Presentation_final.pptx
- Количество слайдов: 38
 Transition metal luminescent complexes: new ideas in synthesis and applications Sergey P. Tunik, Institute of Chemistry, Laboratory of Transition Metal Clusters St. Petersburg University ICFM 2015, Novosibirsk
Transition metal luminescent complexes: new ideas in synthesis and applications Sergey P. Tunik, Institute of Chemistry, Laboratory of Transition Metal Clusters St. Petersburg University ICFM 2015, Novosibirsk
![Coinage metals chemistry, a very old story [Cu. Cl 2]− [Ag(NH 3)2]+ [Au(CN)2]− Angew. Coinage metals chemistry, a very old story [Cu. Cl 2]− [Ag(NH 3)2]+ [Au(CN)2]− Angew.](https://present5.com/presentation/-104014181_425074527/image-2.jpg) Coinage metals chemistry, a very old story [Cu. Cl 2]− [Ag(NH 3)2]+ [Au(CN)2]− Angew. Chem. – 3 papers, Chem. Comm. – 5 Papers, Inorg. Chem. - 7 Papers, Organometallics - 2 Papers, 20 papers in other international journals
Coinage metals chemistry, a very old story [Cu. Cl 2]− [Ag(NH 3)2]+ [Au(CN)2]− Angew. Chem. – 3 papers, Chem. Comm. – 5 Papers, Inorg. Chem. - 7 Papers, Organometallics - 2 Papers, 20 papers in other international journals
![[Cu]+, 4 Ph. C 2 H, 4 NEt 2 H, 4[Au. C 2 Ph]n [Cu]+, 4 Ph. C 2 H, 4 NEt 2 H, 4[Au. C 2 Ph]n](https://present5.com/presentation/-104014181_425074527/image-3.jpg) [Cu]+, 4 Ph. C 2 H, 4 NEt 2 H, 4[Au. C 2 Ph]n Au-Au 2. 97 Å Yield> 85% Au-Cu 2. 68 Å [Cu]+, Ph. C 2 H, NEt 2 H, [Au. C 2 Ph]n +NEt 2 H 9[Au. C 2 Ph]n + 3 Ph. C 2 H + 3 Ph 2 PC 6 H 4 PPh 2 M-M contact hold together 21 + 6[Cu] 15 metal ions Au(I) and Cu(I) St. Petersburg University Angew. Chem. Int. Ed. 2008, 47, 3942 – 3945
[Cu]+, 4 Ph. C 2 H, 4 NEt 2 H, 4[Au. C 2 Ph]n Au-Au 2. 97 Å Yield> 85% Au-Cu 2. 68 Å [Cu]+, Ph. C 2 H, NEt 2 H, [Au. C 2 Ph]n +NEt 2 H 9[Au. C 2 Ph]n + 3 Ph. C 2 H + 3 Ph 2 PC 6 H 4 PPh 2 M-M contact hold together 21 + 6[Cu] 15 metal ions Au(I) and Cu(I) St. Petersburg University Angew. Chem. Int. Ed. 2008, 47, 3942 – 3945
