240e3cccff58e0f18ace8cf6fae8292a.ppt
- Количество слайдов: 86

ระบบการตามสอบสนคาเกษตรแ ละอาหาร ในประเทศคคา พรพรหม ชยฤทธไชย ผอำนวยการ ศนยสารสนเทศ สำนกงานมาตรฐานสนคาเกษตรและอาหารแ หงชาต Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

Main topics • ความสำคญของระบบตามสอบสนคาเ กษตรและอาหาร • มาตรฐานกฎระเบยบของประเทศคคา – ระบบการตามสอบในยโรป – ระบบการตามสอบในสหรฐ • ความกาวหนาการตามสอบของไทย Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

ความสำคญ ของระบบตามสอบสนคาเกษตรและ อาหาร • • Frequent outbreak Economic loss Trade requirement PL law Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร
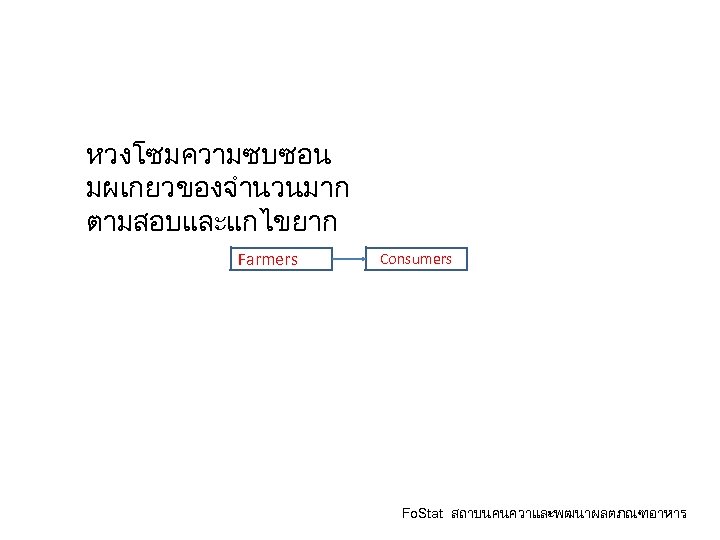
หวงโซมความซบซอน มผเกยวของจำนวนมาก ตามสอบและแกไขยาก Farmers Consumers Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร
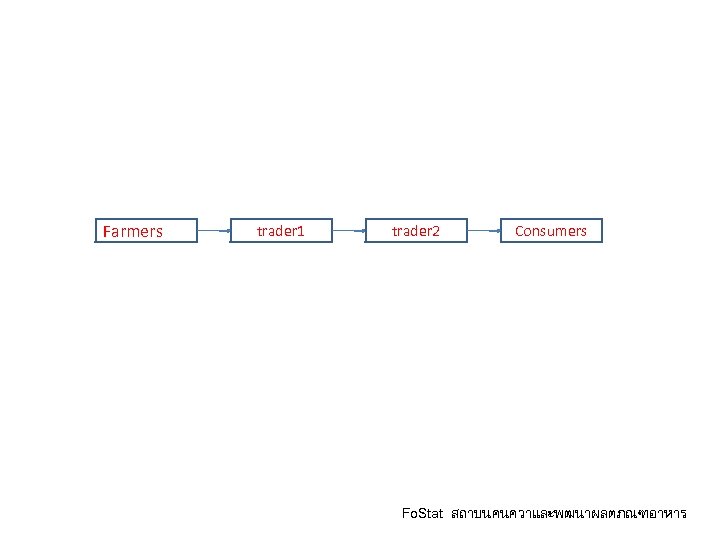
Farmers trader 1 trader 2 Consumers Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร
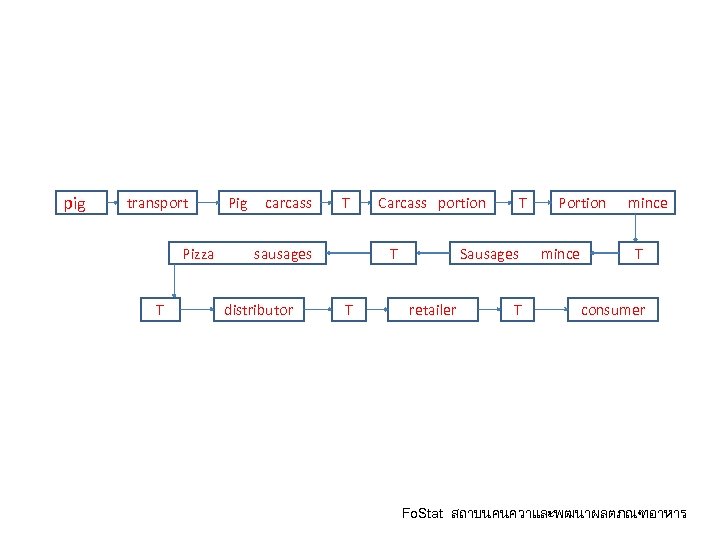
pig transport Pig carcass T Pizza sausages T distributor Carcass portion T T T Portion mince Sausages mince retailer T T consumer Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร
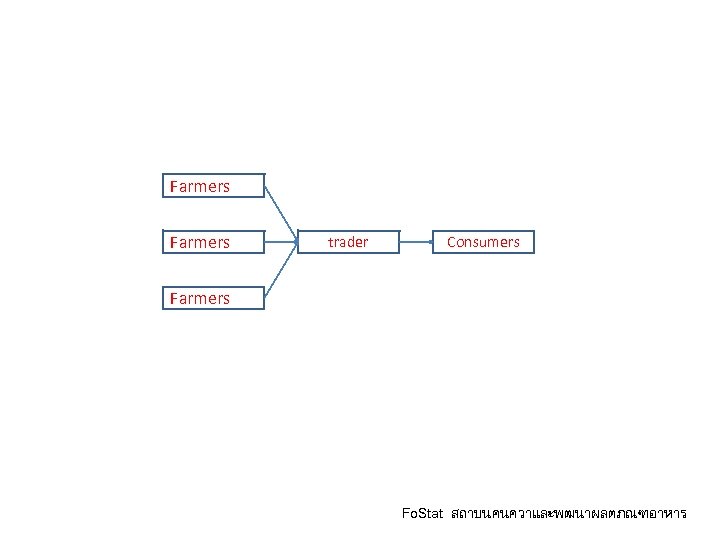
Farmers trader Consumers Farmers Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร
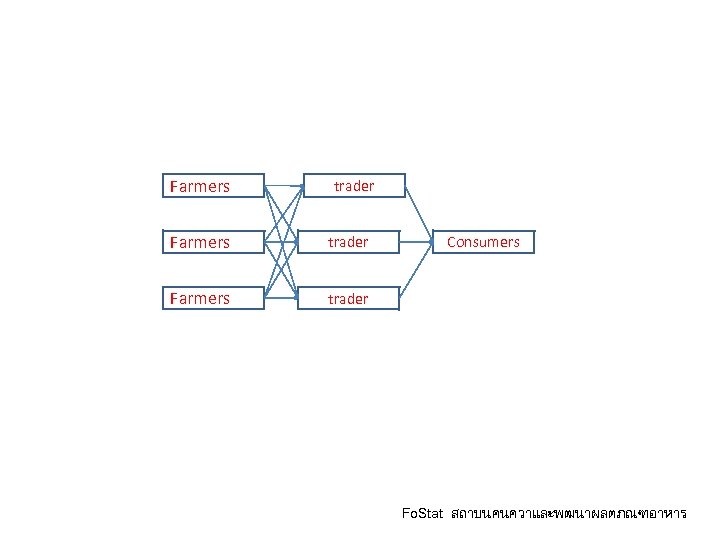
