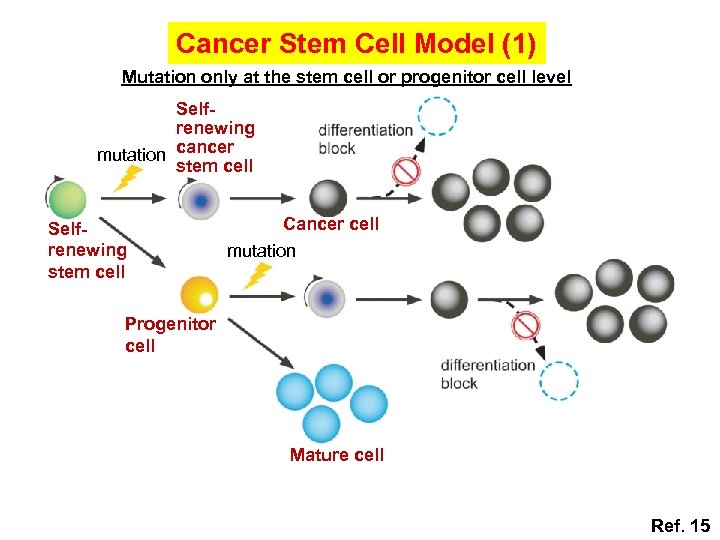
Cancer Stem Cell Model (1) Mutation only at the stem cell or progenitor cell level Selfrenewing mutation cancer stem cell Selfrenewing stem cell Cancer cell mutation Progenitor cell Mature cell Ref. 15
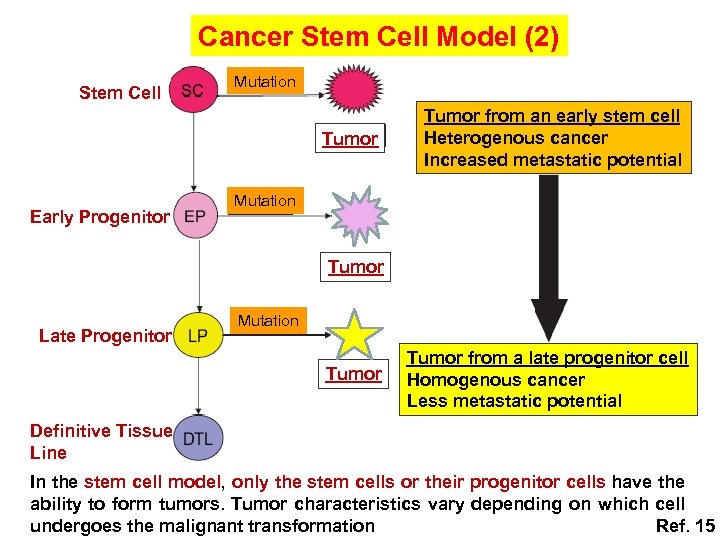
Cancer Stem Cell Model (2) Stem Cell Mutation Tumor Early Progenitor Tumor from an early stem cell Heterogenous cancer Increased metastatic potential Mutation Tumor Late Progenitor Mutation Tumor from a late progenitor cell Homogenous cancer Less metastatic potential Definitive Tissue Line In the stem cell model, only the stem cells or their progenitor cells have the ability to form tumors. Tumor characteristics vary depending on which cell undergoes the malignant transformation Ref. 15
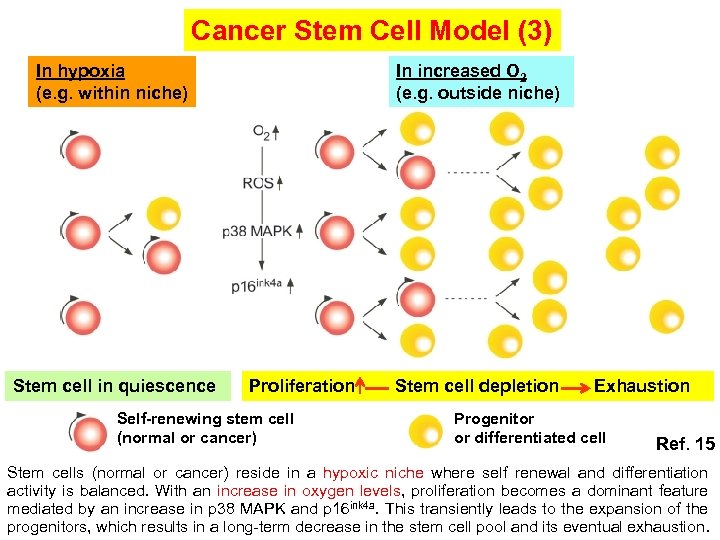
Cancer Stem Cell Model (3) In increased O 2 (e. g. outside niche) In hypoxia (e. g. within niche) Stem cell in quiescence Proliferation Self-renewing stem cell (normal or cancer) Stem cell depletion Exhaustion Progenitor or differentiated cell Ref. 15 Stem cells (normal or cancer) reside in a hypoxic niche where self renewal and differentiation activity is balanced. With an increase in oxygen levels, proliferation becomes a dominant feature mediated by an increase in p 38 MAPK and p 16 ink 4 a. This transiently leads to the expansion of the progenitors, which results in a long-term decrease in the stem cell pool and its eventual exhaustion.
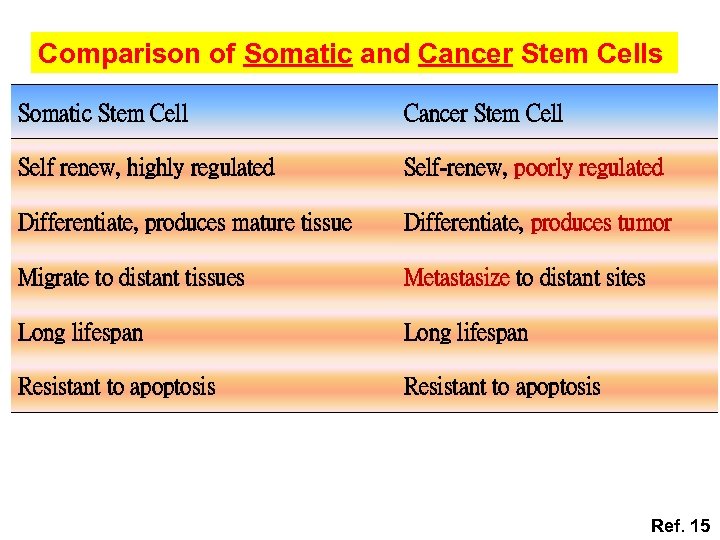
Comparison of Somatic and Cancer Stem Cells Somatic Stem Cell Cancer Stem Cell Self renew, highly regulated Self-renew, poorly regulated Differentiate, produces mature tissue Differentiate, produces tumor Migrate to distant tissues Metastasize to distant sites Long lifespan Resistant to apoptosis Ref. 15
Stem cell - Oral Epithelia • According to the progression model, the development of most of OSCC takes months or years. • As normal human oral epithelia have a rate of renewal estimated to be about 14 -24 days, most epithelial cells do not exist long enough to accumulate the genetic changes necessary for the development of an OSCC. • The hierarchical stem cell structure present in human oral epithelia indicates that stem cells are the only long-time residents of oral epithelia and, consequently, the only cells able to accumulate the necessary number of genetic changes for malignancy to develop
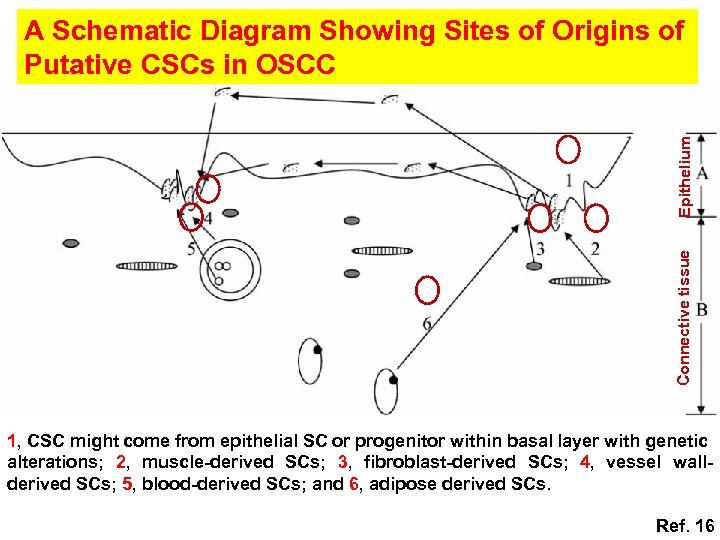
Connective tissue Epithelium A Schematic Diagram Showing Sites of Origins of Putative CSCs in OSCC 1, CSC might come from epithelial SC or progenitor within basal layer with genetic alterations; 2, muscle-derived SCs; 3, fibroblast-derived SCs; 4, vessel wallderived SCs; 5, blood-derived SCs; and 6, adipose derived SCs. Ref. 16
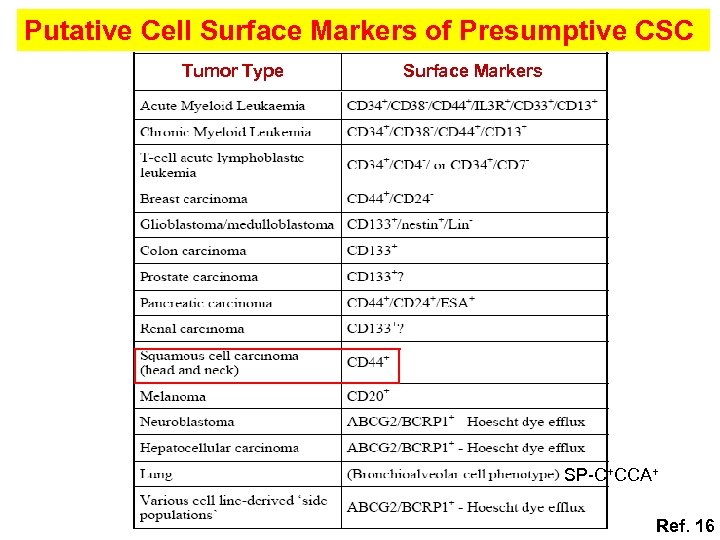
Putative Cell Surface Markers of Presumptive CSC Tumor Type Surface Markers SP-C+CCA+ Ref. 16

Frequencies of CSCs in Various Human Cancers Human cancer Recipient mice Cancer stem cell frequency (%) Ref. 16
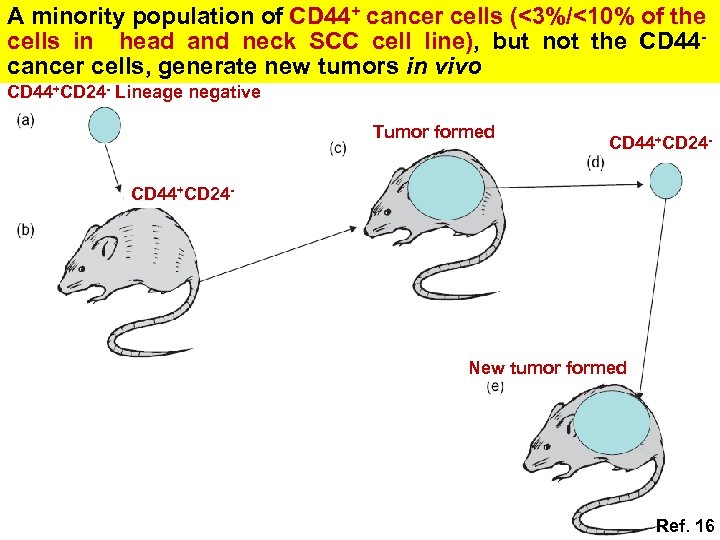
A minority population of CD 44+ cancer cells (<3%/<10% of the cells in head and neck SCC cell line), but not the CD 44 cancer cells, generate new tumors in vivo CD 44+CD 24 - Lineage negative Tumor formed CD 44+CD 24 - New tumor formed Ref. 16