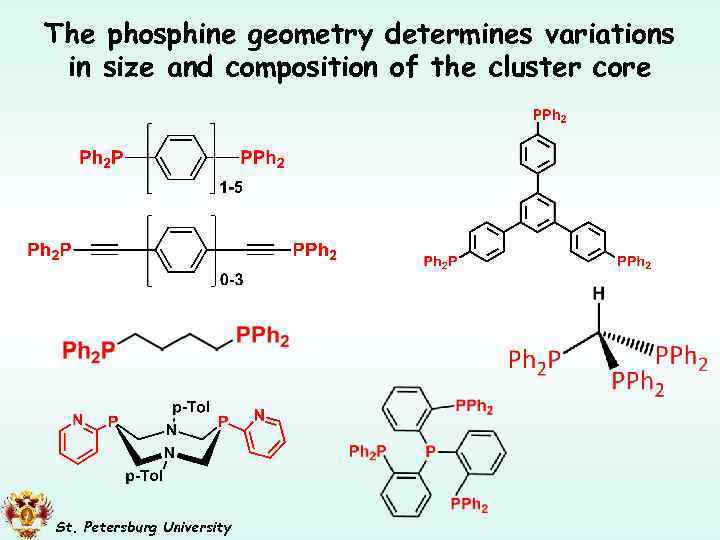 The phosphine geometry determines variations in size and composition of the cluster core St. Petersburg University
The phosphine geometry determines variations in size and composition of the cluster core St. Petersburg University
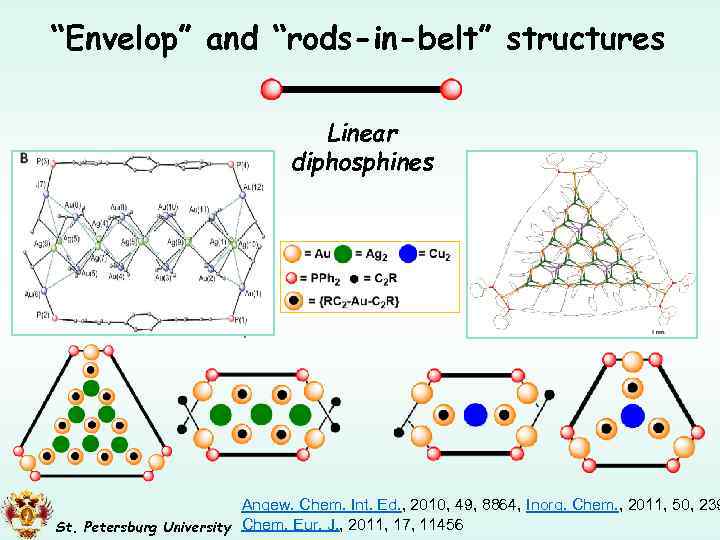 “Envelop” and “rods-in-belt” structures Linear diphosphines + Au(I) + Ag(I) + HC 2 R + Au(I) + Cu(I) + HC 2 R Angew. Chem. Int. Ed. , 2010, 49, 8864, Inorg. Chem. , 2011, 50, 239 St. Petersburg University Chem. Eur. J. , 2011, 17, 11456
“Envelop” and “rods-in-belt” structures Linear diphosphines + Au(I) + Ag(I) + HC 2 R + Au(I) + Cu(I) + HC 2 R Angew. Chem. Int. Ed. , 2010, 49, 8864, Inorg. Chem. , 2011, 50, 239 St. Petersburg University Chem. Eur. J. , 2011, 17, 11456
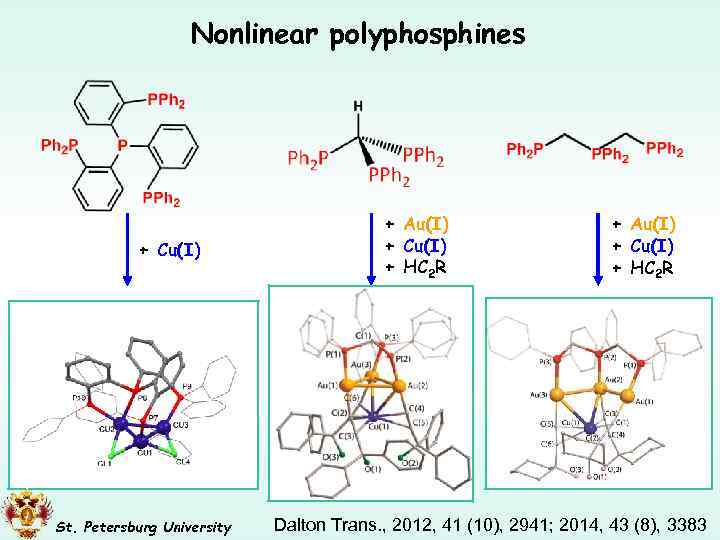 Nonlinear polyphosphines + Cu(I) St. Petersburg University + Au(I) + Cu(I) + HC 2 R Dalton Trans. , 2012, 41 (10), 2941; 2014, 43 (8), 3383
Nonlinear polyphosphines + Cu(I) St. Petersburg University + Au(I) + Cu(I) + HC 2 R Dalton Trans. , 2012, 41 (10), 2941; 2014, 43 (8), 3383
![+ + [Cu(NCMe)4]+ +Et 3 N [Cu(NCMe)4]+ +2 St. Petersburg University Eur. J. Inorg. + + [Cu(NCMe)4]+ +Et 3 N [Cu(NCMe)4]+ +2 St. Petersburg University Eur. J. Inorg.](https://present5.com/presentation/-104014181_425074527/image-7.jpg) + + [Cu(NCMe)4]+ +Et 3 N [Cu(NCMe)4]+ +2 St. Petersburg University Eur. J. Inorg. Chem. , 2012, 25, 4048
+ + [Cu(NCMe)4]+ +Et 3 N [Cu(NCMe)4]+ +2 St. Petersburg University Eur. J. Inorg. Chem. , 2012, 25, 4048
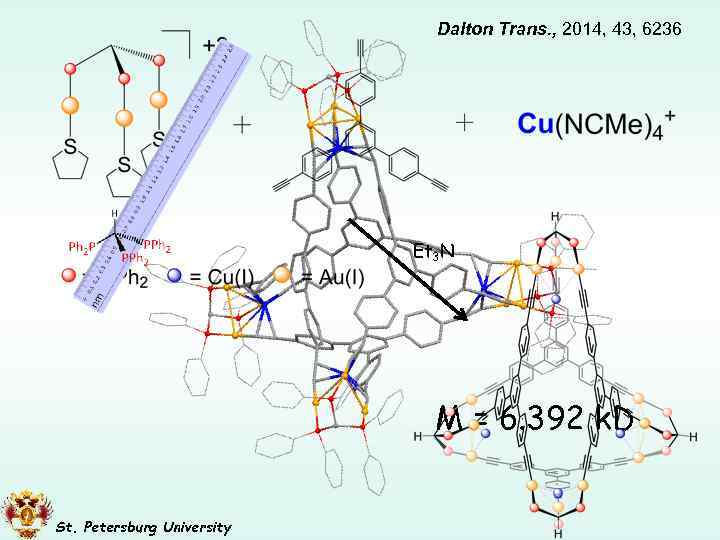 Dalton Trans. , 2014, 43, 6236 Et 3 N M = 6. 392 k. D St. Petersburg University
Dalton Trans. , 2014, 43, 6236 Et 3 N M = 6. 392 k. D St. Petersburg University
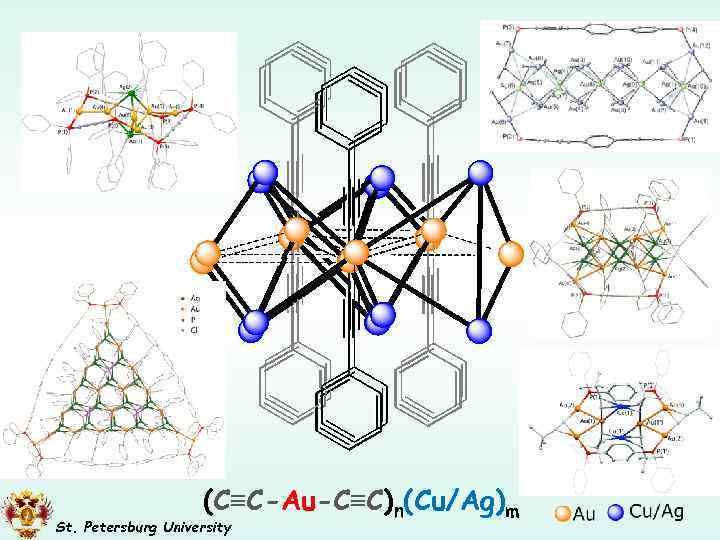 (C≡C-Au-C≡C)n(Cu/Ag)m St. Petersburg University
(C≡C-Au-C≡C)n(Cu/Ag)m St. Petersburg University