Farmers trader Consumers Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร
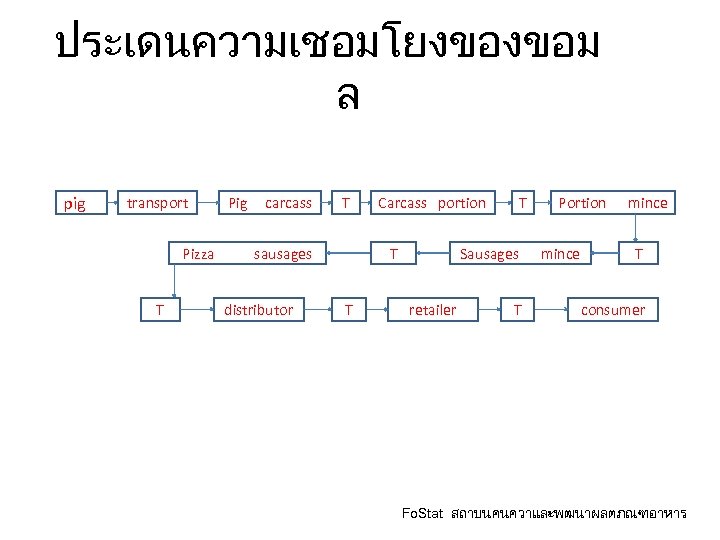
ประเดนความเชอมโยงของขอม ล pig transport Pig carcass T Pizza sausages T distributor Carcass portion T T T Portion mince Sausages mince retailer T T consumer Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร
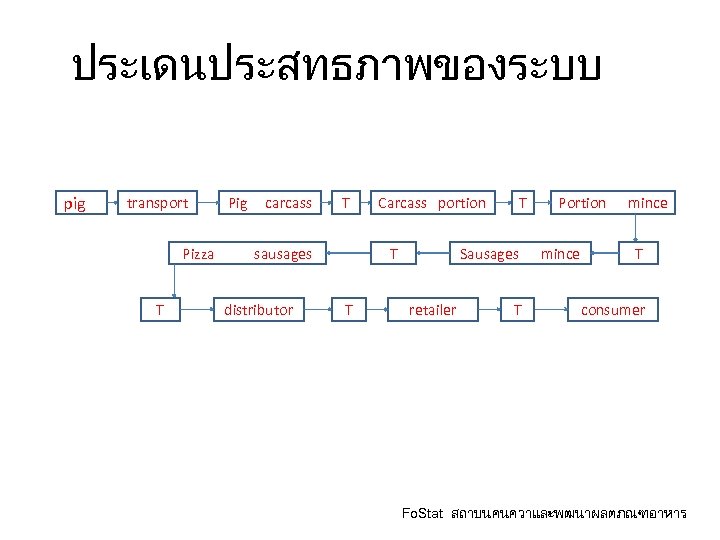
ประเดนประสทธภาพของระบบ pig transport Pig carcass T Pizza sausages T distributor Carcass portion T T T Portion mince Sausages mince retailer T T consumer Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร
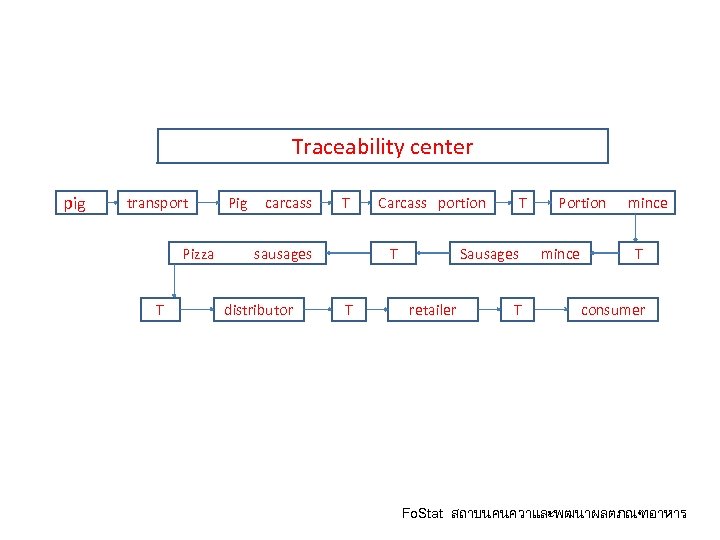
Traceability center pig transport Pig carcass T Pizza sausages T distributor Carcass portion T T T Portion mince Sausages mince retailer T T consumer Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร
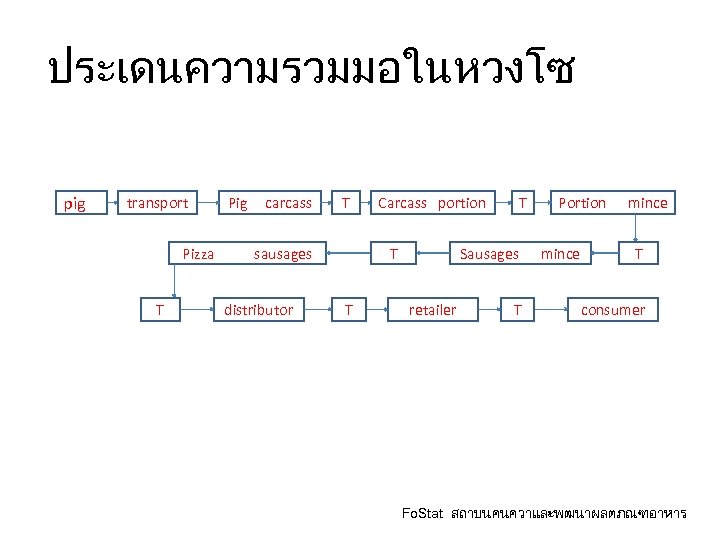
ประเดนความรวมมอในหวงโซ pig transport Pig carcass T Pizza sausages T distributor Carcass portion T T T Portion mince Sausages mince retailer T T consumer Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

ประโยชน สบหาสาเหตไดรวดเรว จำกดขอบเขตของปญหา แกไขปญหาไดตรงจด ปองกนไมใหเกดขนไดอก สรางความโปรงใส ผบรโภคมนใจในระบบความปลอดภ ย • ลดผลกระทบ • • • Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