Potential Mechanisms of CSC Formation A MUTATION Stem/progenitor cells Differentiated cells Progenitors Self renewal CSC (A) Mutation. The cancer stem cells might appear after mutations in specific stem cells or early stem cells progenitors. It is also possible that CSC can be derived from Ref. 16 differentiated cells.
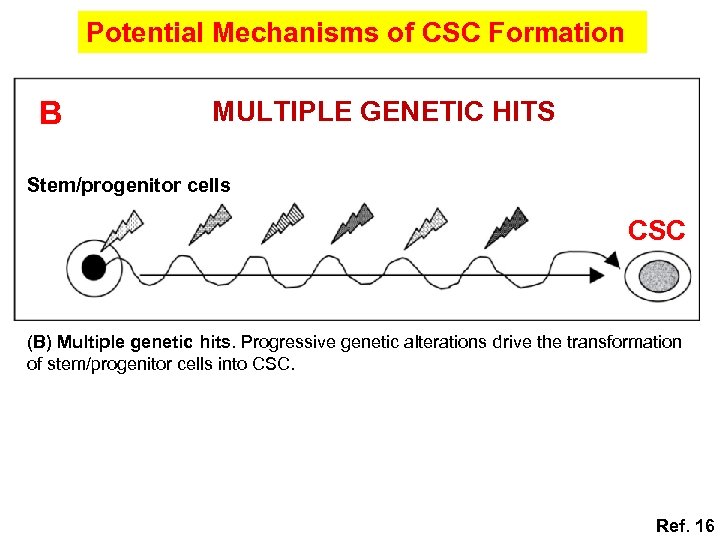
Potential Mechanisms of CSC Formation B MULTIPLE GENETIC HITS Stem/progenitor cells CSC (B) Multiple genetic hits. Progressive genetic alterations drive the transformation of stem/progenitor cells into CSC. Ref. 16
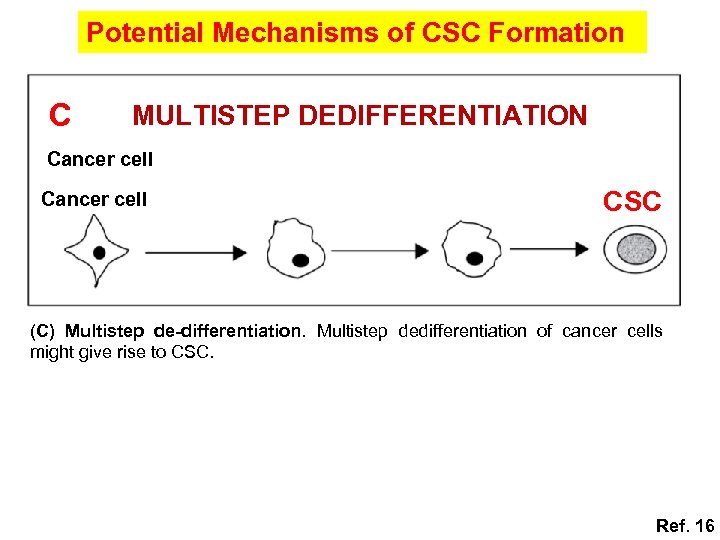
Potential Mechanisms of CSC Formation C MULTISTEP DEDIFFERENTIATION Cancer cell CSC (C) Multistep de-differentiation. Multistep dedifferentiation of cancer cells might give rise to CSC. Ref. 16
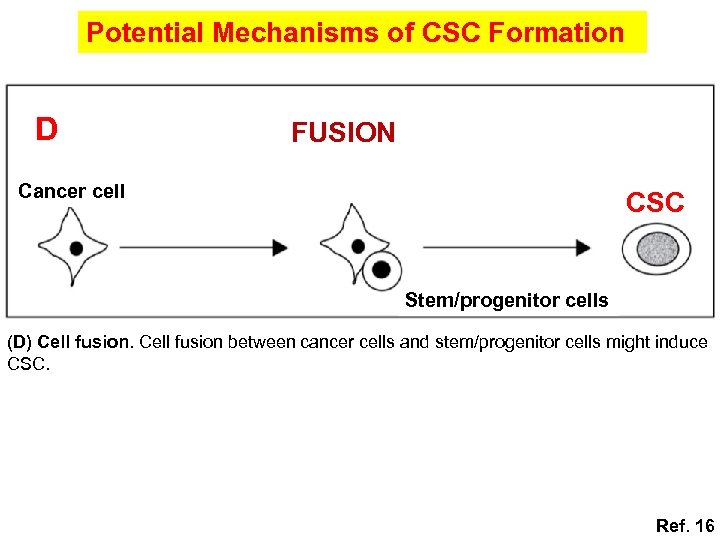
Potential Mechanisms of CSC Formation D FUSION Cancer cell CSC Stem/progenitor cells (D) Cell fusion between cancer cells and stem/progenitor cells might induce CSC. Ref. 16
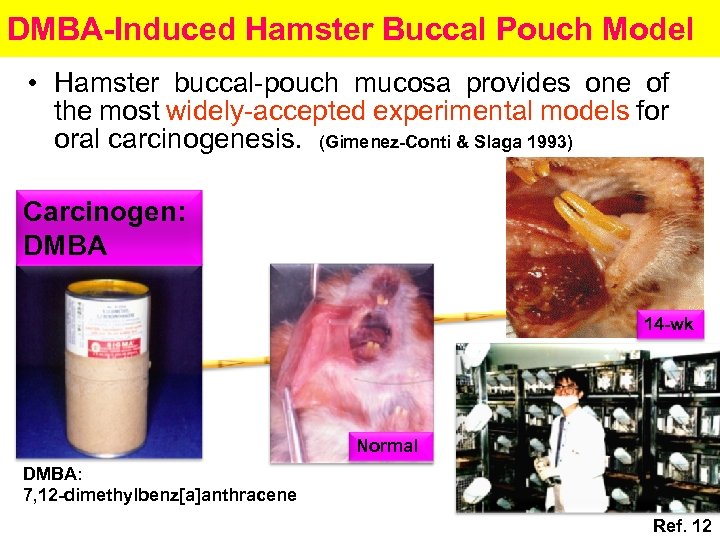
DMBA-Induced Hamster Buccal Pouch Model • Hamster buccal-pouch mucosa provides one of the most widely-accepted experimental models for oral carcinogenesis. (Gimenez-Conti & Slaga 1993) Carcinogen: DMBA 14 -wk Normal DMBA: 7, 12 -dimethylbenz[a]anthracene Ref. 12
DMBA-Induced Hamster Buccal Pouch Model • Despite anatomical and histological differences between (hamster) pouch mucosa and human buccal tissue, experimental carcinogenesis protocols for the former induce premalignant changes and carcinomas that are similar to the development of premalignancy and malignancy in human oral mucosa. (Morris 1961) Animal Study Human Study Ref. 12
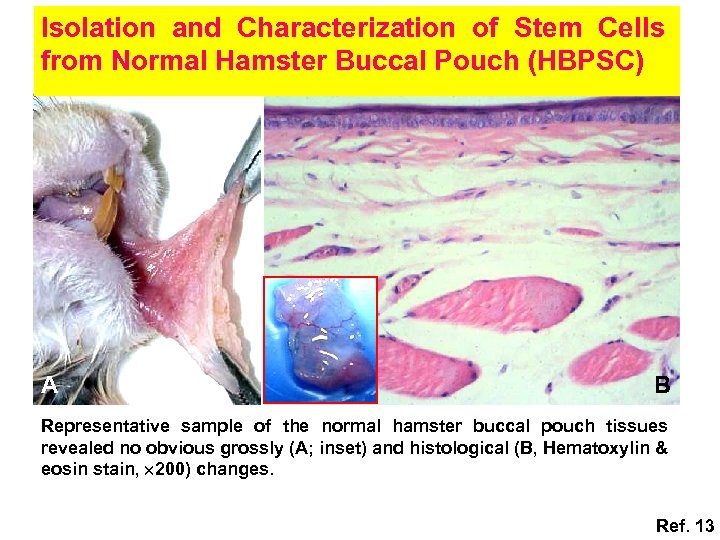
Isolation and Characterization of Stem Cells from Normal Hamster Buccal Pouch (HBPSC) A B Representative sample of the normal hamster buccal pouch tissues revealed no obvious grossly (A; inset) and histological (B, Hematoxylin & eosin stain, 200) changes. Ref. 13
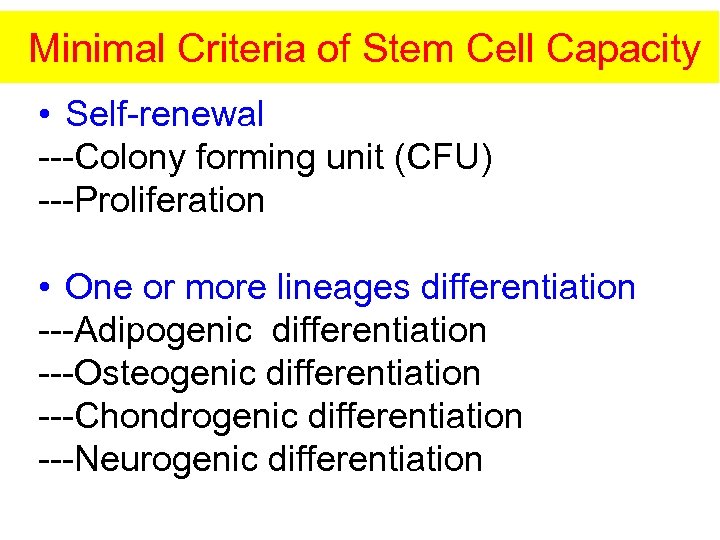
Minimal Criteria of Stem Cell Capacity • Self-renewal ---Colony forming unit (CFU) ---Proliferation • One or more lineages differentiation ---Adipogenic differentiation ---Osteogenic differentiation ---Chondrogenic differentiation ---Neurogenic differentiation
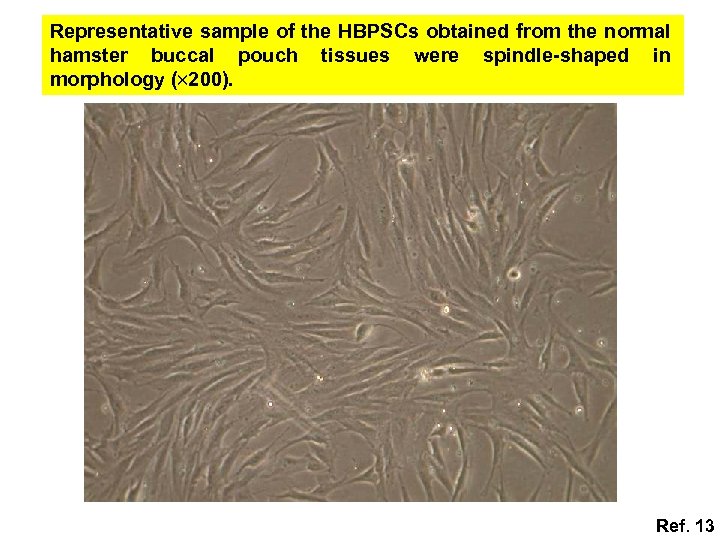
Representative sample of the HBPSCs obtained from the normal hamster buccal pouch tissues were spindle-shaped in morphology ( 200). Ref. 13
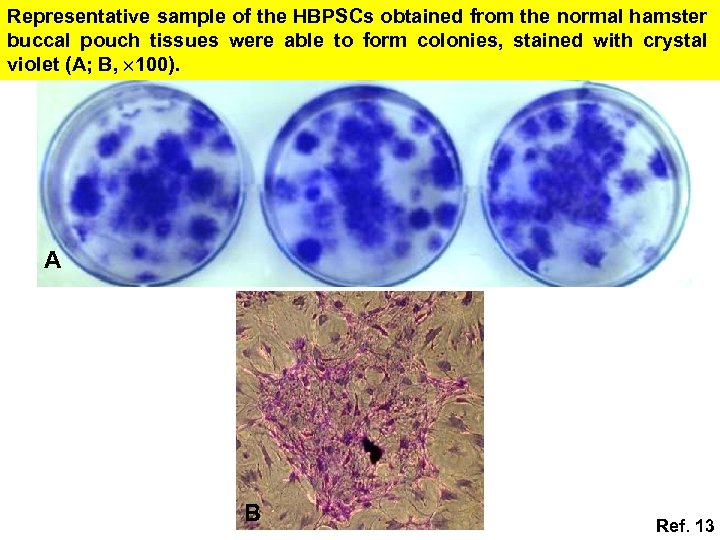
Representative sample of the HBPSCs obtained from the normal hamster buccal pouch tissues were able to form colonies, stained with crystal violet (A; B, 100). A B Ref. 13