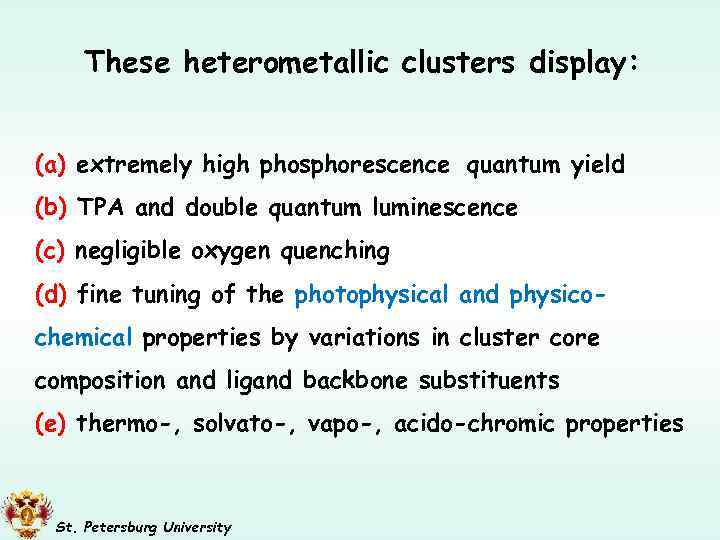 These heterometallic clusters display: (a) extremely high phosphorescence quantum yield (b) TPA and double quantum luminescence (c) negligible oxygen quenching (d) fine tuning of the photophysical and physicochemical properties by variations in cluster core composition and ligand backbone substituents (e) thermo-, solvato-, vapo-, acido-chromic properties St. Petersburg University
These heterometallic clusters display: (a) extremely high phosphorescence quantum yield (b) TPA and double quantum luminescence (c) negligible oxygen quenching (d) fine tuning of the photophysical and physicochemical properties by variations in cluster core composition and ligand backbone substituents (e) thermo-, solvato-, vapo-, acido-chromic properties St. Petersburg University
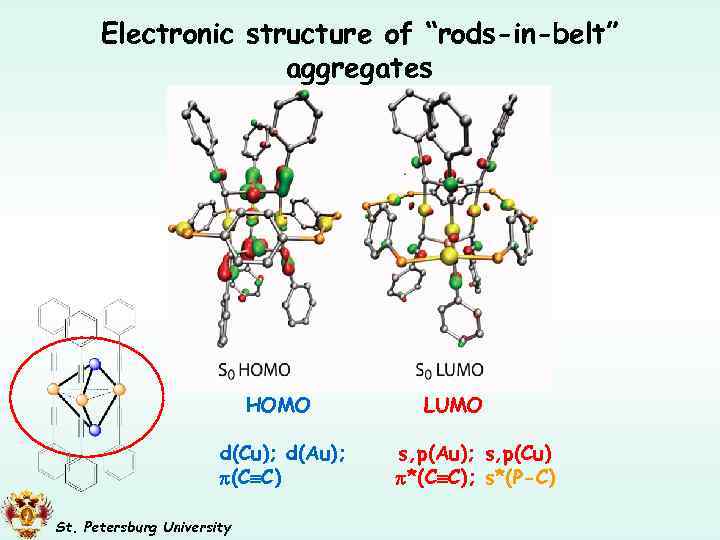 Electronic structure of “rods-in-belt” aggregates HOMO d(Cu); d(Au); (C C) St. Petersburg University LUMO s, p(Au); s, p(Cu) *(C C); s*(P-C)
Electronic structure of “rods-in-belt” aggregates HOMO d(Cu); d(Au); (C C) St. Petersburg University LUMO s, p(Au); s, p(Cu) *(C C); s*(P-C)
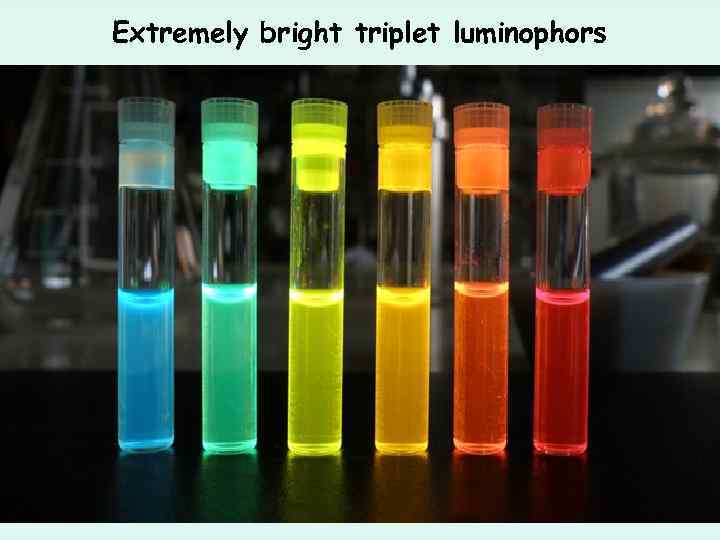 Extremely bright triplet luminophors 490 nm 605 nm 594 nm 649 nm Φ = 100 % Φ = 96 % Φ = 100 % Φ = 54 %
Extremely bright triplet luminophors 490 nm 605 nm 594 nm 649 nm Φ = 100 % Φ = 96 % Φ = 100 % Φ = 54 %
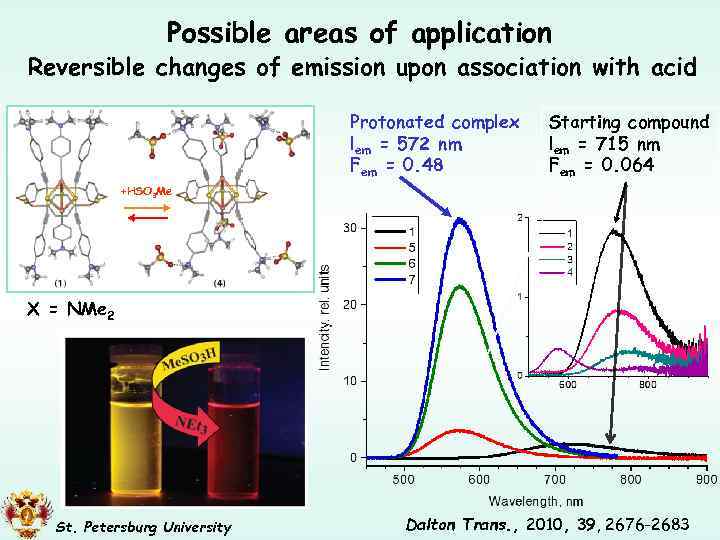 Possible areas of application Reversible changes of emission upon association with acid Protonated complex lem = 572 nm Fem = 0. 48 Starting compound lem = 715 nm Fem = 0. 064 +HSO 3 Me X = NMe 2 St. Petersburg University Dalton Trans. , 2010, 39, 2676 -2683
Possible areas of application Reversible changes of emission upon association with acid Protonated complex lem = 572 nm Fem = 0. 48 Starting compound lem = 715 nm Fem = 0. 064 +HSO 3 Me X = NMe 2 St. Petersburg University Dalton Trans. , 2010, 39, 2676 -2683
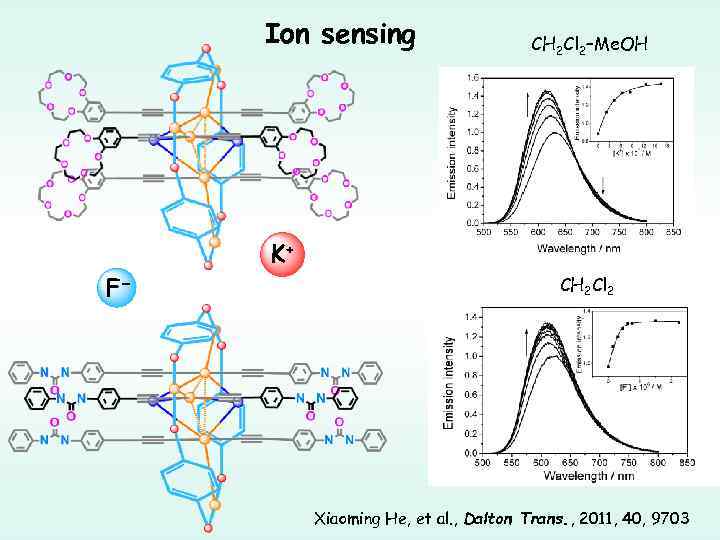 Ion sensing CH 2 Cl 2–Me. OH K+ F− CH 2 Cl 2 Xiaoming He, et al. , Dalton Trans. , 2011, 40, 9703
Ion sensing CH 2 Cl 2–Me. OH K+ F− CH 2 Cl 2 Xiaoming He, et al. , Dalton Trans. , 2011, 40, 9703