อะไรคอสงทตองการ • ประสทธภาพของระบบ • ระบบกฎหมายทรดกม • ความรวมมอของทกหนวย ในหวงโซ Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

การตามสอบในสหภาพยโรป • White paper http: //eur-lex. europa. eu/Lex. Uri. Serv/site/en/com/1999/com 1999_0719 en 01. pdf • General Food Law http: //eur-lex. europa. eu/pri/en/oj/dat/2002/l_03120020201 en 00010024. pdf • Traceability implementation http: //ec. europa. eu/food/animal/diseases/traces/what_is/index_en. htm http: //www. trace. eu. org/menu/project/ Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

White paper Para 10, Chap 2 Principles of Food Safety Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

The EU White Paper on Food Safety introduced proposals “ to transform EU food policy into a proactive, dynamic, coherent and comprehensive instrument to ensure a high level of human health and consumer protection ”. “ A successful food policy demands the traceability of feed and food and their ingredients ”. Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

General Food Law Regulation 178/2002 http: //eur-lex. europa. eu/pri/en/oj/dat/2002/l_03120020201 en 00010024. pdf Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

European Regulation • Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety [See amending acts]. • http: //www. southwestfarmer. co. uk/news/farmingnews/4864256. Pig_producers_ welcome_changes_to_European_labelling_rules/ • http: //www. food. gov. uk/newsarchive/2010/jan/coolresearch Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

178/2002 EU General Food Law Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

• Article 3 of Regulation 178/2002/EC, the General Food Law, defines ‘Traceability' as being the ability to trace and follow a food, feed, food-producing animal or substance intended to be, or expected to be incorporated into a food or feed, through all stages of production, processing and distribution. Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

• Traceability is a fundamental requirement of all Quality Management Systems that require documented procedures aimed at product identification, from the purchase of the starting materials throughout the whole production process and shipment. A sound knowledge of the food chain, feed included, is needed to identify the critical control points in the chain and to implement appropriate controls. Traceability as such does not improve food/feed safety, but establishes the transparency needed for efficient control measures. All traceability measures should be part of the quality system of the food/feed producer and not handled separately Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

• The general principles of food law under Regulation 178/2002 require all feed and food businesses “ to have in place systems so that they can identify the supplier of foods, feeds, food producing animals and to whom they have supplied such products ”. Since 1 st January 2006, food sector operators must comply with the provisions of the aforementioned Regulation: • The general duty of compliance (Articles 14 and 17), • Traceability (Article 18), and • Incident management (Article 19) Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

• Prior to January 2006, Industry Guidelines on traceability were developed by the European food and drink industry. These are particularly relevant for SMEs, which count for the fast majority of the CIAA Membership (more then 90% of CIAA Members are SMEs). • Practical industry experiences confirm that the principles of traceability must be simple and efficient to be able to be met by all operators in the food chain. Existing requirements, such as the indication of batch identification (lot numbers), product labelling, or, in the case of animal derived food, the use of health marking, are all relevant tools. • The requirement for traceability must rely on the responsibility of each operator in the food chain. Each operator has to record and archive relevant information for traceability. A reference which enables a link with this information must be passed on to the next operator. • Industry operators have put such systems in place. Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

EU Food labelling • Council Directive 2000/13/EC • Commission Directive 2001/101/EC of 26 November 2001 regulating the definition of meat for labelling purpose, where meat is used as an ingredient in foodstuffs, and by Directive 2003/89/EC regard indication of the ingredients present in foodstuffs. • Under reviewed. Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

• Introducing the proposal, health commissioner Kyprianou explained "Food labels can have a huge influence on consumers' purchasing decisions. Confusing, overloaded or misleading labels can be more of a hindrance than a help to the consumer. Today's proposal aims to ensure that food labels carry the essential information in a clear and legible way, so that EU citizens are empowered to make balanced dietary choices". Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

• Food labels revisited • One day not so far in the future, Europeans may be able to buy a loaf of bread knowing where the flour came from. Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

• The EU is considering expanding the use of food labels to show where the product was farmed. The step follows a Europewide consultation on the issue of food quality. Farmers, producers and consumers voiced strong support for greater use of ‘place-of-farming’ labels. Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

• Such labels indicate the country of harvest, not where the product was processed. They are already mandatory for some foods sold in the EU, including unprocessed beef, poultry, fruit, vegetables, eggs, honey, wine and olive oil. Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

• In a paper spelling out its position, the commission says it will take into account the concerns of processors and retailers, who worry they will have a hard time tracking down the origins of ingredients in processed food. Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

• The EU has some of the most stringent farming requirements in the world. But many consumers question the quality of products from outside the EU, more so in the wake of several scares involving imported food in recent years. Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

• European farmers like the labels because they add appeal to their products, both in the EU and in the global marketplace. Many want the labels to be even more precise, showing the particular region where the product was farmed. Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

• The paper also calls for changes to clear up confusion caused by the proliferation of other kinds of food labels in the EU. Many countries, producers and retailers have adopted schemes that are different from those used by the EU. Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

• The commission wants to abolish the EU label for identifying and protecting the names of traditional products. There have been just 20 registrations since the scheme was set up in 1992. They include a traditional Finnish biscuit, mozzarella cheese produced in the Italian tradition and certain Belgian beers. Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

• EU labels referring to a product’s geographical origin would also be revised. Examples of products carrying this logo include Camembert cheese from the Normandy region of France; prosciutto from Parma, Italy; Kalamata olive oil from Greece; Scotch beef from the UK and bratwurst sausage from Nuremberg, Germany. Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

• Meanwhile, an EU logo for organic foods is being developed. Starting in 2010, it will be mandatory for all products sold as organic in the EU. Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร
Traceability implementation • EU TR@CES • Trace project Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

EU TR@CES • EU TR@CES (TRAde Control and Expert System) is a trans-European network for veterinary health which notifies, certifies and monitors imports, exports and trade in animals and animal products. (decision 2002/459/EC) http: //ec. europa. eu/food/animal/diseases/traces/what_is/index_en. htm Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

Trace project • Trace project aims to improve the health and well-being of European citizens by delivering improved traceability of food products. (5 year project sponsored by the European Commission) • will provide consumers with added confidence in the authenticity of European food through complete traceability along entire fork to farm food chains. • will develop cost effective analytical methods integrated within sectorspecific and -generic traceability systems that will enable the determination and the objective verification of the origin of food. • will focus firstly on mineral water, cereals, honey, meat and chicken but will have wider applicability to other commodities. http: //www. trace. eu. org/menu/project/ Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