Cytoplasmic keratin (A, 200) and vimentin (B, 200) stainings were noted for the representative sample of the HBPSCs obtained from the normal hamster buccal pouch tissues. A B Ref. 13
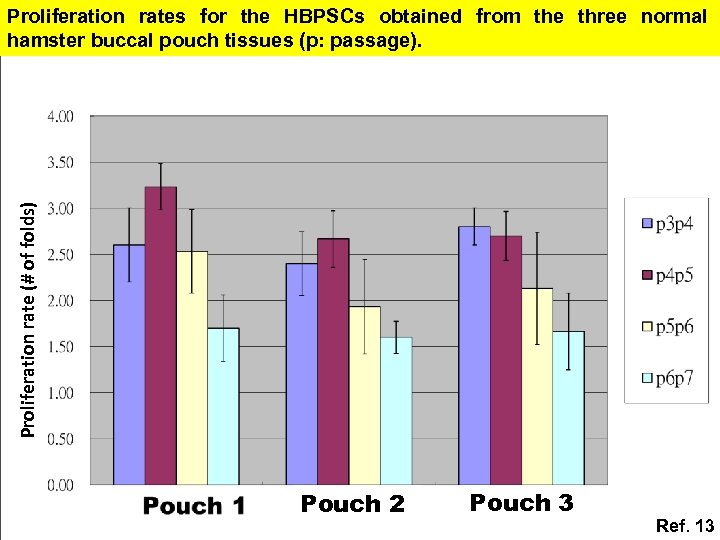
Proliferation rate (# of folds) Proliferation rates for the HBPSCs obtained from the three normal hamster buccal pouch tissues (p: passage). Pouch 2 Pouch 3 Ref. 13
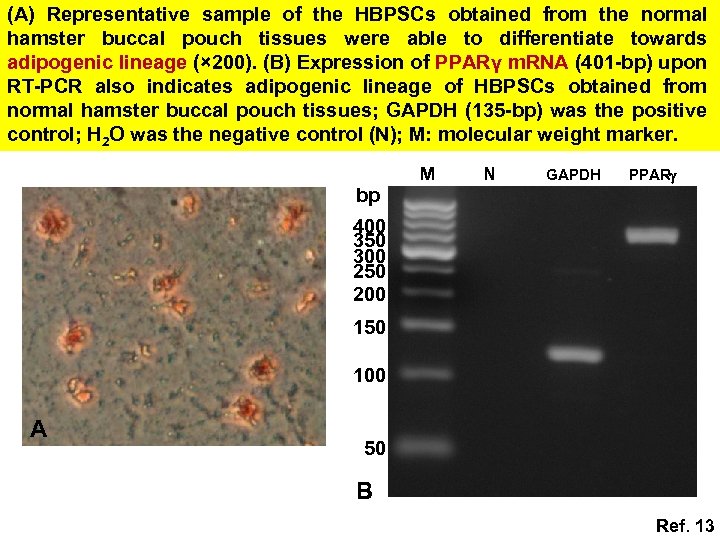
(A) Representative sample of the HBPSCs obtained from the normal hamster buccal pouch tissues were able to differentiate towards adipogenic lineage (× 200). (B) Expression of PPARγ m. RNA (401 -bp) upon RT-PCR also indicates adipogenic lineage of HBPSCs obtained from normal hamster buccal pouch tissues; GAPDH (135 -bp) was the positive control; H 2 O was the negative control (N); M: molecular weight marker. bp M N GAPDH PPAR 400 350 300 250 200 150 100 A 50 B Ref. 13

Representative sample of the HBPSCs obtained from the normal hamster buccal pouch tissues were able to differentiate towards chondrogenic lineage (× 200); inset: a yellowish chondroid pellet (~3 mm in diameter). Representative sample of the HBPSCs obtained from the normal hamster buccal pouch tissues were able to differentiate towards osteogenic lineage (× 200). Ref. 13
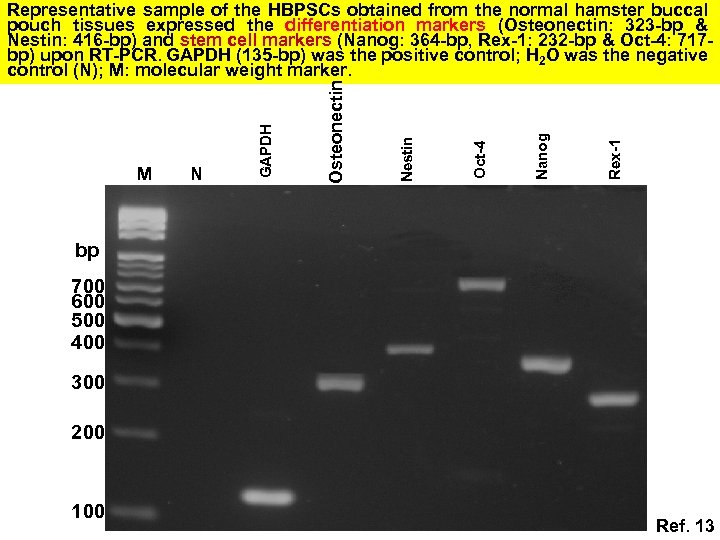
Rex-1 Nanog Oct-4 Nestin N Osteonectin M GAPDH Representative sample of the HBPSCs obtained from the normal hamster buccal pouch tissues expressed the differentiation markers (Osteonectin: 323 -bp & Nestin: 416 -bp) and stem cell markers (Nanog: 364 -bp, Rex-1: 232 -bp & Oct-4: 717 bp) upon RT-PCR. GAPDH (135 -bp) was the positive control; H 2 O was the negative control (N); M: molecular weight marker. bp 700 600 500 400 300 200 100 Ref. 13

Representative sample of the HBPSCs obtained from the normal hamster buccal pouch tissues showed high expression for surface markers: CD 29, CD 90, and CD 105 but very low expression for CD 14, CD 34, and CD 45 (Black/blue line: isotype control, Red line: marker of interest; Max: maximum). 100 93. 6 85. 8 % of Max CD 29 CD 90 100 % of Max CD 45 CD 105 100 1. 5 51. 3 1. 7 CD 34 % of Max 100 0. 9 CD 14 Ref. 13