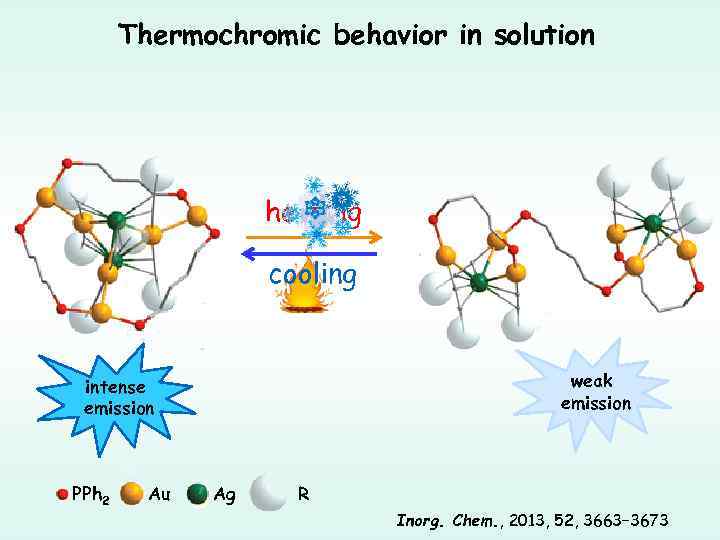 Thermochromic behavior in solution heating cooling weak emission intense emission PPh 2 Au Ag R Inorg. Chem. , 2013, 52, 3663− 3673
Thermochromic behavior in solution heating cooling weak emission intense emission PPh 2 Au Ag R Inorg. Chem. , 2013, 52, 3663− 3673
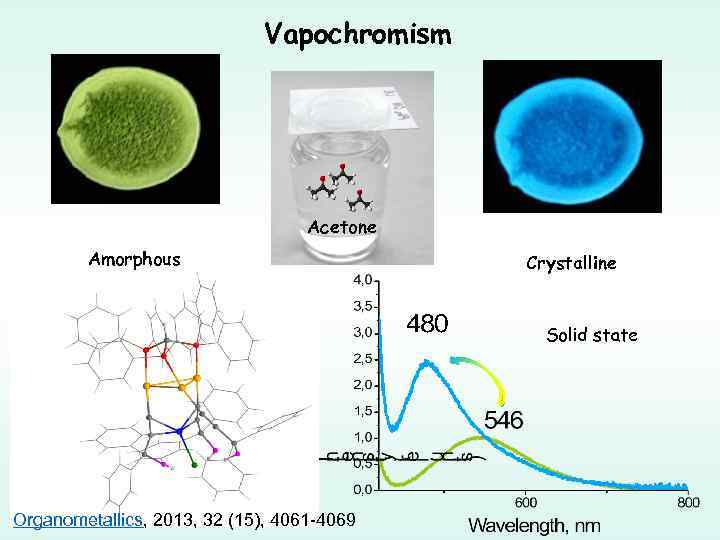 Vapochromism Acetone Amorphous Crystalline Solid state Organometallics, 2013, 32 (15), 4061 -4069
Vapochromism Acetone Amorphous Crystalline Solid state Organometallics, 2013, 32 (15), 4061 -4069
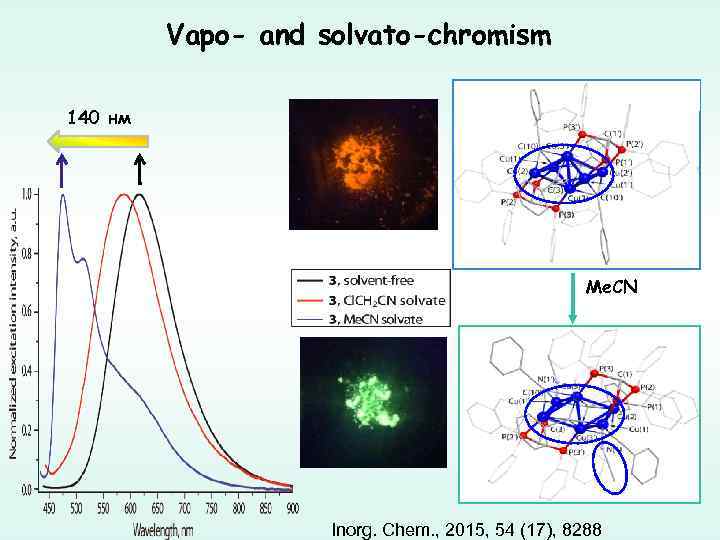 Vapo- and solvato-chromism 140 нм Me. CN Inorg. Chem. , 2015, 54 (17), 8288
Vapo- and solvato-chromism 140 нм Me. CN Inorg. Chem. , 2015, 54 (17), 8288
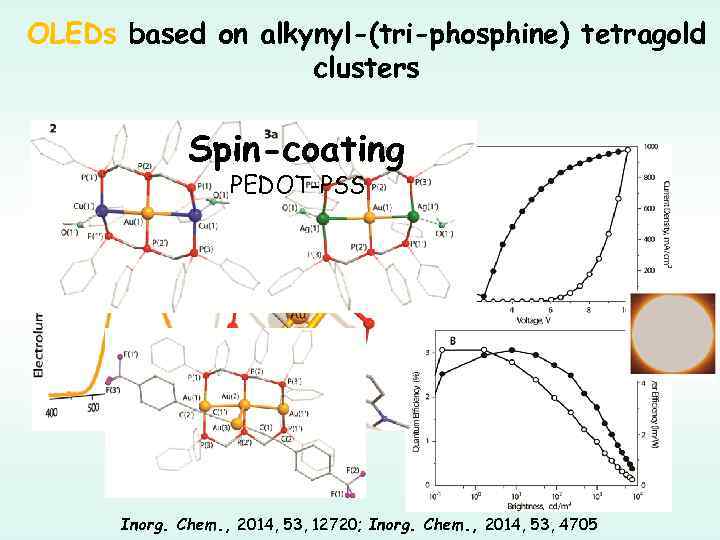 OLEDs based on alkynyl-(tri-phosphine) tetragold clusters Spin-coating PEDOT-PSS Inorg. Chem. , 2014, 53, 12720; Inorg. Chem. , 2014, 53, 4705
OLEDs based on alkynyl-(tri-phosphine) tetragold clusters Spin-coating PEDOT-PSS Inorg. Chem. , 2014, 53, 12720; Inorg. Chem. , 2014, 53, 4705
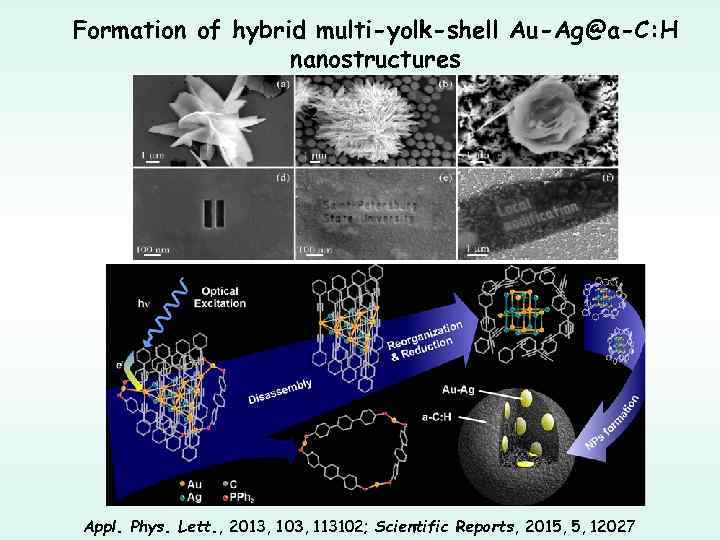 Formation of hybrid multi-yolk-shell Au-Ag@a-C: H nanostructures Appl. Phys. Lett. , 2013, 103, 113102; Scientific Reports, 2015, 5, 12027
Formation of hybrid multi-yolk-shell Au-Ag@a-C: H nanostructures Appl. Phys. Lett. , 2013, 103, 113102; Scientific Reports, 2015, 5, 12027
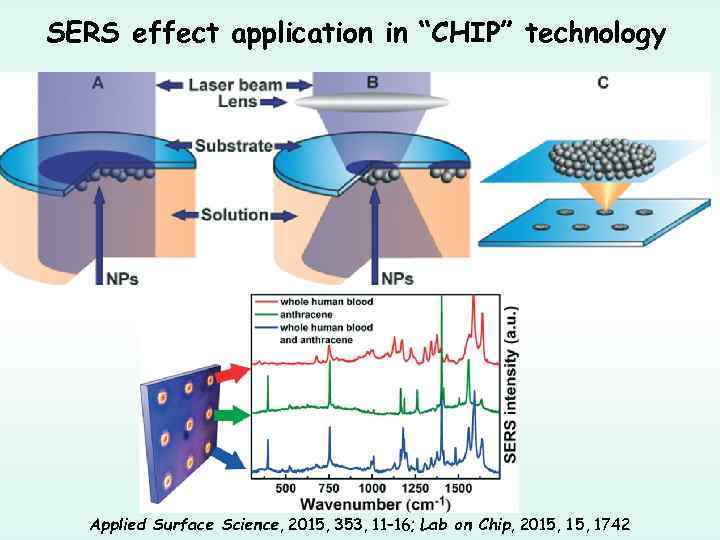 SERS effect application in “CHIP” technology Applied Surface Science, 2015, 353, 11– 16; Lab on Chip, 2015, 1742
SERS effect application in “CHIP” technology Applied Surface Science, 2015, 353, 11– 16; Lab on Chip, 2015, 1742
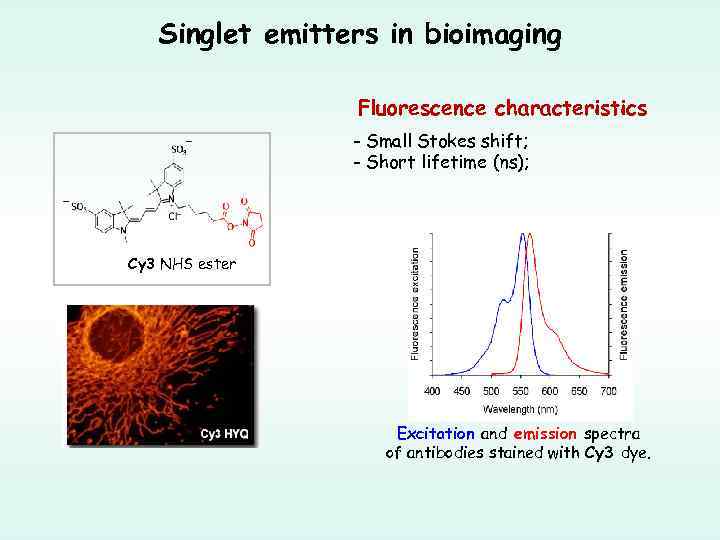 Singlet emitters in bioimaging Fluorescence characteristics - Small Stokes shift; - Short lifetime (ns); Cy 3 NHS ester Excitation and emission spectra of antibodies stained with Cy 3 dye.
Singlet emitters in bioimaging Fluorescence characteristics - Small Stokes shift; - Short lifetime (ns); Cy 3 NHS ester Excitation and emission spectra of antibodies stained with Cy 3 dye.