Overview of the TRACE project • • • • The TRACE project is a five year research project funded by the European Commission through the Sixth Framework Programme that aims to deliver traceability systems and methods that will enhance consumer confidence in the authenticity of European food. It has done this though the development and implementation of improvements in traceability and verification procedures. Some of the key outputs of the project are: Specification prediction models – results of extensive multi-disciplinary scientific research that have linked components in food with those in its immediate environment. Fingerprinting procedures that can verify species, variety and production origin An open source molecular biological database containing information on methods and sequences for use in authenticating food A traceability language that allows different traceability systems to communicate with each other Testing of developed traceability systems within industry A wikipedia for food traceability An extensive consumer behaviour study on food traceability Web-based interactive communication vehicles for dissemination to stakeholders Over 50 peer reviewed research papers The establishment of an international network of researchers and stakeholders that are exploiting TRACE technologies through implementation in the food industry The project has also provided training in the subject areas to 49 trainees from 10 countries and hosted 12 workshops and 6 international conferences. Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

Improved food chain traceability as a result of TRACE • • Traceability is the ability to trace the history, application or location of an entity by means of recorded identifications. For a given process or product, we want to know all raw materials and ingredients that went into it. For a given raw material or ingredient, we want to know all processes and products that it went into. In a supply chain with many links, this means recording information in each link and passing it along with the product. In TRACE, we have developed tools, methods and guidelines to aid in the recording and passing on of this type of food product information. This includes: ‘Good Traceability Practice' guidelines both for the food industry in general and for specific sectors Software tools for recording food product information A standardized language (Trace. Core XML) for enabling electronic data interchange between links in the supply chain for food products Data dictionaries for various food sectors (naming and meaning of terms) A common and re-usable framework (Trace. Food Framework) encompassing all of the above, a All these tools and methods have been implemented in pilot chains in the food industry. A dynamic repository of related knowledge and experiences has been set up (Trace. Food Wiki), and industry training courses and presentation materials have been developed. This presentation gives an overall, non-technical overview of how food chain traceability has -, or can be improved as a result of these activities and results Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร
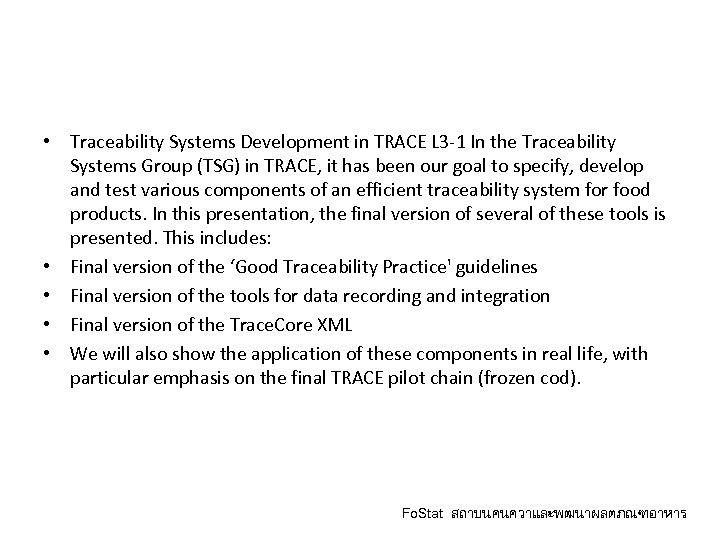
• Traceability Systems Development in TRACE L 3 -1 In the Traceability Systems Group (TSG) in TRACE, it has been our goal to specify, develop and test various components of an efficient traceability system for food products. In this presentation, the final version of several of these tools is presented. This includes: • Final version of the ‘Good Traceability Practice' guidelines • Final version of the tools for data recording and integration • Final version of the Trace. Core XML • We will also show the application of these components in real life, with particular emphasis on the final TRACE pilot chain (frozen cod). Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

• • Exploitation of traceability outputs after TRACE ? L 3 -2 This presentation focuses on the TSG products that will live on after TRACE. The presentation will consist of three main parts: the Trace. Food wiki, the foodtraceability website, and the Trace. Core model. During the last year the Trace. Food Framework and GTP have been transformed into the Trace. Food wiki. A wiki based tool for scientific collaboration in food traceability. Our goal with this tool is to provide a hub for cooperation between both countries and businesses. The content includes: Fundamentals of traceability, both principles and definitions, The good traceability practice guide, both generic and for certain sectors, tools, including the work on the Trace. Core model and process mapping methods, traceability projects, both national and international, and traceability references. The wiki can be found at http: //www. tracefood. org. In addition to the Trace. Food wiki, we have also developed the foodtraceability website which provides an easier and interactive approach to traceability. This site is structured around different actors view on traceability and includes viewpoints from: the consumer, public authorities, quality assurance, supply chain management, operational and IT. Within each viewpoint we have small introductory flash videos explaining the basics of traceability from the viewpoint of the actor, the user can also read more in depth information, test the principles in interactive flash games, access relevant training material and read more about what other actors think of the current actors related to traceability. The website can be found at http: //www. foodtraceability. eu. A comprehensive XSD Schema, called Trace. Core XML (TCX) version 2. 2, is now finished and finalized. The TCX has been tested in various scenarios in the entire fork to farm food chain and the tests have verified the usability of the standard. However, Trace. Core. XML adoption has had limited success so far. The group behind TCX has therefore discussed how TCX could get more momentum in the industry in the future. After the Trace project ends in December 2009 there are no more funds to further develop, support and maintain the format. It has therefore been important to try to identify commercially driven processes that can support the further development of the format. The group has now decided that EPCglobal probably is the best community to approach. Also, in order to be able to map the traceability model behind Trace. Core onto more than only one format (not necessarily only XML), the TCX group has also created an abstract model to represent the Trace. Core data model and traceability model. Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

Consumers' perception of food traceability in Europe • • The introduction of improved food traceability systems has aimed to restore consumer confidence in food safety and quality, in part by being able to provide consumers with more information about the origins of foods and food ingredients. However little is known about consumer attitudes and beliefs associated with traceability, nor their preferences for information provision. Given the heterogeneity of European consumers, it is not surprising that they have different perceptions and expectations regarding and understanding the concept of ‘traceability'. Consumers may also perceive traceability differently for different product types. The present paper is based upon an analysis of focus groups in 12 countries across Europe. It explains how European consumers understand the traceability of food products as well as their expectations of traceability for different types of food product (meat and honey have been used as examples). Labelling schemes for these two types of products are also examined. The results showed that dissimilarities exist in consumers' perceptions of traceability in different countries. Some dissimilarity also exists between consumers' expectations of traceability and the information they require. However, labels are still seen as an important way of communicating with consumers, although the participants claimed that these labels need to be understandable and more easily accessible to facilitate consumer understanding. Hence, consumer information needs and requirements regarding traceability are investigated. Semi-structured interviews with consumers in four European countries focused on the need for traceability, the preferred means of communication, labelling, and bodies held responsible for traceability and dealing with fraud. Furthermore, consumer perceptions regarding traceability were investigated by means-end-chain laddering. Consumers in four European countries were questioned about the benefits they associate with traceability related attributes. The benefits consumers associate with traceability are in terms of health, quality, safety and control, of which the latter was associated with trust and confidence. These benefits were similarly important in the countries investigated. Cross-national differences were also observed. Importantly, both quality and safety were shown to be related to traceability in the consumers' minds with quality implying safety. The results show that traceability may contribute to improving consumer confidence. The most important aspects of traceability which should be incorporated into communication with consumers are discussed. Keywords : Consumer, traceability, food quality, food safety, focus groups, laddering interviews, labels Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