DMBA-Induced Hamster Buccal Pouch Model Isolation of normal HBPSC, we may follow in vitro the sequential changes of the normal HBPSCs during multistep oral carcinogenesis or the alternations of these cells upon irradiation treatment and/or chemotherapy. Hence, the isolated normal HBPSCs, would provide a potential avenue for the future study of CSCs of buccal SCCs.
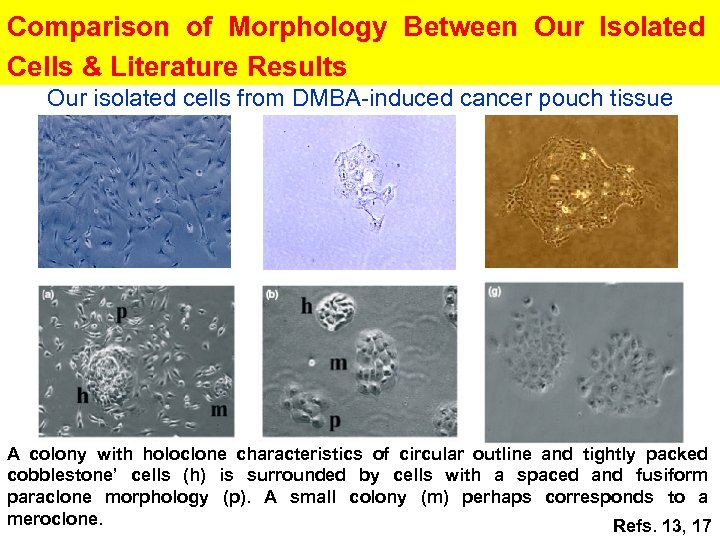
Comparison of Morphology Between Our Isolated Cells & Literature Results Our isolated cells from DMBA-induced cancer pouch tissue A colony with holoclone characteristics of circular outline and tightly packed cobblestone’ cells (h) is surrounded by cells with a spaced and fusiform paraclone morphology (p). A small colony (m) perhaps corresponds to a meroclone. Refs. 13, 17
Hallmarks of CSCs (1) Self-renewal, stem cell marker expression, aberrant differentiation, and tumor-initiating potential OSCC-driven squamospheres demonstrated: (1) A number of stem cell markers, such as CK 5, OCT 4, SOX 2, nestin, and CD 44, Bmi-1, CD 133, ALDH 1 (2) Single-dissociated squamosphere cells were able to form new squamospheres within 1 week of reseeding (3) Serum treatment led HNSCC-driven squamospheres to be non-tumorigenic differentiated cancer cells (4) Injection of as few as 100 undifferentiated squamosphere cells in nude mice gave rise to tumor formation CSCs is known to be significantly resistant to various chemotherapeutic agents (cisplatin, 5 -fluorouracil (FU), paclitaxel, and doxetaxel)
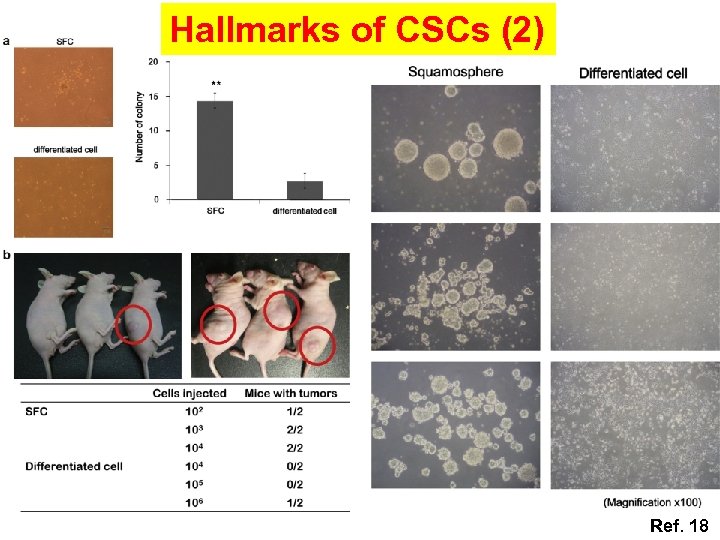
Hallmarks of CSCs (2) Ref. 18
第一站總結 1. In stochastic model, clonal variants, including stromal cells derived from tumor cells, generate a microenvironment for tumor cells, and support tumor progression after tumor cells undergo clonal evolution 請注意以下的重點提要 2. CSCs may originate from normal somatic stem cells, it has been estimated that 3 to 6 genetic events are required to transform a normal human cell into a cancer cell 3. Accumulated evidences have identified that CSCs in SCCs of head and neck region including oral cavity function in initiation, maintenance, growth, and metastasis of tumors Cancer development: Stochastic clonal evolution model VS cancer stem cells
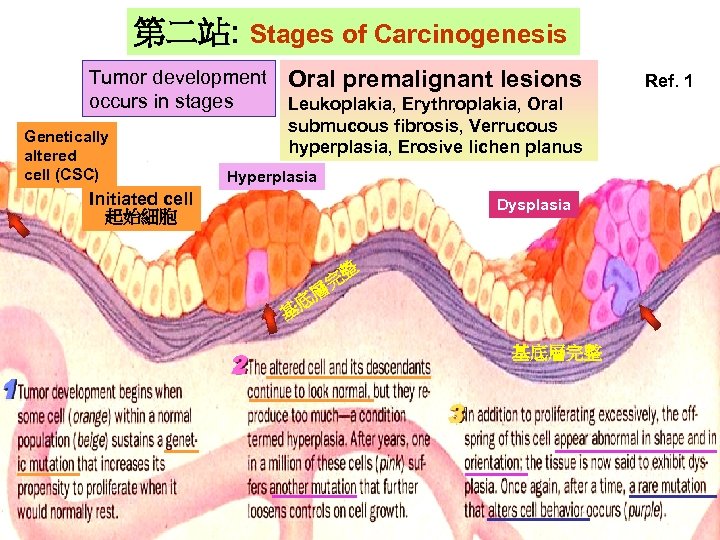
第二站: Stages of Carcinogenesis Tumor development occurs in stages Genetically Gentically altered cell (CSC) altered cell Oral premalignant lesions Leukoplakia, Erythroplakia, Oral submucous fibrosis, Verrucous hyperplasia, Erosive lichen planus Hyperplasia Initiated cell 起始細胞 Hyperlasia Dysplasia 整 完 層 基 底 基底層完整 Ref. 1
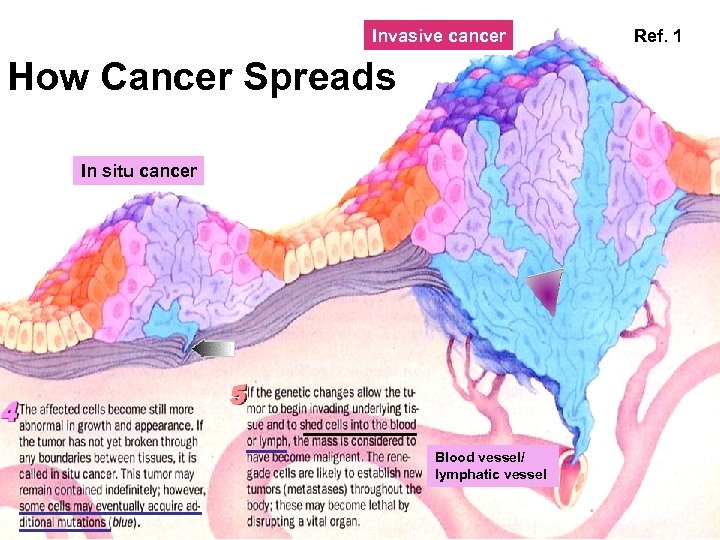
Invasive cancer How Cancer Spreads In situ cancer Blood vessel/ lymphatic vessel Ref. 1
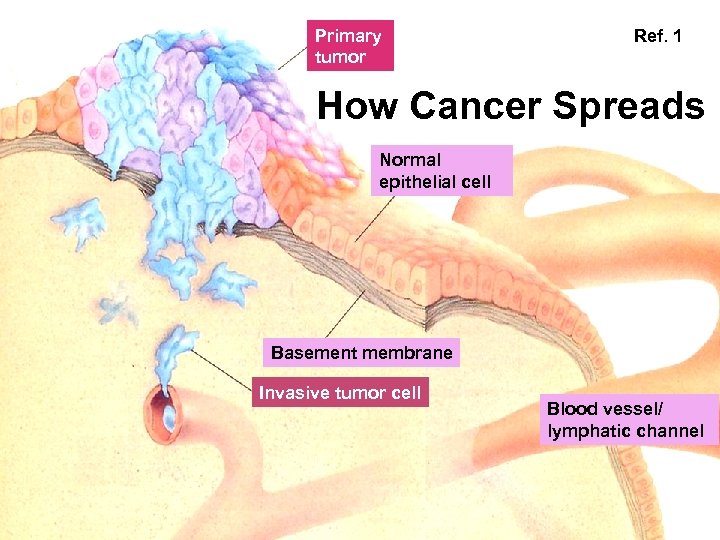
Primary tumor Ref. 1 How Cancer Spreads Normal epithelial cell Basement membrane Invasive tumor cell Blood vessel/ lymphatic channel
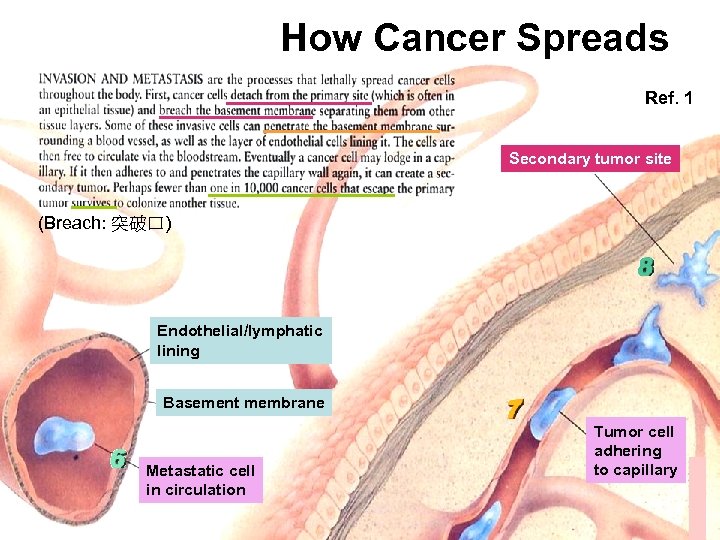
How Cancer Spreads Ref. 1 Secondary tumor site (Breach: 突破口) Endothelial/lymphatic lining Basement membrane Metastatic cell in circulation Tumor cell adhering to capillary
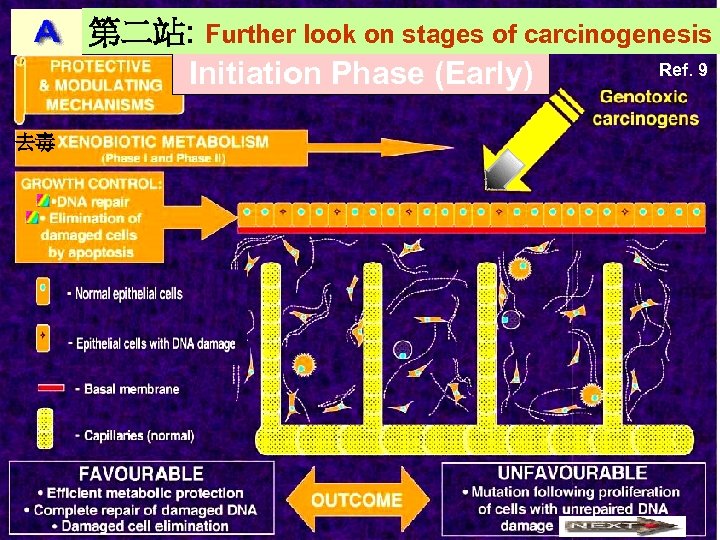
第二站: Further look on stages of carcinogenesis Ref. 9 Initiation Phase (Early) 去毒
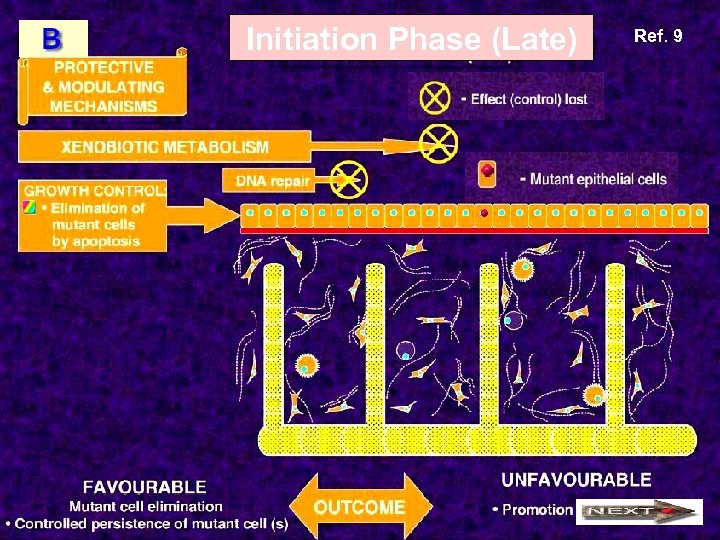
Initiation Phase (Late) Ref. 9
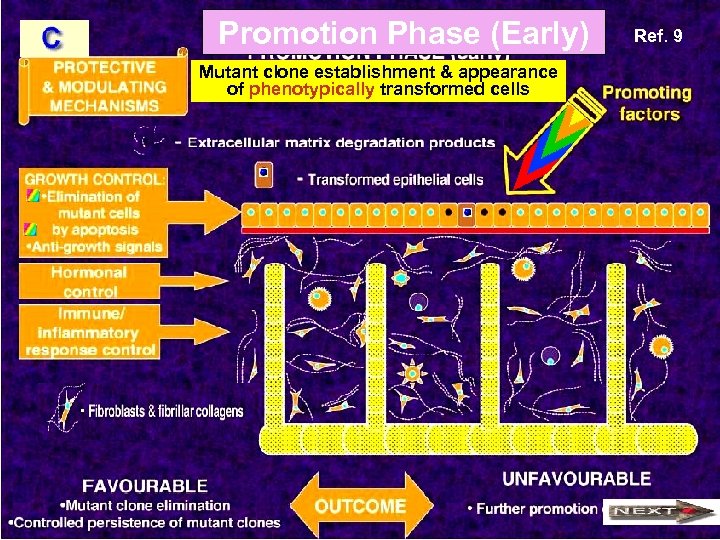
Promotion Phase (Early) Mutant clone establishment & appearance of phenotypically transformed cells Ref. 9
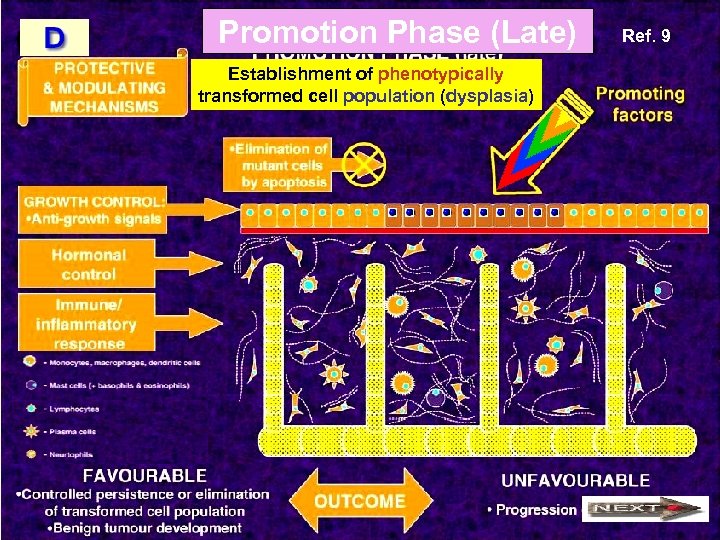
Promotion Phase (Late) Establishment of phenotypically transformed cell population (dysplasia) Ref. 9
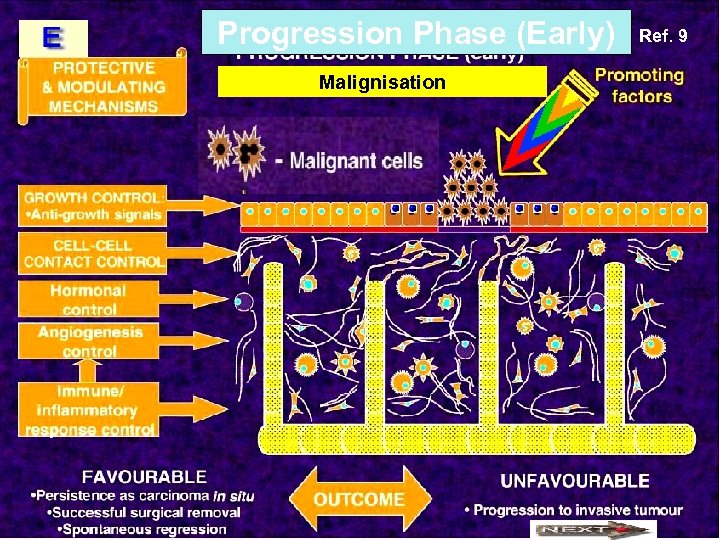
Progression Phase (Early) Malignisation Ref. 9
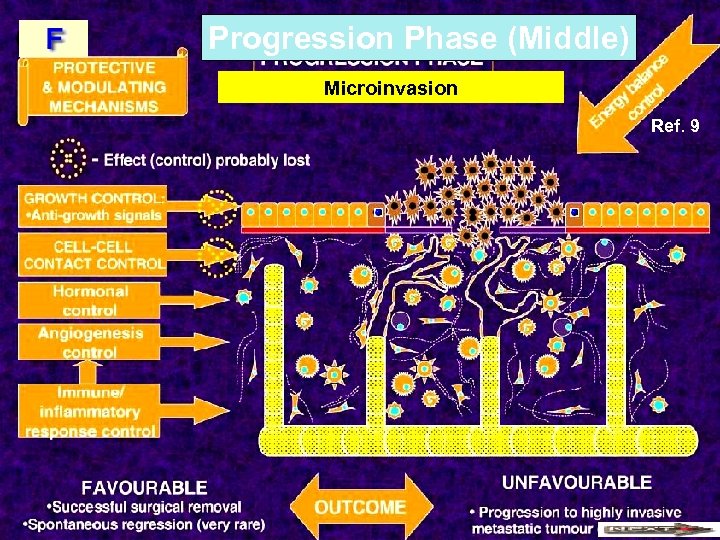
Progression Phase (Middle) Microinvasion Ref. 9
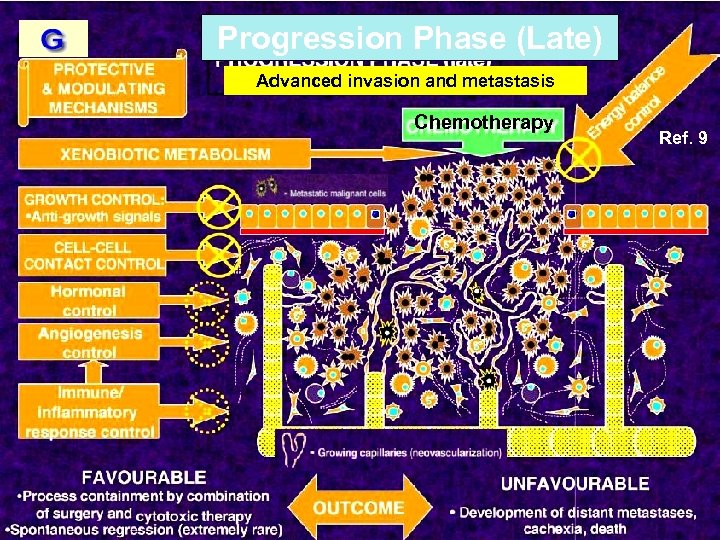
Progression Phase (Late) Advanced invasion and metastasis Chemotherapy Ref. 9
第二站總結 癌症形成是階段性的 Initiation 能力 (early, late) vs正常細胞有自衛 Genetically altered cell (CSC) 請注意以下的重點提要 (late) Progression (middle) Progression Promotion (early) Hyperplasia Microinvasion Invasive cancer Promotion (late) Dysplaisa Progression (early) In situ cancer Progression (late) Metastasis Tumor development occurs in stages Normal cell has self-defense
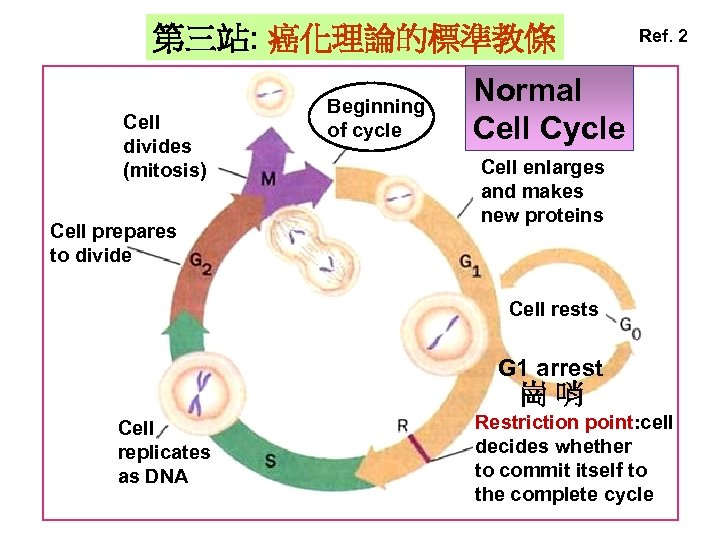
第三站: 癌化理論的標準教條 Cell divides (mitosis) Cell prepares to divide Beginning of cycle Ref. 2 Normal Cell Cycle Cell enlarges and makes new proteins Cell rests G 1 arrest 崗哨 Cell replicates as DNA Restriction point: cell decides whether to commit itself to the complete cycle
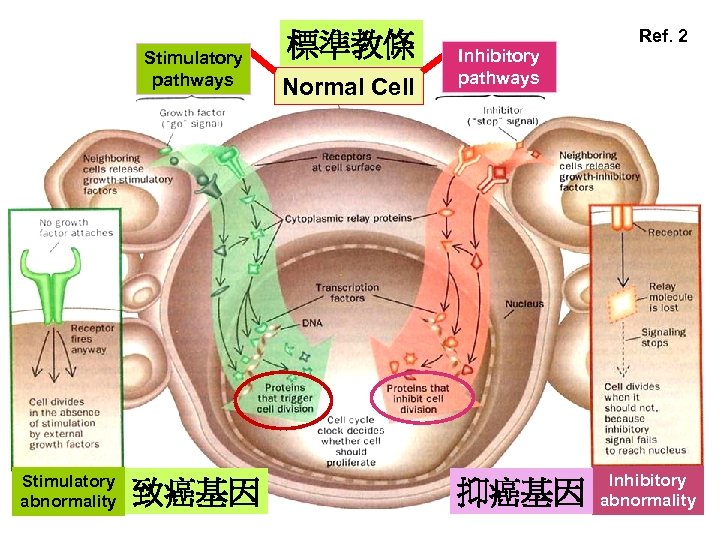
Stimulatory pathways Stimulatory abnormality 致癌基因 標準教條 Normal Cell Ref. 2 Inhibitory pathways 抑癌基因 Inhibitory abnormality
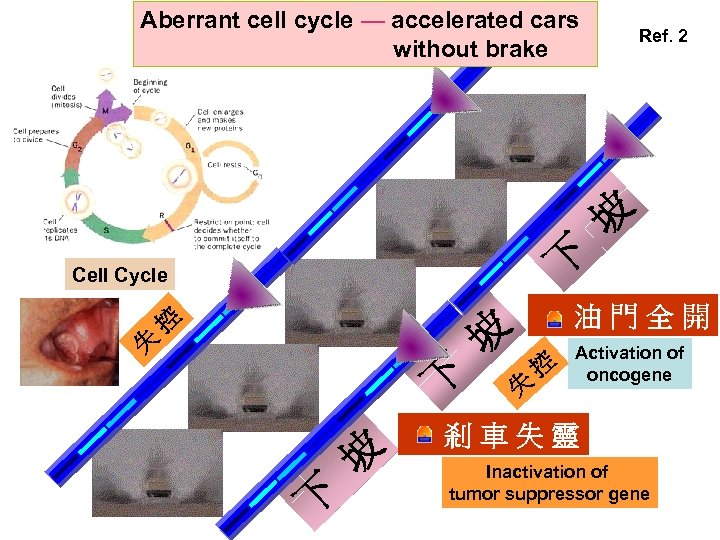
Aberrant cell cycle — accelerated cars without brake Ref. 2 坡 下 Cell Cycle 失 控 油門全開 坡 下 失 控 Activation of oncogene 剎車失靈 Inactivation of tumor suppressor gene
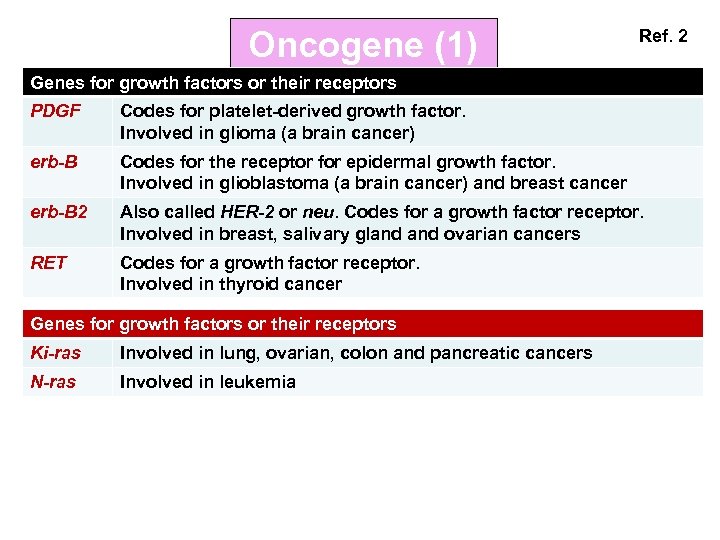
Oncogene (1) Ref. 2 Genes for growth factors or their receptors PDGF Codes for platelet-derived growth factor. Involved in glioma (a brain cancer) erb-B Codes for the receptor for epidermal growth factor. Involved in glioblastoma (a brain cancer) and breast cancer erb-B 2 Also called HER-2 or neu. Codes for a growth factor receptor. Involved in breast, salivary gland ovarian cancers RET Codes for a growth factor receptor. Involved in thyroid cancer Genes for growth factors or their receptors Ki-ras Involved in lung, ovarian, colon and pancreatic cancers N-ras Involved in leukemia
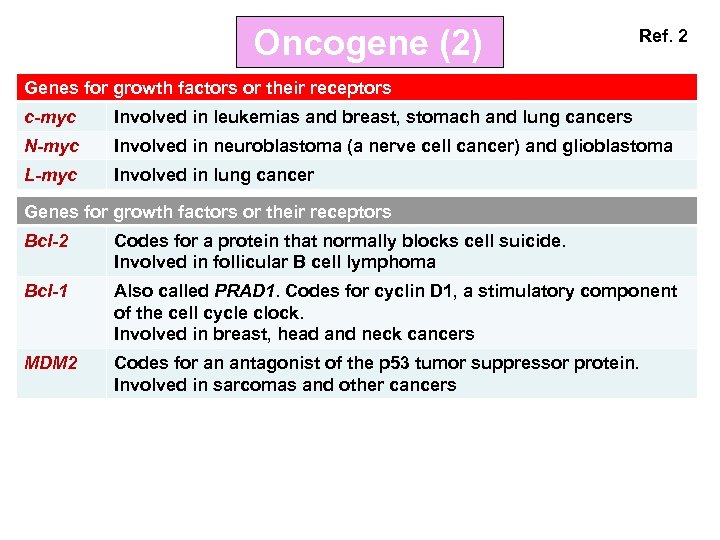
Oncogene (2) Ref. 2 Genes for growth factors or their receptors c-myc Involved in leukemias and breast, stomach and lung cancers N-myc Involved in neuroblastoma (a nerve cell cancer) and glioblastoma L-myc Involved in lung cancer Genes for growth factors or their receptors Bcl-2 Codes for a protein that normally blocks cell suicide. Involved in follicular B cell lymphoma Bcl-1 Also called PRAD 1. Codes for cyclin D 1, a stimulatory component of the cell cycle clock. Involved in breast, head and neck cancers MDM 2 Codes for an antagonist of the p 53 tumor suppressor protein. Involved in sarcomas and other cancers
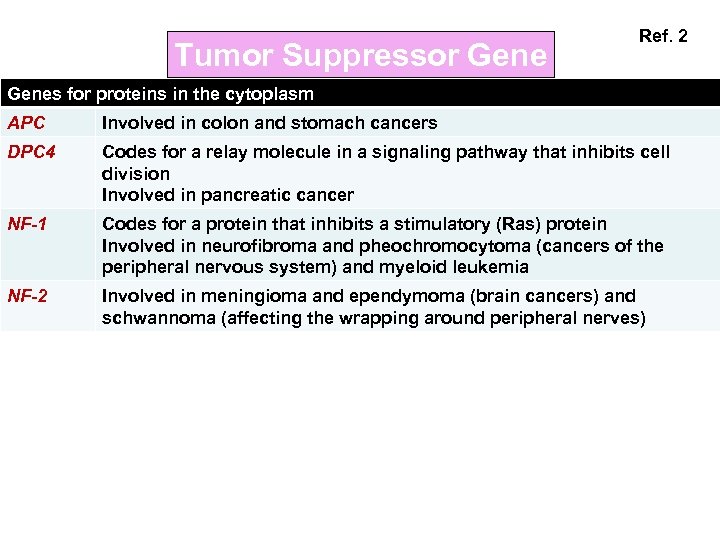
Tumor Suppressor Gene Ref. 2 Genes for proteins in the cytoplasm APC Involved in colon and stomach cancers DPC 4 Codes for a relay molecule in a signaling pathway that inhibits cell division Involved in pancreatic cancer NF-1 Codes for a protein that inhibits a stimulatory (Ras) protein Involved in neurofibroma and pheochromocytoma (cancers of the peripheral nervous system) and myeloid leukemia NF-2 Involved in meningioma and ependymoma (brain cancers) and schwannoma (affecting the wrapping around peripheral nerves)
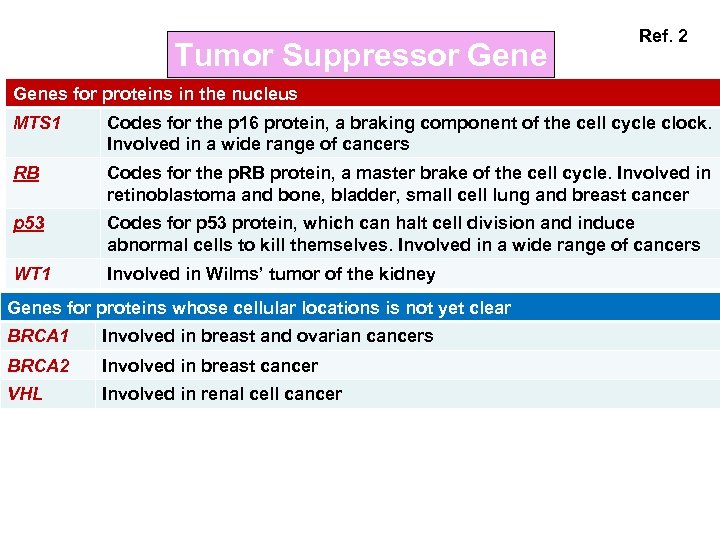
Tumor Suppressor Gene Ref. 2 Genes for proteins in the nucleus MTS 1 Codes for the p 16 protein, a braking component of the cell cycle clock. Involved in a wide range of cancers RB Codes for the p. RB protein, a master brake of the cell cycle. Involved in retinoblastoma and bone, bladder, small cell lung and breast cancer p 53 Codes for p 53 protein, which can halt cell division and induce abnormal cells to kill themselves. Involved in a wide range of cancers WT 1 Involved in Wilms’ tumor of the kidney Genes for proteins whose cellular locations is not yet clear BRCA 1 Involved in breast and ovarian cancers BRCA 2 Involved in breast cancer VHL Involved in renal cell cancer