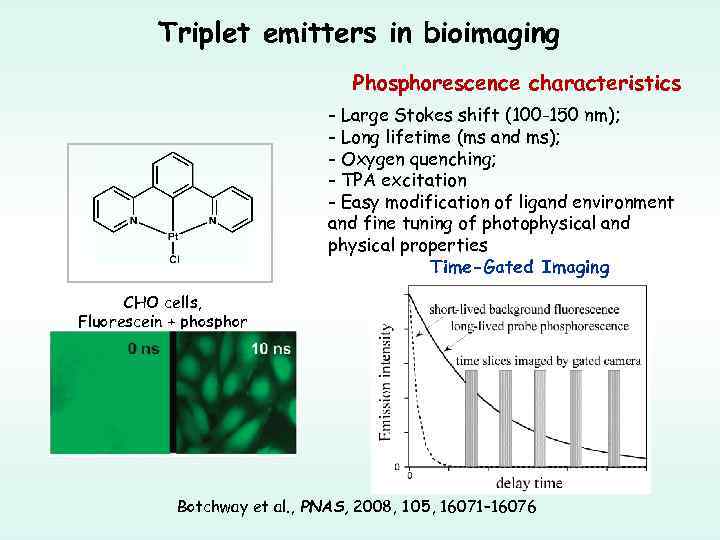 Triplet emitters in bioimaging Phosphorescence characteristics - Large Stokes shift (100 -150 nm); - Long lifetime (ms and ms); - Oxygen quenching; - TPA excitation - Easy modification of ligand environment and fine tuning of photophysical and physical properties Time-Gated Imaging CHO cells, Fluorescein + phosphor Botchway et al. , PNAS, 2008, 105, 16071– 16076
Triplet emitters in bioimaging Phosphorescence characteristics - Large Stokes shift (100 -150 nm); - Long lifetime (ms and ms); - Oxygen quenching; - TPA excitation - Easy modification of ligand environment and fine tuning of photophysical and physical properties Time-Gated Imaging CHO cells, Fluorescein + phosphor Botchway et al. , PNAS, 2008, 105, 16071– 16076
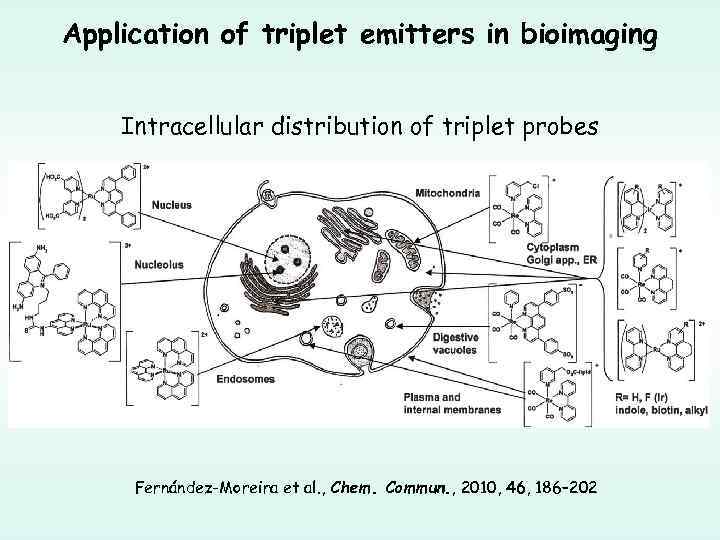 Application of triplet emitters in bioimaging Intracellular distribution of triplet probes Fernández-Moreira et al. , Chem. Commun. , 2010, 46, 186– 202
Application of triplet emitters in bioimaging Intracellular distribution of triplet probes Fernández-Moreira et al. , Chem. Commun. , 2010, 46, 186– 202
 Polynuclear heterometallic complexes ü High emission quantum yield ü Double quantum luminescence under excitation in “transparency window” ü No oxygen quenching ü Fine tuning of photophysical and physico-chemical characteristics through variations in cluster core composition and ligand environment St. Petersburg University
Polynuclear heterometallic complexes ü High emission quantum yield ü Double quantum luminescence under excitation in “transparency window” ü No oxygen quenching ü Fine tuning of photophysical and physico-chemical characteristics through variations in cluster core composition and ligand environment St. Petersburg University
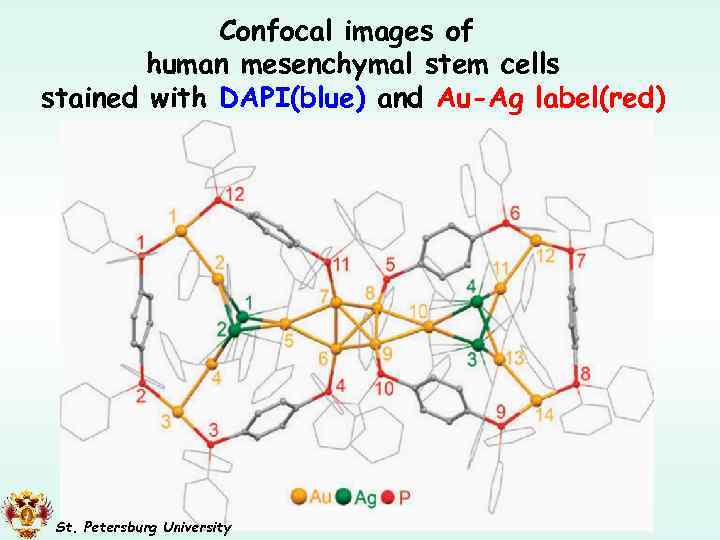 Confocal images of human mesenchymal stem cells stained with DAPI(blue) and Au-Ag label(red) Si. O 2 206 GM Chem. Commun. , 2010, 46, 1440– 1442 St. Petersburg University