Traceability in USA • FDA • USDA/FSIS • States, local, tribal food safety agencies and law enforcement agencies, Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

กฎหมายเกยวกบการตามสอบในส หรฐ • Federal Food, Drug, and Cosmetic Act • the Public Health Security and Bioterrorism Preparedness and Response Act – Section 305 (Registration of Food Facilities) – Section 306 (Establishment and Maintenance of Records) – Section 307 (Prior Notice of Imported Food Shipments) http: //www. fda. gov/Regulatory. Information/Legislation/default. htm • Food Safety Enhancement Act (SEC. 107 Traceability of food) http: //www. govtrack. us/congress/bill. xpd? bill=h 111 -2749 • Food Safety Modernization Act (SEC. 210 Traceback requirements) H. R. 875: Food Safety Modernization Act of 2009 Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

CFR • The Code of Federal Regulations (CFR) is the codification of the general and permanent rules and regulations (sometimes called administrative law) published in the Federal Register by the executive departments and agencies of the Federal Government of the United States. The CFR is published by the Office of the Federal Register, an agency of the National Archives and Records Administration (NARA). • The CFR is divided into 50 titles that represent broad areas subject to Federal regulation. • From wikipedia Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

List of regulation titles • • • • • • • Title 1: General Provisions Title 2: Grants and Agreements Title 3: The President Title 4: Accounts Title 5: Administrative Personnel Title 6: Homeland Security Title 7: Agriculture Title 8: Aliens and Nationality Title 9: Animals and Animal Products Title 10: Energy Title 11: Federal Elections Title 12: Banks and Banking Title 13: Business Credit and Assistance Title 14: Aeronautics and Space (also known as the Federal Aviation Regulations, administered by the Federal Aviation Administration) Title 15: Commerce and Foreign Trade Title 16: Commercial Practices Title 17: Commodity and Securities Exchanges Title 18: Conservation of Power and Water Resources Title 19: Customs Duties Title 20: Employees' Benefits Title 21: Food and Drugs (administered by the US Food and Drug Administration and the US Drug Enforcement Administration) Title 22: Foreign Relations Title 23: Highways Title 24: Housing and Urban Development Title 25: Indians Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

• • • • • • • Title 26: Internal Revenue Title 27: Alcohol, Tobacco Products and Firearms Title 28: Judicial Administration Title 29: Labor Title 30: Mineral Resources Title 31: Money and Finance: Treasury Title 32: National Defense Title 33: Navigation and Navigable Waters Title 34: Education Title 35: Reserved (formerly Panama Canal) Title 36: Parks, Forests, and Public Property Title 37: Patents, Trademarks, and Copyrights Title 38: Pensions, Bonuses, and Veterans' Relief Title 39: Postal Service Title 40: Protection of Environment (administered by the United States Environmental Protection Agency) Title 41: Public Contracts and Property Management Title 42: Public Health Title 43: Public Lands: Interior Title 44: Emergency Management and Assistance Title 45: Public Welfare Title 46: Shipping Title 47: Telecommunication (also known as the "FCC Rules", administered by the Federal Communications Commission) Title 48: Federal Acquisition Regulations System Title 49: Transportation Title 50: Wildlife and Fisheries Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

Recent development • Traceability Reviewed by FDA/FSIS (December 2009) • Federal food safety agencies need to increase the speed and accuracy of traceback investigations and traceforward operations. • FDA commissioned IFT to examine traceability system (report published in January 2010) • http: //www. fsis. usda. gov/OPPDE/rdad/FRPubs/Docket_No. FDA-2009 -0523. htm Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

FDA/FSIS Reviews • FDA and FSIS intend the public meeting to stimulate and focus a discussion about the core elements of product tracing systems, gaps in current product tracing systems, and mechanisms to enhance product tracing systems for food. FDA and FSIS also intend the public meeting to improve the ability of FDA and FSIS to use the information in such systems to identify the source of contamination during outbreaks of foodborne illness, and to improve the ability of all persons in the supply chain to more quickly identify food that is (or potentially is) contaminated and remove it from the market during traceforward operations. This discussion will help FDA and FSIS determine what short and long term steps each agency should take to enhance the current tracing system. Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

กฎหมายทเกยวของ • FDA Several sections in the FFDCA (such as sections 301, 402, 403, 412, 414, 416, 417 and 704(a)) (21 U. S. C. 321, 342, 343, 350(a), 350(c), 350(e), 350(f), and 374(a)) and section 361 of the Public Health Service Act (42 U. S. C. 264) provide authority for, or are otherwise relevant to, product tracing systems. Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

21 CFR part 1 subpart j Establishment, Maintenance, Availability of Records • Requires certain persons who manufacture, process, pack, transport, distribute, receive, hold, or import food to establish and maintain certain records identifying the immediate previous source of all food received, as well as the immediate subsequent recipient of all food released. The regulations describe the information that must be established and maintained, how long it must be maintained, and how quickly it must be available to FDA when FDA has a reasonable belief that an article of food is adulterated and presents a threat of serious adverse health consequences or death to humans or animals. The regulations also describe persons (e. g. , farms and restaurants) who are excluded from some or all of the requirements. Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

• Code of Federal Regulations, - Title 21, Food Labeling 1 • Title 21 – parts 100 – 1692 • Title 21 --parts 170 to 1993 • Federal Food, Drug, and Cosmetic Act (FD&C Act) Chapter IV: Food 4 • Fair Packaging and Labeling Act of 19665 • http: //www. fda. gov/Food/Labeling. Nutrition/Food. Labeling. Guidance. Regul atory. Information/default. htm Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

• • CHAPTER I--FOOD AND DRUG ADMINISTRATION, DEPARTMENT OF HEALTH AND HUMAN SERVICES (CONTINUED) Part 100 General 101 Food labeling 102 Common or usual name for nonstandardized foods 104 Nutritional quality guidelines for foods 105 Foods for special dietary use 106 Infant formula quality control procedures 107 Infant formula 108 Emergency permit control 109 Unavoidable contaminants in food for human consumption and food-packaging material 110 Current good manufacturing practice in manufacturing, packing, or holding human food 111 Current good manufacturing practice in manufacturing, packaging, labeling, or holding operations for dietary supplements 113 Thermally processed low-acid foods packaged in hermetically sealed containers 114 Acidified foods 115 Shell eggs 119 Dietary supplements that present a significant or unreasonable risk 120 Hazard Analysis and Critical Control Point (HACCP) systems 123 Fish and fishery products 129 Processing and bottling of bottled drinking water 130 Food standards: General 131 Milk and cream 133 Cheeses and related cheese products 135 Frozen desserts 136 Bakery products 137 Cereal flours and related products 139 Macaroni and noodle products 145 Canned fruits 146 Canned fruit juices 150 Fruit butters, jellies, preserves, and related products 152 Fruit pies 155 Canned vegetables 156 Vegetable juices 158 Frozen vegetables 160 Eggs and egg products 161 Fish and shellfish 163 Cacao products 164 Tree nut and peanut products 165 Beverages 166 Margarine 168 Sweeteners and table sirups 169 Food dressings and flavorings http: //www. access. gpo. gov/nara/cfr/waisidx_08/21 cfrv 2_08. html http: //www. fda. gov/Food/Labeling. Nutrition/Food. Labeling. Guidance. Regulatory. Information/default. htm Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