基因突變地圖 Ref. 2 在各種癌症中發現超過百種以上的突變基因 癌化理論 → 標準教條: 細胞循環中,正常 促進細胞形成 基因 o過 度活化 ,變成致癌基因 ; 抑制細胞形 而 成基因 o發生突變,失去功能 X,成為抑 癌基因 A Subway Map for Cancer Pathways
第三站總結 癌化理論 → 標準教條: 細胞循環中,原來正常的 腫瘤致 癌基因 與 抑癌基因 發生突變而 請注意以下的重點提要 失控; 造成 致癌基因 過度活化及 抑癌 基因 失去功能 Tumor development occurs due to formations of oncogene & tumor suppressor gene
第四站 癌化的四個理論 標準教條 Ref. 2
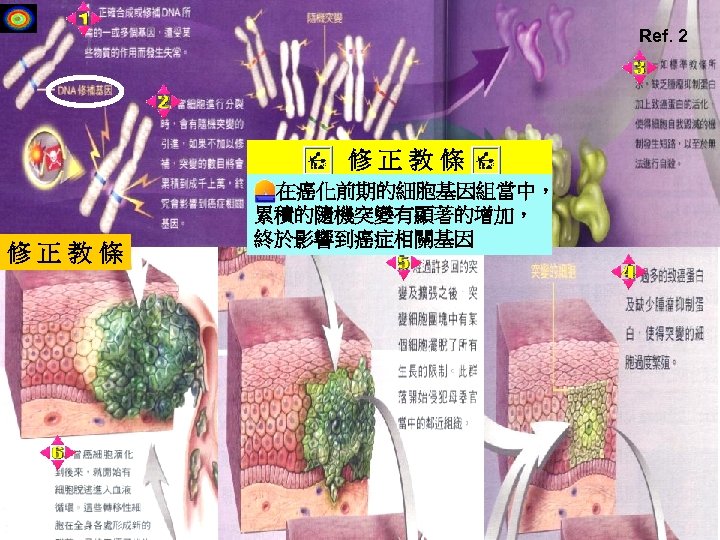
Ref. 2 修正教條 在癌化前期的細胞基因組當中, 累積的隨機突變有顯著的增加, 終於影響到癌症相關基因
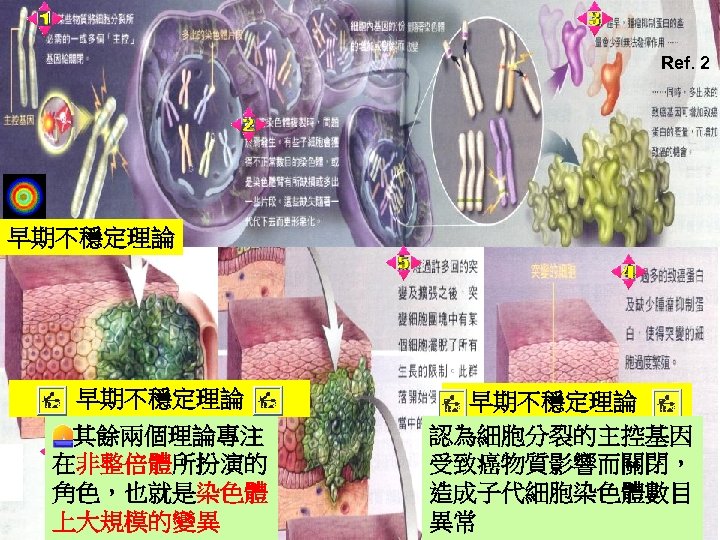
Ref. 2 早期不穩定理論 其餘兩個理論專注 在非整倍體所扮演的 角色,也就是染色體 上大規模的變異 早期不穩定理論 認為細胞分裂的主控基因 受致癌物質影響而關閉, 造成子代細胞染色體數目 異常
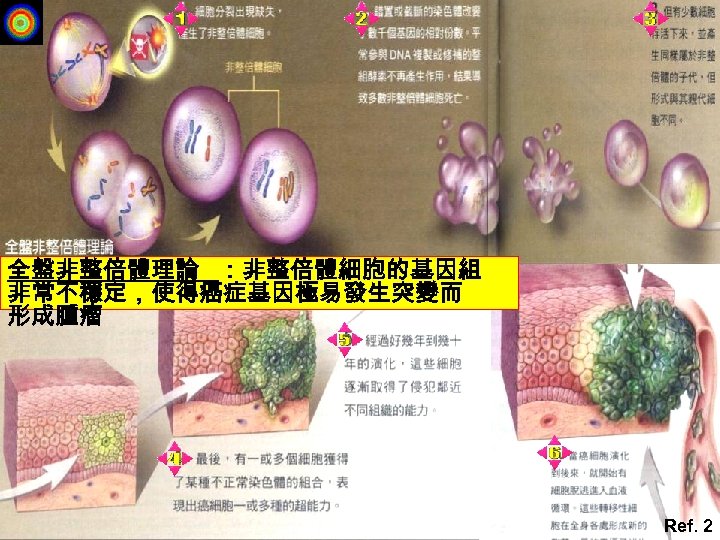
全盤非整倍體理論 :非整倍體細胞的基因組 非常不穩定,使得癌症基因極易發生突變而 形成腫瘤 Ref. 2
Ref. 2 隨染色體起舞 癌症是一種基因的疾病 然而癌症的複雜情況, 卻不能用簡單的「基因 突變」來描述。 最近理論認為,染色體 的異常可能才是細胞邁 向癌症之路的第一步。
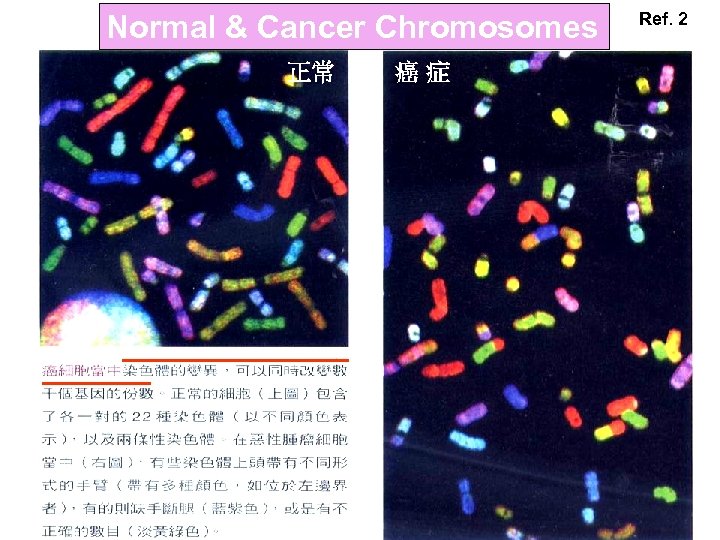
Normal & Cancer Chromosomes 正常 癌症 Ref. 2
第四站總結 請注意以下的重點提要 癌化的四個理論:(1)致癌基因、抑癌基因;(2) 修 正 教 條;(3)早期不穩定理論;(4)全盤非整 倍體理論
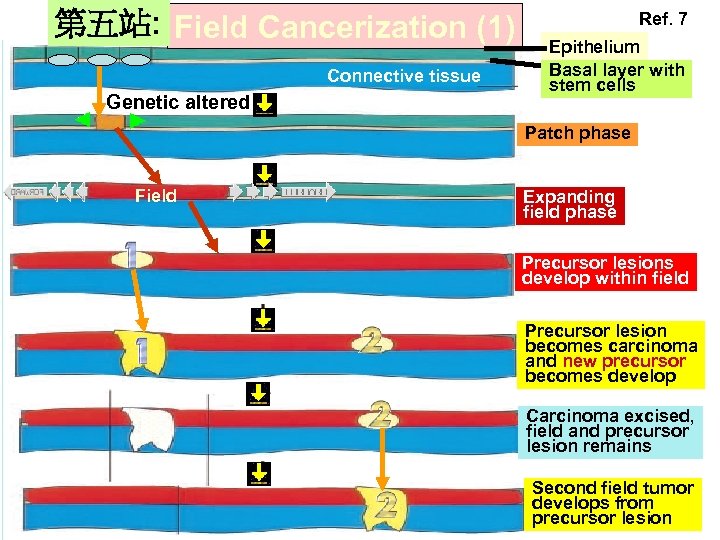
第五站: Field Cancerization (1) Connective tissue Genetic altered Ref. 7 Epithelium Basal layer with stem cells Patch phase Field Expanding field phase Precursor lesions develop within field Precursor lesion becomes carcinoma and new precursor becomes develop Carcinoma excised, field and precursor lesion remains Second field tumor develops from precursor lesion
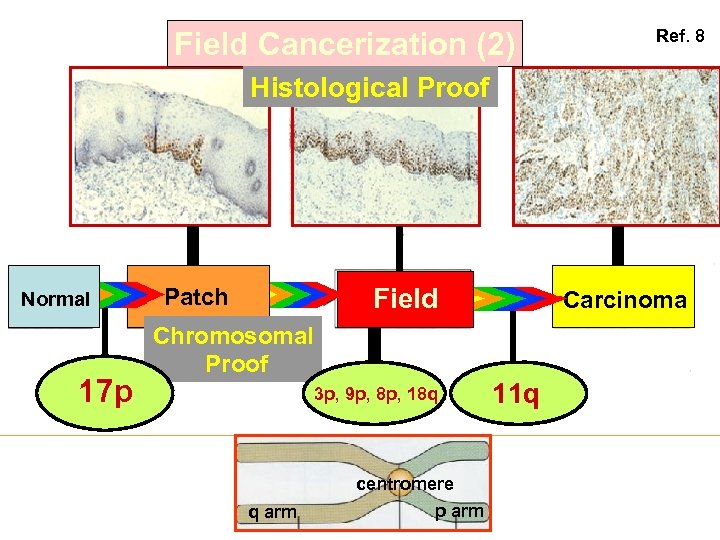
Field Cancerization (2) Ref. 8 Histological Proof Normal 17 p Field Patch Carcinoma Chromosomal Proof 3 p, 9 p, 8 p, 18 q centromere q arm p arm 11 q
第五站總結 瞭解 Field cancerization的形成 : Normal→Patch→Field→Cancer 請注意以下的重點提要 瞭解 Field cancerization的重要 : 腫瘤切除要有足夠的 safe margin Formation of field cancerization Meaning of field cancerization
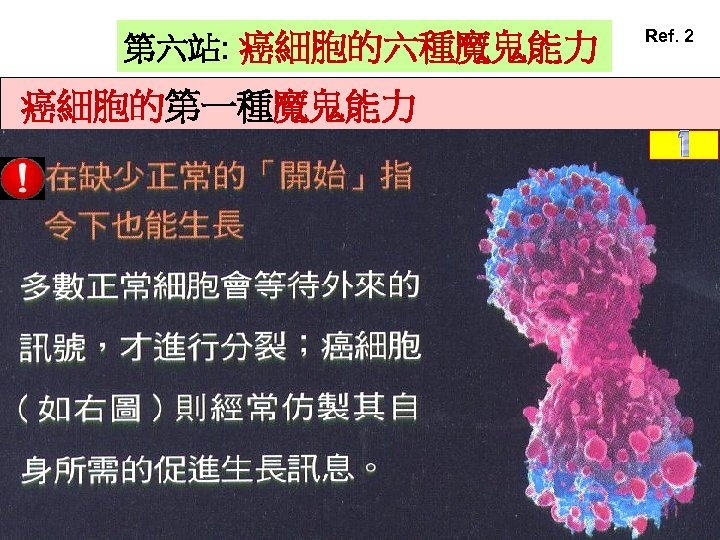
第六站: 癌細胞的六種魔鬼能力 癌細胞的第一種魔鬼能力 Ref. 2
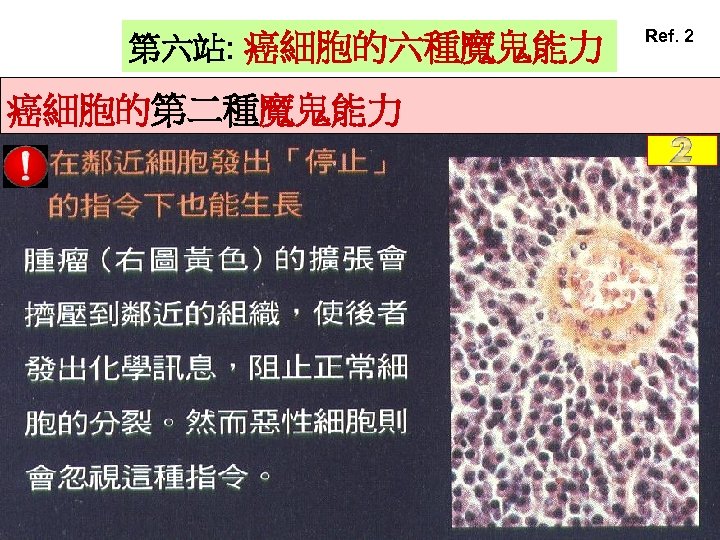
第六站: 癌細胞的六種魔鬼能力 癌細胞的第二種魔鬼能力 Ref. 2
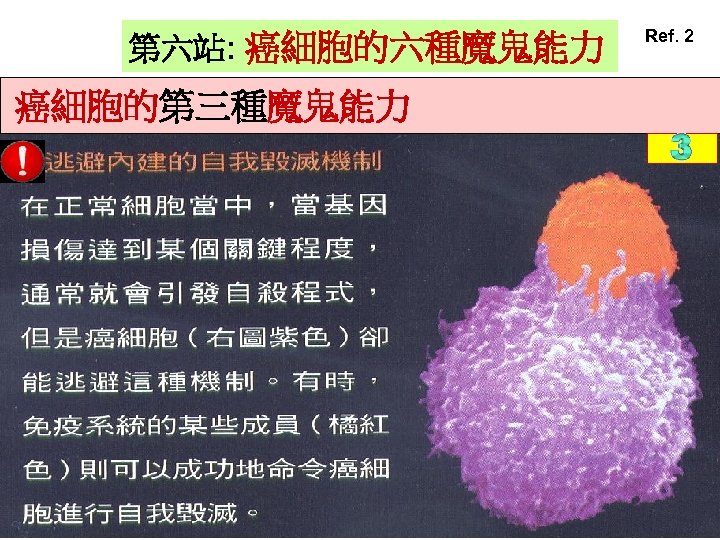
第六站: 癌細胞的六種魔鬼能力 癌細胞的第三種魔鬼能力 Ref. 2