Confocal images of human mesenchymal stem cells stained with DAPI(blue) and Au-Ag label(red) Si. O 2 206 GM Chem. Commun. , 2010, 46, 1440– 1442 St. Petersburg University
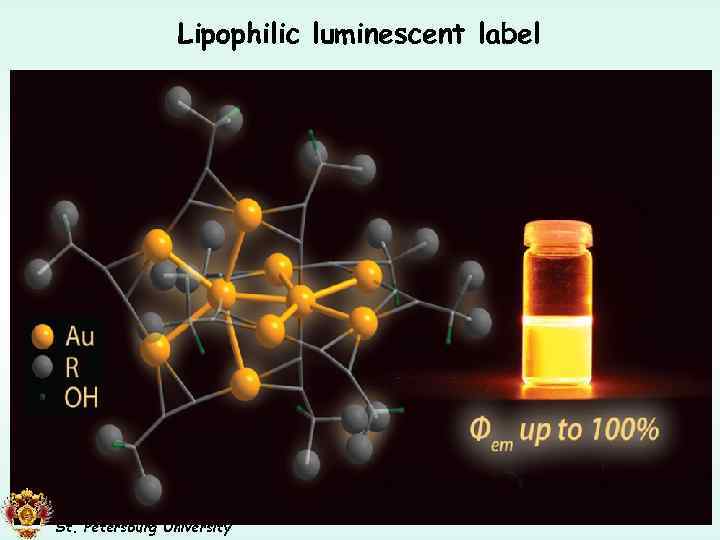 Lipophilic luminescent label Subcutaneous Adipose tissue (pigeon) Small Intestine Wall (pigeon) Adipose tissue (fat droplets) Pseudocolors: Red: Phosphor; Blue: Nuclei (DAPI); Green: Autofluorescence Muscle 100 nm St. Petersburg University
Lipophilic luminescent label Subcutaneous Adipose tissue (pigeon) Small Intestine Wall (pigeon) Adipose tissue (fat droplets) Pseudocolors: Red: Phosphor; Blue: Nuclei (DAPI); Green: Autofluorescence Muscle 100 nm St. Petersburg University
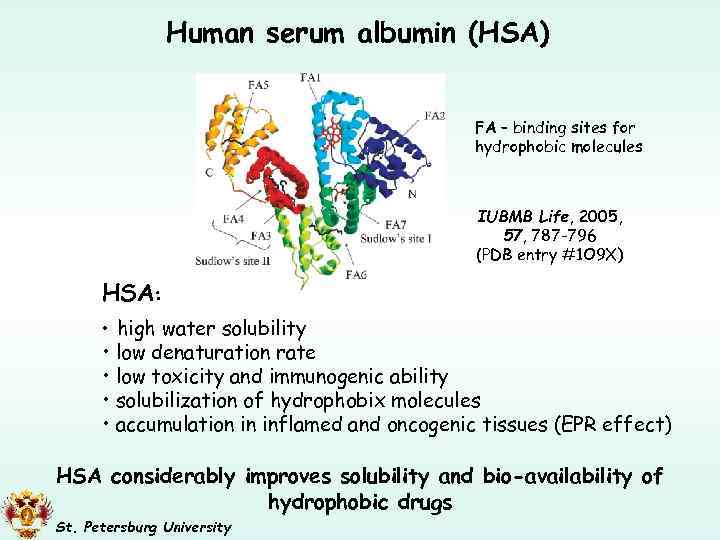 Human serum albumin (HSA) FA – binding sites for hydrophobic molecules IUBMB Life, 2005, 57, 787 -796 (PDB entry #1 O 9 X) HSA: • high water solubility • low denaturation rate • low toxicity and immunogenic ability • solubilization of hydrophobix molecules • accumulation in inflamed and oncogenic tissues (EPR effect) HSA considerably improves solubility and bio-availability of hydrophobic drugs St. Petersburg University
Human serum albumin (HSA) FA – binding sites for hydrophobic molecules IUBMB Life, 2005, 57, 787 -796 (PDB entry #1 O 9 X) HSA: • high water solubility • low denaturation rate • low toxicity and immunogenic ability • solubilization of hydrophobix molecules • accumulation in inflamed and oncogenic tissues (EPR effect) HSA considerably improves solubility and bio-availability of hydrophobic drugs St. Petersburg University
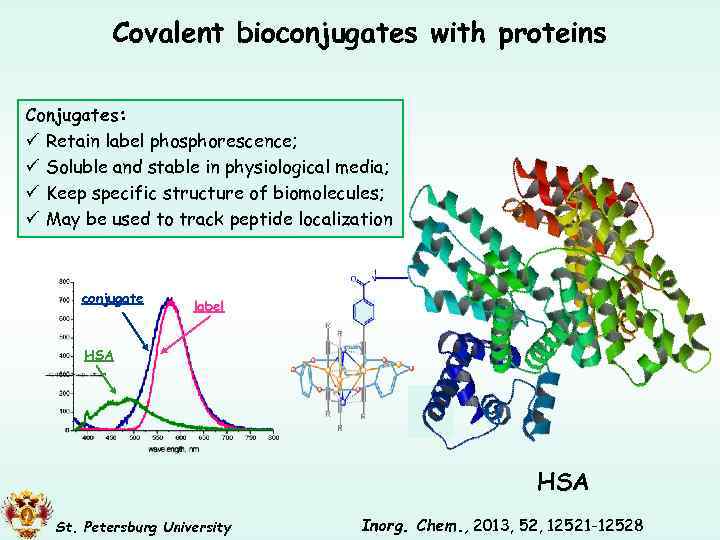 Covalent bioconjugates with proteins Conjugates: ü Retain label phosphorescence; ü Soluble and stable in physiological media; ü Keep specific structure of biomolecules; ü May be used to track peptide localization conjugate label HSA St. Petersburg University Inorg. Chem. , 2013, 52, 12521 -12528