• 21 CFR 101. 3 และ 21 CFR 501. 3 Identity labeling of food in packaged form – Requires the principal display panel of a food in package form to bear a statement of the identity of the commodity. Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

• 21 CFR 101. 5 และ 21 CFR 501. 5 Food; name and place of business of manufacturer, packer, or distributor. – Requires the label of a food in packaged form to specify conspicuously the name and place of business of the manufacturer, packer, or distributor. Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

• 21 CFR 106. 90 Infant Formula Quality Control Procedures – Requires product coding for all infant formulas. Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

• 21 CFR part 111 Current Good Manufacturing Practice in Manufacturing, Packaging, Labeling, or Holding Operations for Dietary Supplements – Requires, among other things, identification of each lot of received components in a manner that allows tracing the lot to the supplier and the date received; using this unique identifier when recording the disposition of the lot of received components; establishing a batch, lot or control number for each finished batch of dietary supplements; and being able to determine the complete manufacturing history and control of the packaged and labeled dietary supplement through distribution. Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

• 21 CFR, 113. 60(c) ; 21 CFR, 114. 80(b) Thermally Processed Low-Acid Foods Packaged In Hermetically Sealed Containers; Acidified Foods – A product code must be established and included on the package of a food that is a thermally processed low-acid food packaged in a hermetically sealed container (Sec. 113. 60(c)) or an acidified food (Sec. 114. 80(b)). Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

• Section 417 of the FFDCA establishes requirements for FDA to establish a Reportable Food Registry (RFR). A ``reportable food'' is an article of food (other than dietary supplements or infant formula) for which there is a reasonable probability that the use of, or exposure to, such article of food will cause serious adverse health consequences or death to humans or animals. The purpose of the RFR is to provide a ``reliable mechanism to track patterns of adulteration in food [which] would support efforts by the Food and Drug Administration to target limited inspection resources to protect the public health'' (Public Law 110 -085, section 1005(a)(4)). In accordance with section 417 of the FFDCA, FDA implemented on September 8, 2009, the RFR electronic portal by which instances of reportable food must be submitted to FDA by responsible parties and may be submitted by public health officials. Information as to the immediate prior source of the food and/or ingredients and the immediate subsequent recipient(s) of the food may be required to be submitted through the electronic portal. FDA has issued a guidance document (Ref. 11) containing questions and answers relating to the requirements under section 417 of the FFDCA . Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร
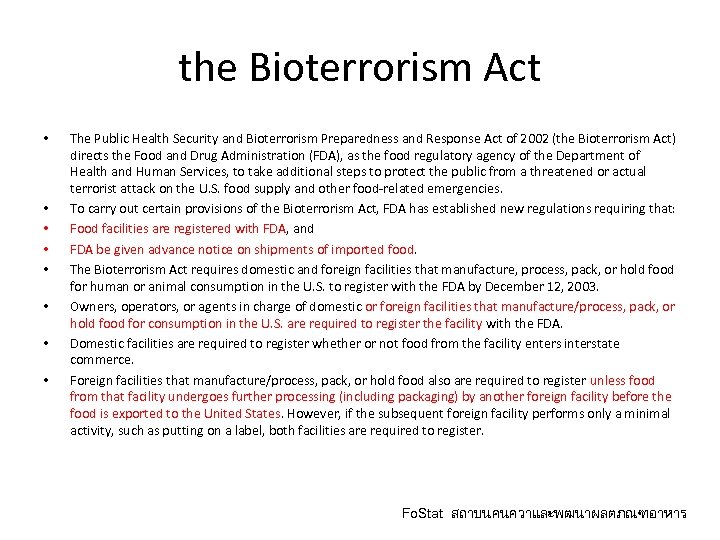
the Bioterrorism Act • • The Public Health Security and Bioterrorism Preparedness and Response Act of 2002 (the Bioterrorism Act) directs the Food and Drug Administration (FDA), as the food regulatory agency of the Department of Health and Human Services, to take additional steps to protect the public from a threatened or actual terrorist attack on the U. S. food supply and other food-related emergencies. To carry out certain provisions of the Bioterrorism Act, FDA has established new regulations requiring that: Food facilities are registered with FDA, and FDA be given advance notice on shipments of imported food. The Bioterrorism Act requires domestic and foreign facilities that manufacture, process, pack, or hold food for human or animal consumption in the U. S. to register with the FDA by December 12, 2003. Owners, operators, or agents in charge of domestic or foreign facilities that manufacture/process, pack, or hold food for consumption in the U. S. are required to register the facility with the FDA. Domestic facilities are required to register whether or not food from the facility enters interstate commerce. Foreign facilities that manufacture/process, pack, or hold food also are required to register unless food from that facility undergoes further processing (including packaging) by another foreign facility before the food is exported to the United States. However, if the subsequent foreign facility performs only a minimal activity, such as putting on a label, both facilities are required to register. Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร
FSIS • FSIS' statutes have sections that are relevant to product tracing systems for meat, poultry, and egg products subject to FSIS' jurisdiction. – the Federal Meat Inspection Act – the Poultry Products Inspection Act – the Egg Products Inspection Act Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร
• Section 642 of the Federal Meat Inspection Act (21 U. S. C. 601 et seq. ), • Section 460(b) of the Poultry Products Inspection Act (21 U. S. C. 451 et seq. ), and • Section 1040 of the Egg Products Inspection Act (21 U. S. C. 1031 et seq. ) – require certain classes of firms and corporations to maintain, retain, and make available full and correct business records or transactions in food. Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

• The regulations implementing those statutory sections, 9 CFR part 320, 9 CFR part 381, and 9 CFR 590. 200, specify businesses and what types of basic records are required, such as bills of sale, bills of lading, receiving and shipping papers, receipts and inventories. Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร
• Under the Federal Meat Inspection Act, FSIS also has the authority, under certain circumstances, to mandate specified recordkeeping by retail stores for certain violations and to withdraw or modify statutory exemptions for public health reasons (21 U. S. C. 623 and 454, 9 CFR parts 301 and 381). • The United States Code (U. S. C. ) is a compilation and codification of the general and permanent federal law of the United States. It contains 50 titles and is published every six years by the Office of the Law Revision Counsel of the House of Representatives. [ Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