第六站: 癌細胞的六種魔鬼能力 癌細胞的第四種魔鬼能力 Ref. 2
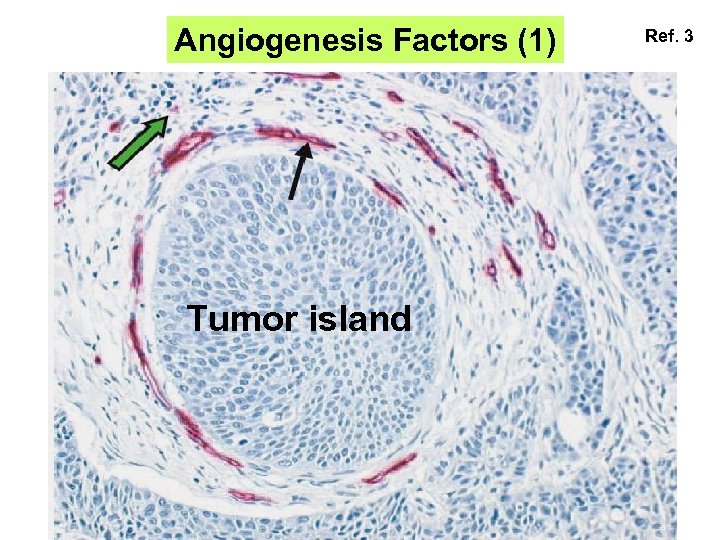
Angiogenesis Factors (1) Tumor island Ref. 3
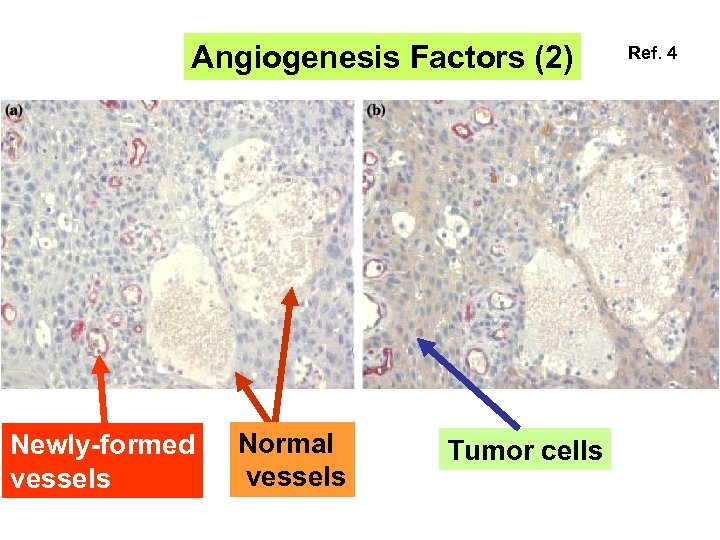
Angiogenesis Factors (2) Newly-formed vessels Normal vessels Tumor cells Ref. 4
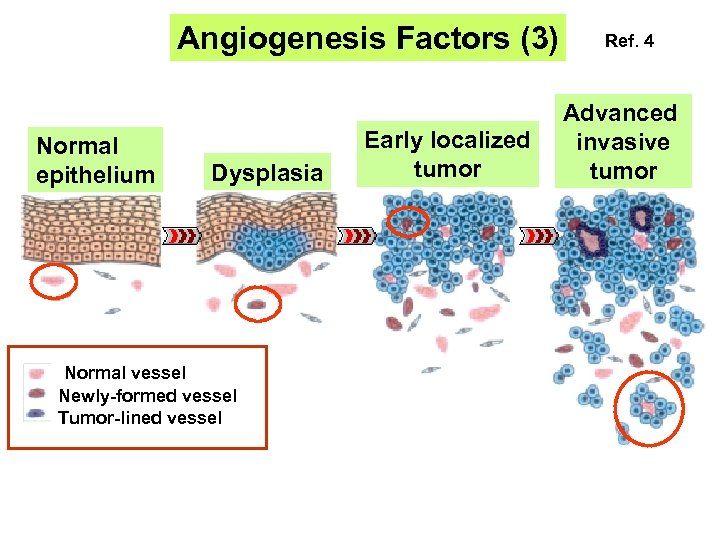
Angiogenesis Factors (3) Normal epithelium Dysplasia Normal vessel Newly-formed vessel Tumor-lined vessel Early localized tumor Ref. 4 Advanced invasive tumor
第六站: 癌細胞的六種魔鬼能力 Ref. 2 癌細胞的第五種魔鬼能力 centromere q arm 藍 p arm
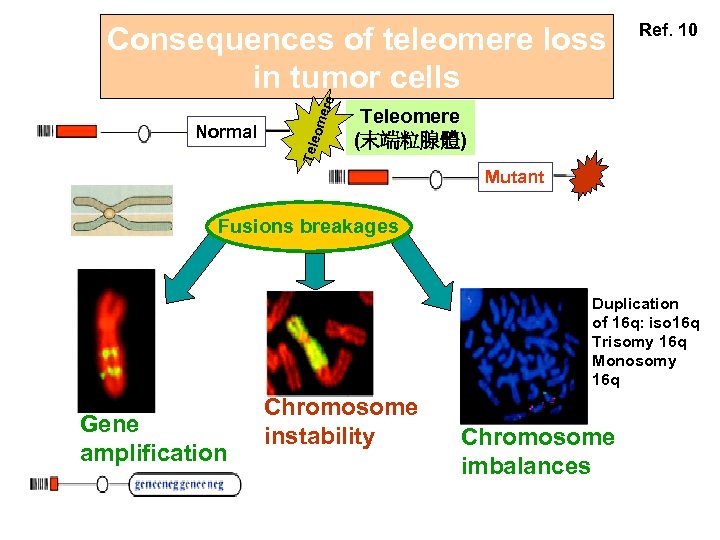
Tele Normal ome re Consequences of teleomere loss in tumor cells Ref. 10 Teleomere (末端粒腺體) Mutant Fusions breakages Duplication of 16 q: iso 16 q Trisomy 16 q Monosomy 16 q Gene amplification Chromosome instability Chromosome imbalances
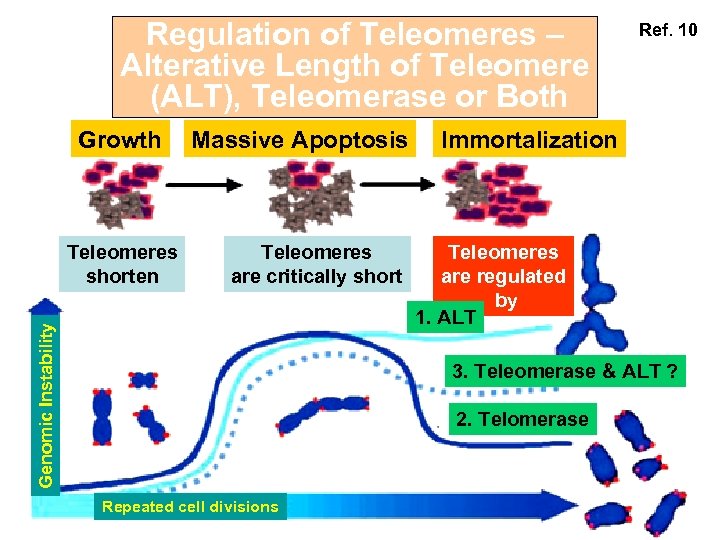
Regulation of Teleomeres – Alterative Length of Teleomere (ALT), Teleomerase or Both Growth Teleomeres are critically short Genomic Instability Teleomeres shorten Massive Apoptosis Ref. 10 Immortalization Teleomeres are regulated by 1. ALT 3. Teleomerase & ALT ? 2. Telomerase Repeated cell divisions
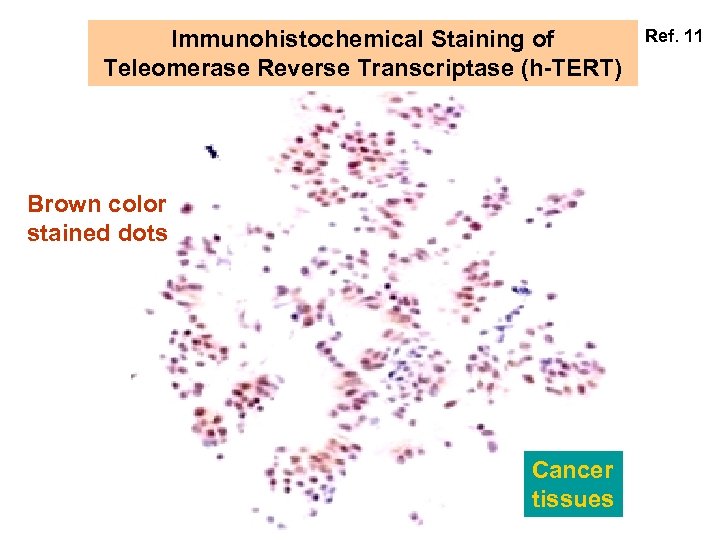
Immunohistochemical Staining of Teleomerase Reverse Transcriptase (h-TERT) Brown color stained dots Cancer tissues Ref. 11
第六站: 癌細胞的六種魔鬼能力 癌細胞的第六種魔鬼能力 Ref. 2
第六站總結 瞭解 癌細胞的六種魔鬼能力 1. Self replication : 2. Loss of contact inhibition 請注意以下的重點提要 3. Escape from apoptosis 4. Ability of angiogenesis 5. Immortalization 6. Ability of invasion & metastasis Understand the six super abilities of cancer cells