Covalent bioconjugates with proteins Conjugates: ü Retain label phosphorescence; ü Soluble and stable in physiological media; ü Keep specific structure of biomolecules; ü May be used to track peptide localization conjugate label HSA St. Petersburg University Inorg. Chem. , 2013, 52, 12521 -12528
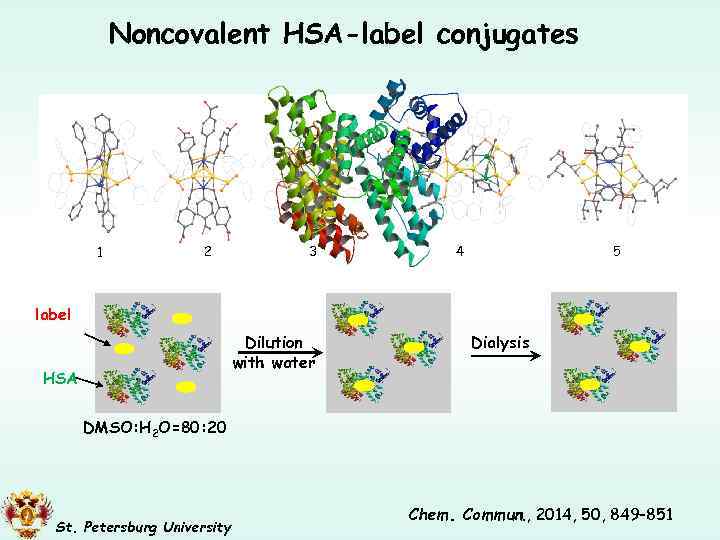 Noncovalent HSA-label conjugates 2 1 label 3 4 5 ЧСА Dilution with water HSA Dialysis DMSO: H 2 O=80: 20 St. Petersburg University Chem. Commun. , 2014, 50, 849– 851
Noncovalent HSA-label conjugates 2 1 label 3 4 5 ЧСА Dilution with water HSA Dialysis DMSO: H 2 O=80: 20 St. Petersburg University Chem. Commun. , 2014, 50, 849– 851
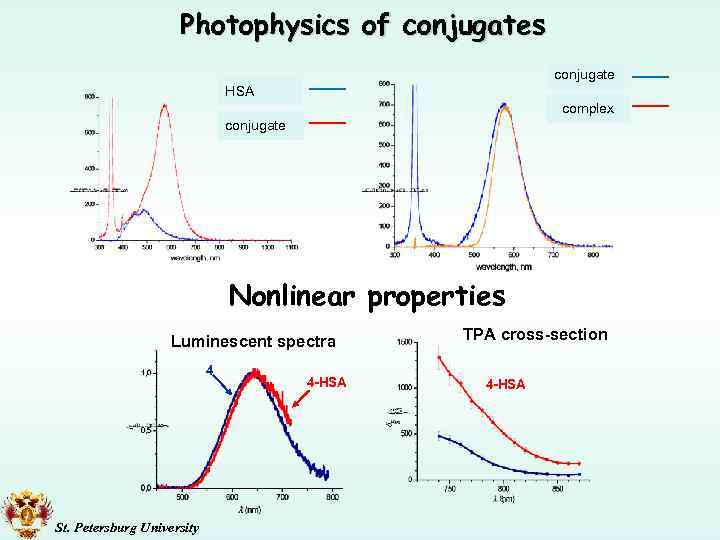 Photophysics of conjugates conjugate HSA complex conjugate Nonlinear properties Luminescent spectra 4 St. Petersburg University 4 -HSA TPA cross-section 4 -HSA
Photophysics of conjugates conjugate HSA complex conjugate Nonlinear properties Luminescent spectra 4 St. Petersburg University 4 -HSA TPA cross-section 4 -HSA
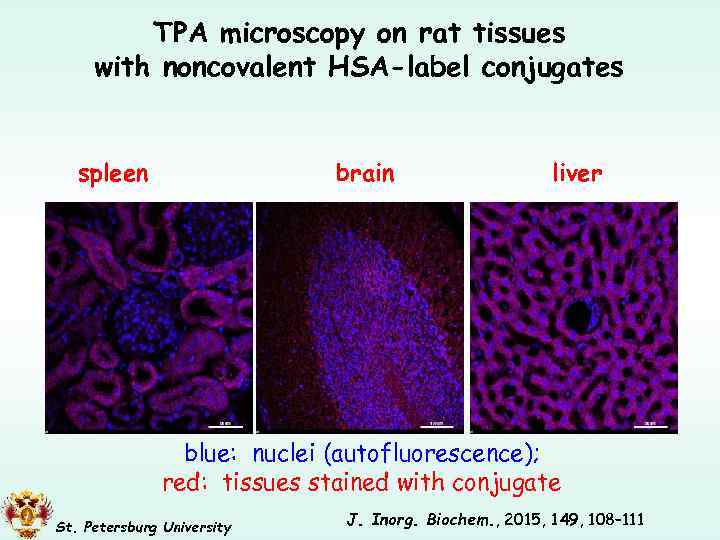 TPA microscopy on rat tissues with noncovalent HSA-label conjugates spleen brain liver blue: nuclei (autofluorescence); red: tissues stained with conjugate St. Petersburg University J. Inorg. Biochem. , 2015, 149, 108– 111
TPA microscopy on rat tissues with noncovalent HSA-label conjugates spleen brain liver blue: nuclei (autofluorescence); red: tissues stained with conjugate St. Petersburg University J. Inorg. Biochem. , 2015, 149, 108– 111
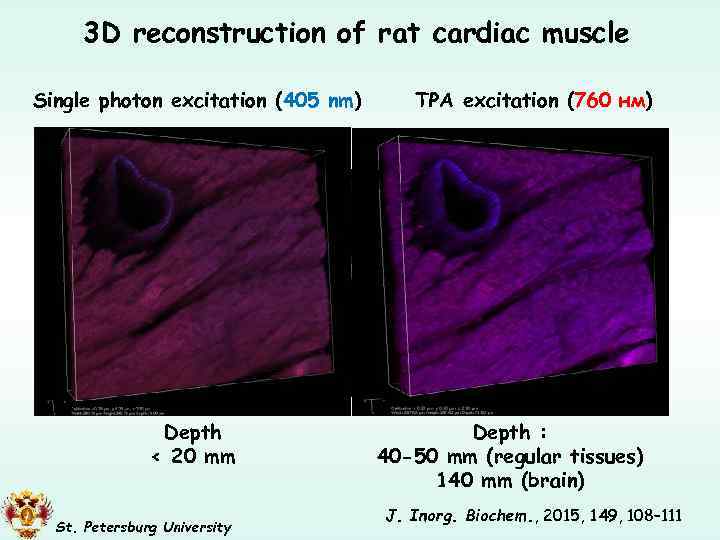 3 D reconstruction of rat cardiac muscle Single photon excitation (405 nm) Depth < 20 mm St. Petersburg University TPA excitation (760 нм) Depth : 40 -50 mm (regular tissues) 140 mm (brain) J. Inorg. Biochem. , 2015, 149, 108– 111
3 D reconstruction of rat cardiac muscle Single photon excitation (405 nm) Depth < 20 mm St. Petersburg University TPA excitation (760 нм) Depth : 40 -50 mm (regular tissues) 140 mm (brain) J. Inorg. Biochem. , 2015, 149, 108– 111
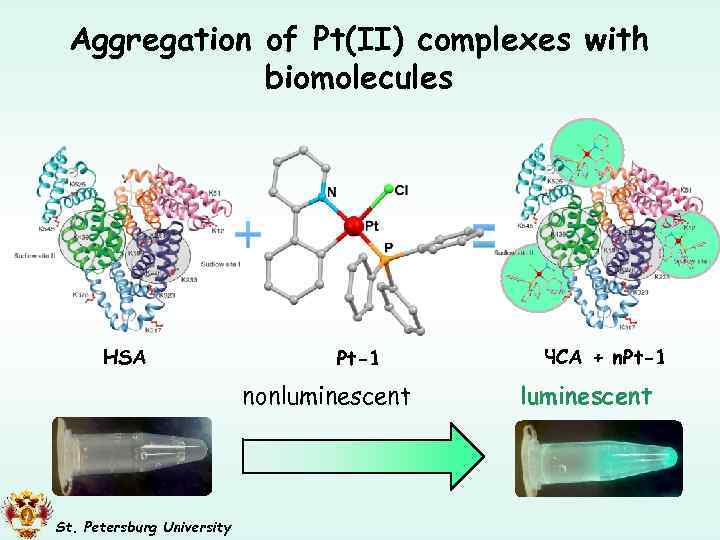 Aggregation of Pt(II) complexes with biomolecules HSA Pt-1 nonluminescent St. Petersburg University ЧСА + n. Pt-1 luminescent