Under FSIS' Hazard Analysis and Critical Control Points (HACCP) regulations (9 CFR part 417), • a meat or poultry establishment is required to keep records related to its HAACP plan, including all records associated with its operation (i. e. , monitoring, verification, and corrective action). The records of these activities are subject to FSIS review and are to be made available to FSIS personnel (9 CFR 417. 5(e) and (f)). Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

Especially relevant are • (1)all records, results, and supporting documentation associated with prerequisite programs ; • (2)the results and records associated with testing conducted for the establishment's business customer; and • (3)results and records associated with an establishment's quality control program. Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

• All of the records generated under the agency's statutory authority facilitate FSIS surveillance and investigation activities, and the control and removal of adulterated, misbranded, or otherwise illegal or unsafe products from commerce. Failure to keep such records negatively affects consumers' health and FSIS food safety and response activities (e. g. , foodborne illness investigations, product trace back, product trace forward, and product recall). Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

ปญหาอปสรรค • ไมสามารถตามสอบไดรวดเรว • การจดเกบบนทกยงไมเปนมาตรฐานเด ยวกน Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

แหลงตรวจสอบขอมล • Through the Listserv and Web page, FSIS is able to provide information to a much broader and more diverse audience. In addition, FSIS offers an electronic mail subscription service which provides automatic and customized access to selected food safety news and information. This service is available at http: //www. fsis. usda. gov/ news_and_events/email_subscription/. Options range from recalls to export information to regulations, directives and notices. Customers can add or delete subscriptions themselves, and have the option to password protect their accounts. Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

ความกาวหนาการตามสอบของไทย • มาตรฐานการตามสอบ มกษ 9028 -2551 หลกการตามสอบสนคาทเปนเครองมอในระบบการตรว จสอบและออกใบรบรองสนคาเกษตรและอาหาร • แผนงาน /โครงการ ในกระทรวงเกษตรและสหกรณ • พฒนาระบบตามสอบดวยระบบอเลกทรอนก ส Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร
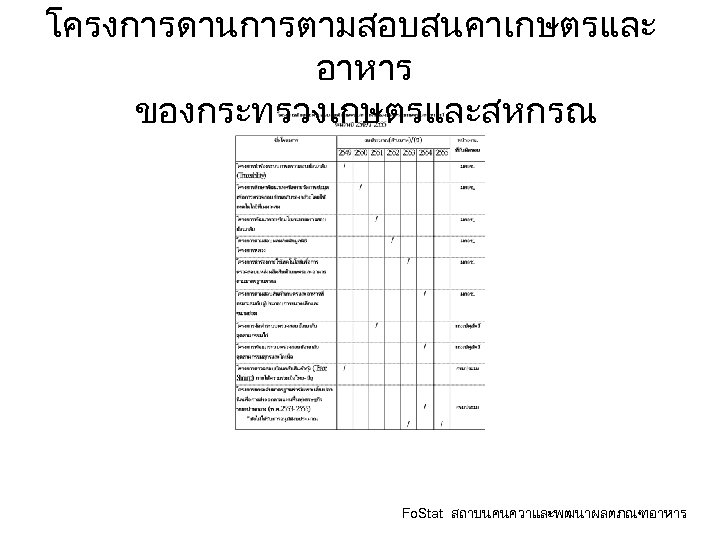
โครงการดานการตามสอบสนคาเกษตรและ อาหาร ของกระทรวงเกษตรและสหกรณ Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

พช 32 ชนด 1) ทเ รยน 2) ลำไย 3) ลน จ 4) สมโ อ 5) มะม วง 6) มงค ด 7) 8) 9) 10) 11) 12) 13) 14) ผกชไทย 15) ผกชฝรง 16) ใบกระเพรา 17) ใบโหระพา 18) ผกแขยง 19) ใบสะระแหน 20) ผกแพรว 21) ตนหอม 22) 23) คนไช 23) ใบบวบก ใบกยไช 24) ใบชะพล ดอกกยไช 25) ผกโขมแ ดง ชะอม 26) ถวฝก ตะไคร ยาว ผกบง 27) หนอไม ผกแวน ฝรง ผกกระเฉด 28) กระเจ ขาวโพดฝกออน ยบเขยว พรกข Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร 29)
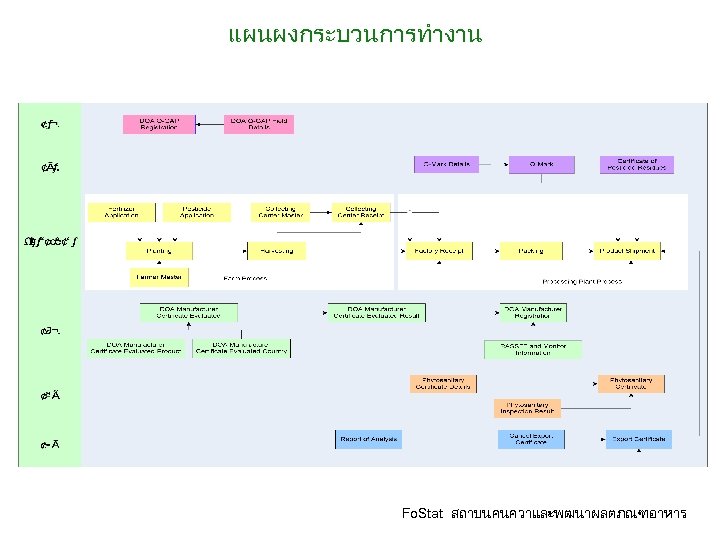
แผนผงกระบวนการทำงาน Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