Causes and Prevention Ref. 1 第七站 What Causes Cancer? The top two causes - tobacco and dietaccount for almost two thirds of all cancer deaths and are amongst most correctable Virus PAPILLOMA VIRUS is a significant cause of cancer Chemical-environment

Most Oral Carcinoma in Taiwan is Associated with Betel Quid Ref. 12

Lancet Oncology 2001; August 印度檳榔包裝 Ref. 6

Lancet Oncology 2003; October 印度的檳榔攤 Ref. 5
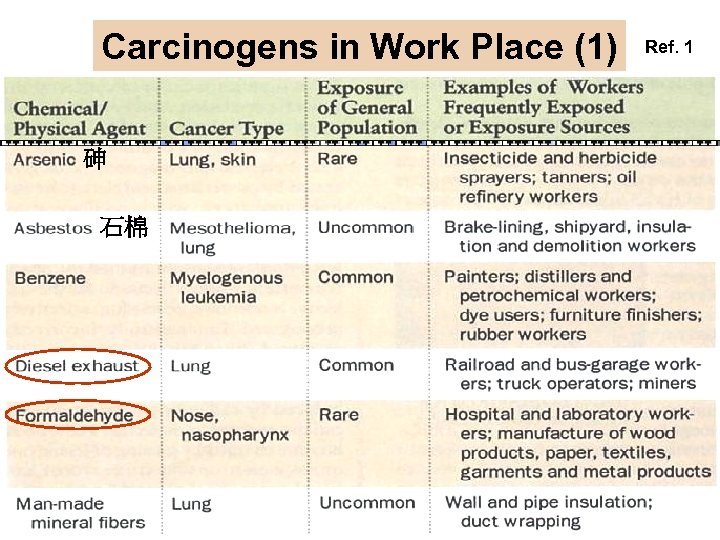
Carcinogens in Work Place (1) 砷 石棉 Ref. 1
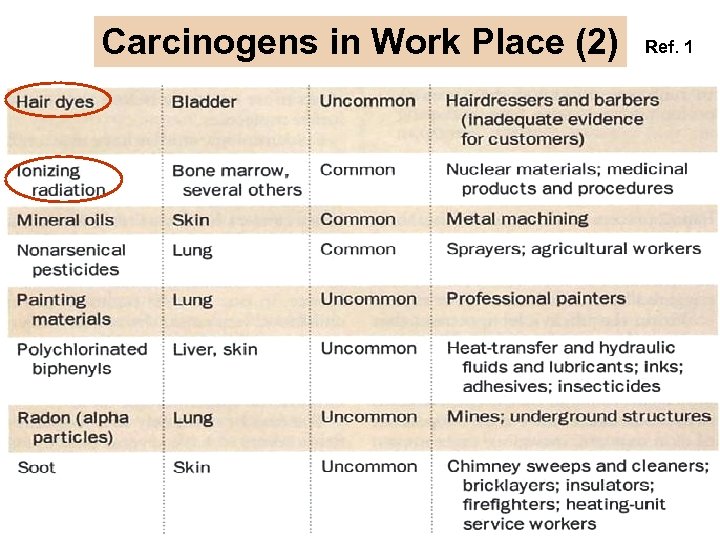
Carcinogens in Work Place (2) Ref. 1
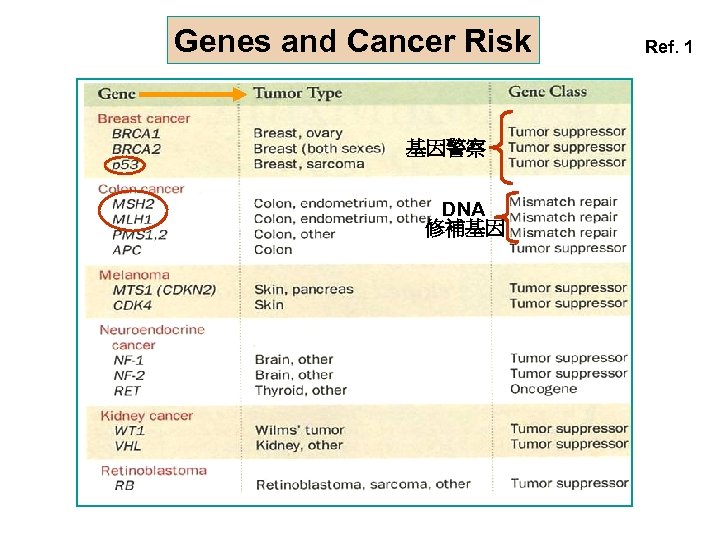
Genes and Cancer Risk 基因警察 DNA 修補基因 Ref. 1
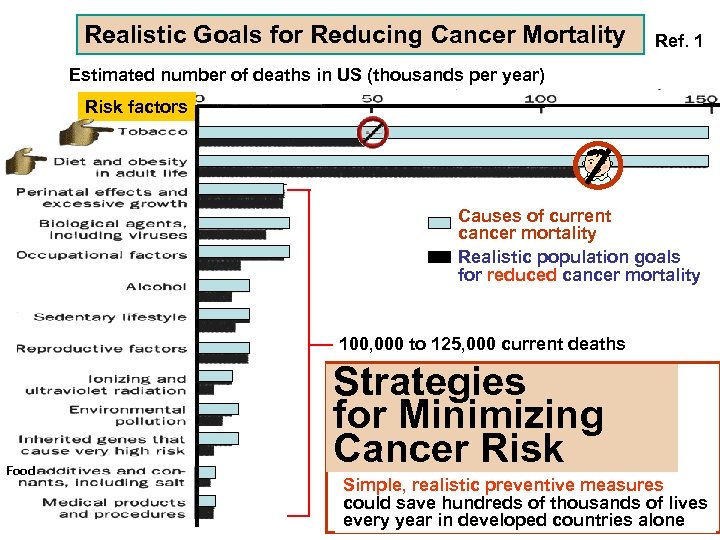
Realistic Goals for Reducing Cancer Mortality Ref. 1 Estimated number of deaths in US (thousands per year) Risk factors Causes of current cancer mortality Realistic population goals for reduced cancer mortality 100, 000 to 125, 000 current deaths Food Strategies for Minimizing Cancer Risk Simple, realistic preventive measures could save hundreds of thousands of lives every year in developed countries alone