Aggregation of Pt(II) complexes with biomolecules HSA Pt-1 nonluminescent St. Petersburg University ЧСА + n. Pt-1 luminescent
![Emission of Pt(II)/HSA aggregates in water solutions [HSA] : [Pt] St. Petersburg University Emission of Pt(II)/HSA aggregates in water solutions [HSA] : [Pt] St. Petersburg University](https://present5.com/presentation/-104014181_425074527/image-34.jpg) Emission of Pt(II)/HSA aggregates in water solutions [HSA] : [Pt] St. Petersburg University
Emission of Pt(II)/HSA aggregates in water solutions [HSA] : [Pt] St. Petersburg University
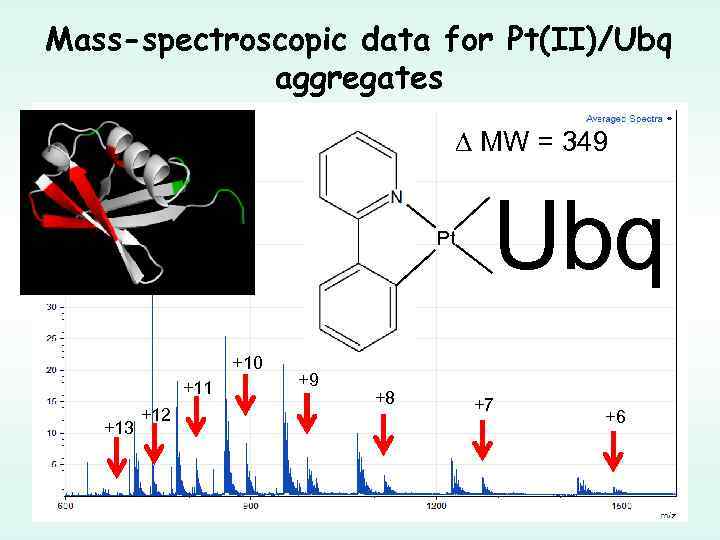 Mass-spectroscopic data for Pt(II)/Ubq aggregates MW = 349 Pt +10 +11 +13 +12 +9 +8 Ubq +7 +6
Mass-spectroscopic data for Pt(II)/Ubq aggregates MW = 349 Pt +10 +11 +13 +12 +9 +8 Ubq +7 +6
 Transition Metal Clusters research group St. Petersburg University
Transition Metal Clusters research group St. Petersburg University
 Author greatly appreciates the contribution of University of Eastern Finland Prof. I. Koshevoy, Dr. M. Haukka, Dr. A. Karttunen IHMC RAS, St. Petersburg Prof. S. Burov, Dr. P. Chelushkin, Prof. T. Tennikova, Dr. E. Vlakh National Taiwan University Prof. Pi-Tai Chou SPb. U internal Grants, RFBR Grants and Finland Academy of Science, for financial support St. Petersburg University
Author greatly appreciates the contribution of University of Eastern Finland Prof. I. Koshevoy, Dr. M. Haukka, Dr. A. Karttunen IHMC RAS, St. Petersburg Prof. S. Burov, Dr. P. Chelushkin, Prof. T. Tennikova, Dr. E. Vlakh National Taiwan University Prof. Pi-Tai Chou SPb. U internal Grants, RFBR Grants and Finland Academy of Science, for financial support St. Petersburg University
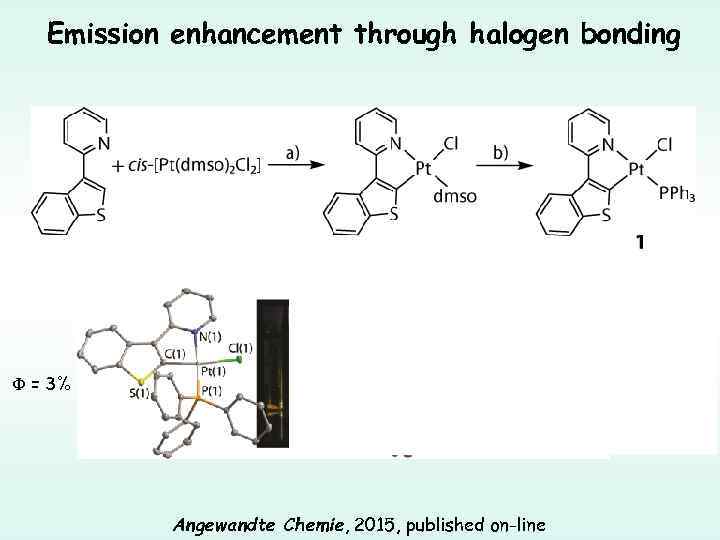 Emission enhancement through halogen bonding F (Br) = 32% F (I) = 65% F = 3% Angewandte Chemie, 2015, published on-line
Emission enhancement through halogen bonding F (Br) = 32% F (I) = 65% F = 3% Angewandte Chemie, 2015, published on-line