ขอมลการใชปย ขอมลการเกบเกยว ขอมลการรบวตถดบเขาโรงงาน ขอมลการบรรจ ขอมลการสงออกสนคาและรายละเอยดสนคา ขอมลการสงออกสนคาและรายละเอยดใบรบร องสารพษตกคาง • ขอมลการสงออกสนคาและรายละเอยดใบรบร องปลอดศตรพช Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร • •
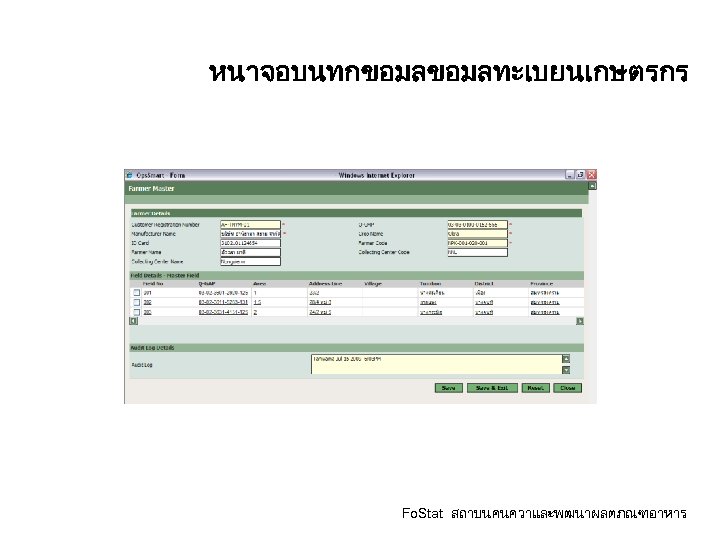
หนาจอบนทกขอมลทะเบยนเกษตรกร Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร
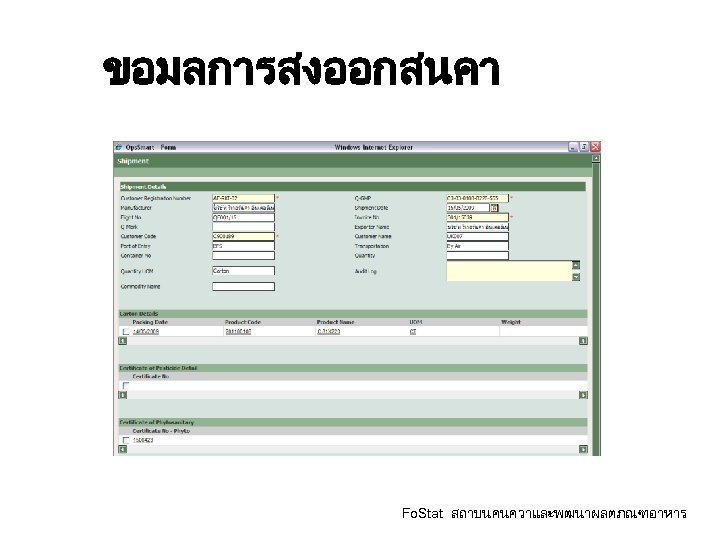
ขอมลการสงออกสนคา Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร
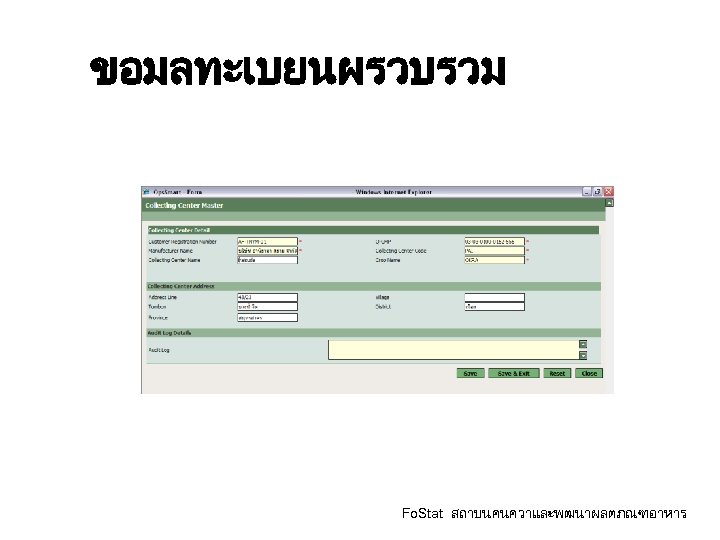
ขอมลทะเบยนผรวบรวม Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

ขอมลการรบวตดบทจด รวบรวม Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร
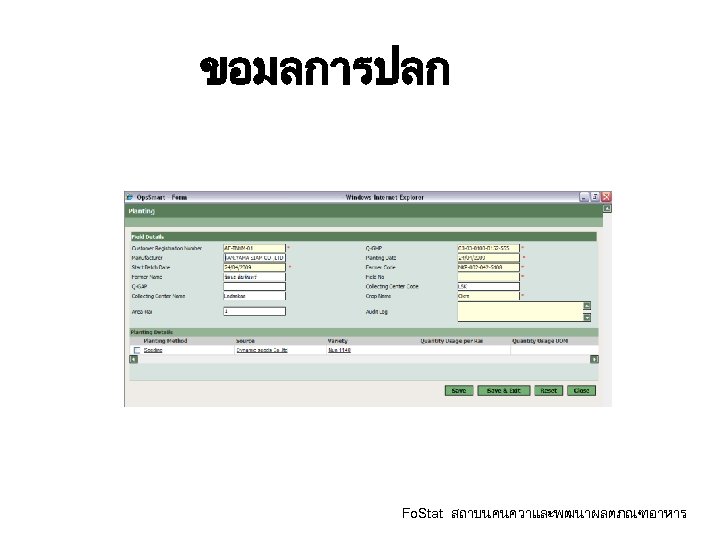
ขอมลการปลก Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร
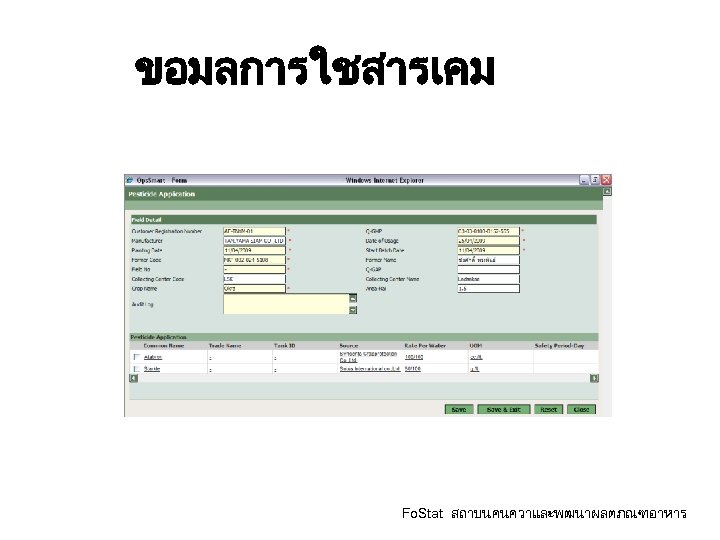
ขอมลการใชสารเคม Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

รปแบบขอมลของผประกอบกา รทนำเขาสระบบตรวจสอบย อนกลบ vรปแบบขอมลทจะนำเขาสระบบไดกำ หนดไว 2 รปแบบดงน § รปแบบ Text ไฟลตามรปแบบททางระบบของกรมวชาการเ กษตรกำหนด § รปแบบ Excel ไฟลตามรปแบบททางระบบของกรมวชาการเ กษตรกำหนด Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

ประโยชนของผประกอบการทเขารวมโคร งการ v ผประกอบการจะไดรบอนญาตใหสามารถใ ชระบบทจดทำขนเพอตรวจสอบยอนกล บสนคาของตนเองได vเพอเตรยมความพรอมของผประกอบการท จะสงออกสนคาในอนาคต ซงสหภาพยโรปจะบงคบใหผประกอบการ ตองมระบบตรวจสอบยอยกลบสนคาได Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร

ขอบคณครบ พรพรหม ชยฤทธไชย ผอำนวยการศนยสารสนเทศ มกอช pchairidchai@acfs. go. th www. acfs. go. th Fo. Stat สถาบนคนควาและพฒนาผลตภณฑอาหาร
240e3cccff58e0f18ace8cf6fae8292a.ppt