Chemoprevention of Cancer Someday people should be able to avoid cancer or delay its onset by taking specially formulated pills or foods Ref. 1
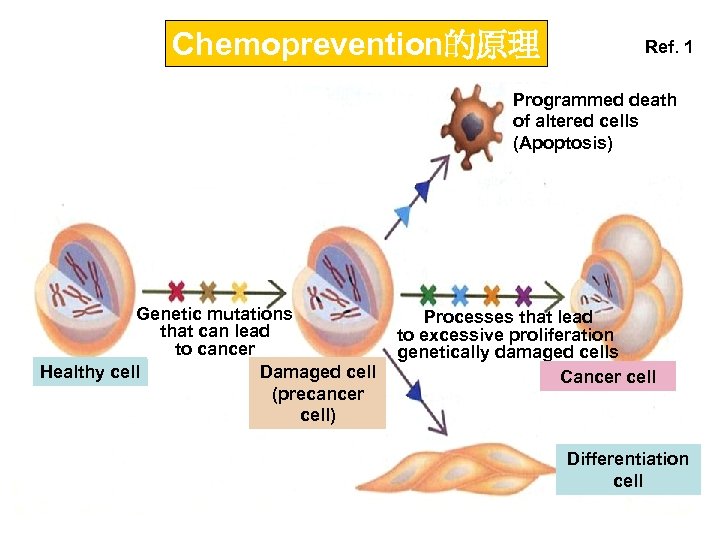
Chemoprevention的原理 Ref. 1 Programmed death of altered cells (Apoptosis) Genetic mutations that can lead to cancer Healthy cell Damaged cell (precancer cell) Processes that lead to excessive proliferation genetically damaged cells Cancer cell Differentiation cell

Ref. 1 Earlier Detection Advances in Cancer Detection Tests to look for the presence of a tumor before any symptoms appear may save more lives than new drug therapies do Chromosome 17 BRCA 1 A family search for BRCA 1 mutation
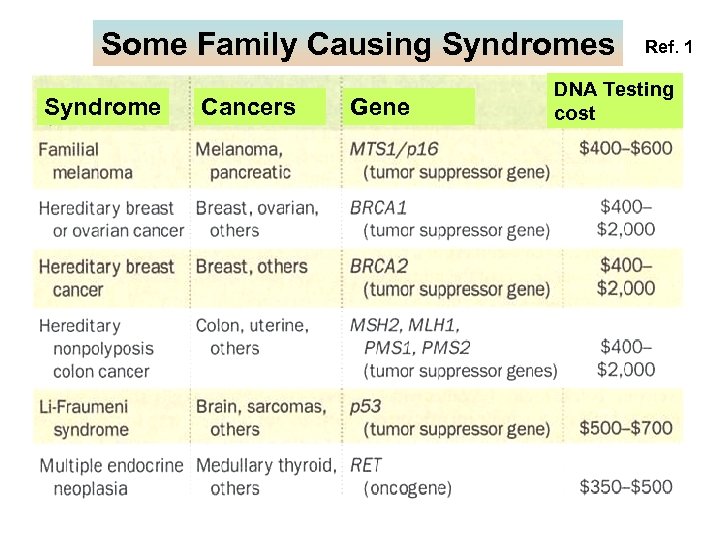
Some Family Causing Syndromes Syndrome Cancers Gene Ref. 1 DNA Testing cost
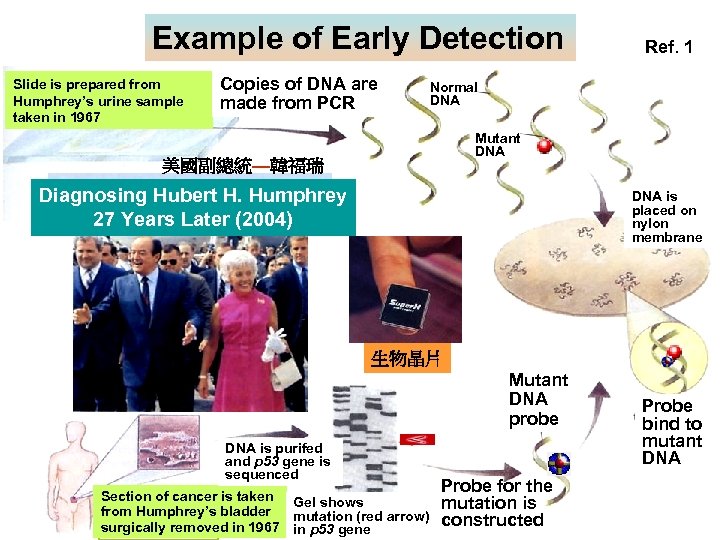
Example of Early Detection Slide is prepared from Humphrey’s urine sample taken in 1967 Copies of DNA are made from PCR Ref. 1 Normal DNA Mutant DNA 美國副總統—韓福瑞 Diagnosing Hubert H. Humphrey 27 Years Later (2004) DNA is placed on nylon membrane 生物晶片 Mutant DNA probe DNA is purifed and p 53 gene is sequenced Section of cancer is taken Gel shows from Humphrey’s bladder mutation (red arrow) surgically removed in 1967 in p 53 gene Probe for the mutation is constructed Probe bind to mutant DNA
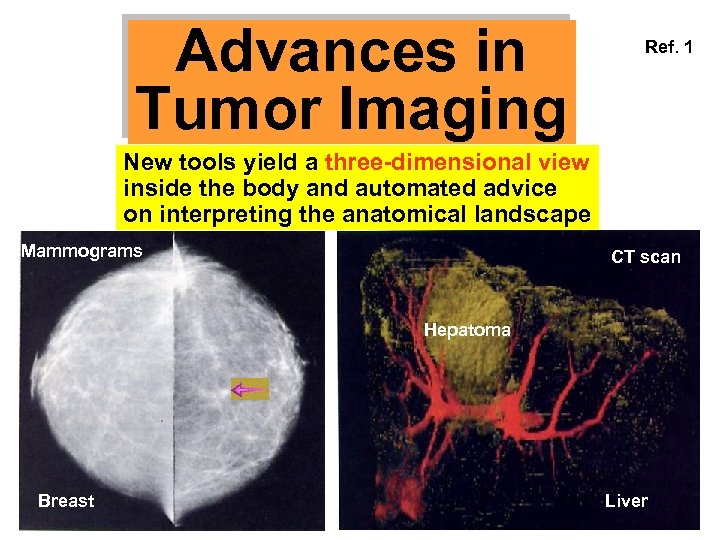
Advances in Tumor Imaging Ref. 1 New tools yield a three-dimensional view inside the body and automated advice on interpreting the anatomical landscape Mammograms CT scan Hepatoma Breast Liver
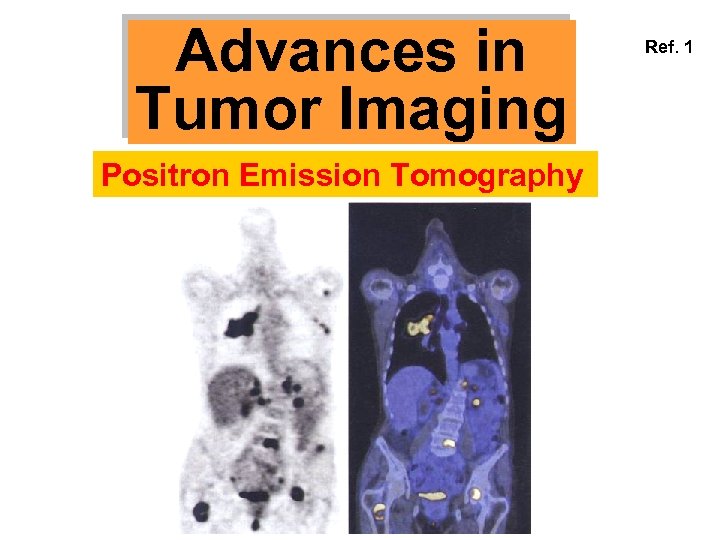
Advances in Tumor Imaging Positron Emission Tomography Ref. 1
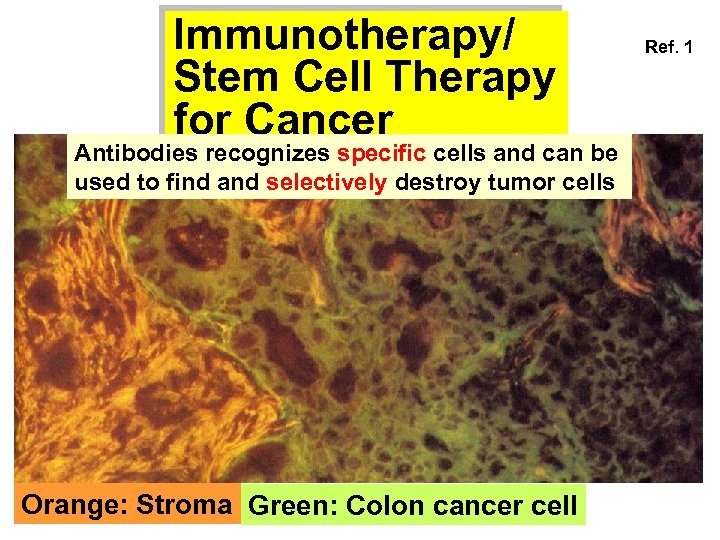
Immunotherapy/ Stem Cell Therapy for Cancer Antibodies recognizes specific cells and can be used to find and selectively destroy tumor cells Orange: Stroma Green: Colon cancer cell Ref. 1
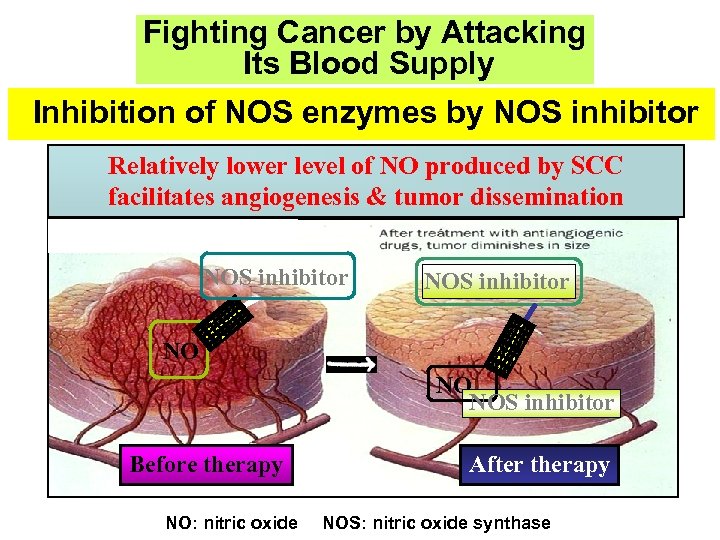
Fighting Cancer by Attacking Its Blood Supply Inhibition of NOS enzymes by NOS inhibitor Relatively lower level of NO produced by SCC facilitates angiogenesis & tumor dissemination NOS inhibitor NO NO NOS inhibitor Before therapy NO: nitric oxide After therapy NOS: nitric oxide synthase
第七站總結 瞭解 以下各點 : 1. Causes and Prevention 2. 3. 4. 5. 6. 7. Carcinogens in Work Place 請注意以下的重點提要 Genes and Cancer Risk Chemoprevention of Cancer Advances in Tumor Imaging Immunotherapy/Stem Cell Therapy Inhibition of Angiogenesis Understand the causes and prevention of cancer

Summaries 瞭解 以下各點 : 1. 2. 3. 4. 5. 6. 7. How cancer arise Stages of carcinogenesis 癌化的標準教條 四種癌化理論 Field cancerization 癌細胞的六種超能力 癌症的預防
d19533f940234244ec456b8fddc91019.ppt