They have five valence electrons in their outermost



















- Размер: 5 Mегабайта
- Количество слайдов: 24
Описание презентации They have five valence electrons in their outermost по слайдам

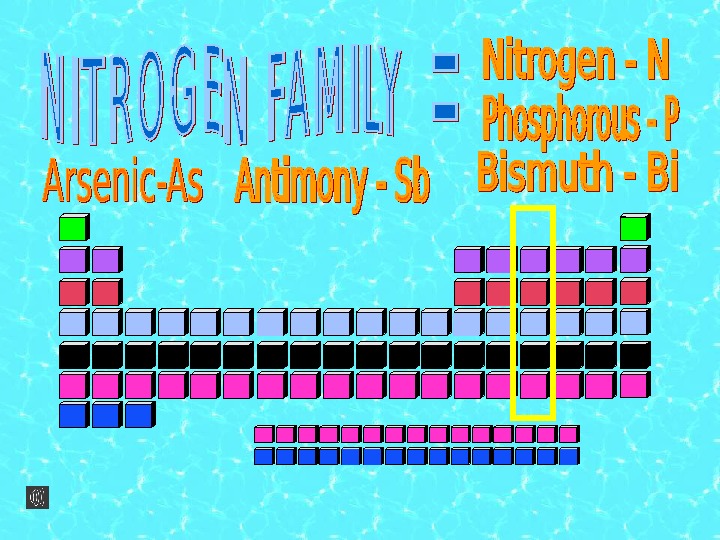
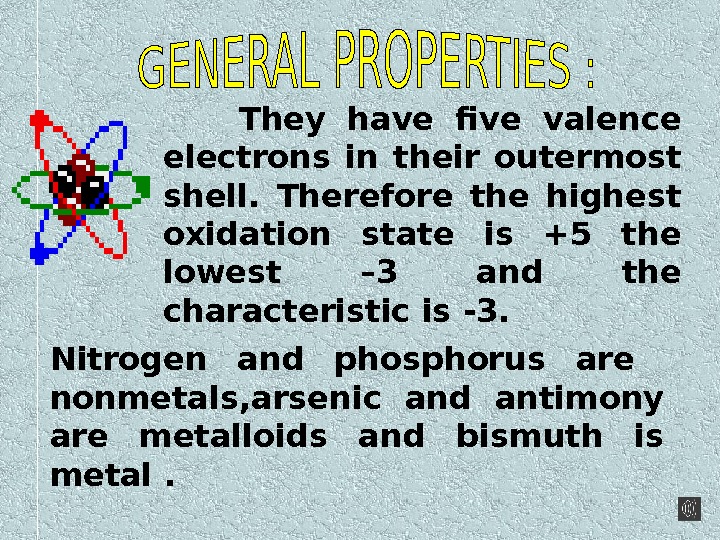
 They have five valence electrons in their outermost shell. Therefore the highest oxidation state is +5 the lowest – 3 and the characteristic is -3. Nitrogen and phosphorus are nonmetals, arsenic and antimony are metalloids and bismuth is metal.
They have five valence electrons in their outermost shell. Therefore the highest oxidation state is +5 the lowest – 3 and the characteristic is -3. Nitrogen and phosphorus are nonmetals, arsenic and antimony are metalloids and bismuth is metal.
 Nitrogen is colorless, and odorless gas. Mineral sources of nitrogen are potassium nitrate, KNO 3 and sodium nitrate, Na. NO 3 (Chili Peter). Together with C, H and O, Nitrogen, is one of the principal elements found in all living matter.
Nitrogen is colorless, and odorless gas. Mineral sources of nitrogen are potassium nitrate, KNO 3 and sodium nitrate, Na. NO 3 (Chili Peter). Together with C, H and O, Nitrogen, is one of the principal elements found in all living matter.
 Pure nitrogen is obtained by fractional distillation of liquid air. Nitrogen composes about 78% by volume of the atmosphere. It is an important element in plants and animals nutrition; It is an important constituent of animal tissue as proteins. .
Pure nitrogen is obtained by fractional distillation of liquid air. Nitrogen composes about 78% by volume of the atmosphere. It is an important element in plants and animals nutrition; It is an important constituent of animal tissue as proteins. .
 The triple bond in nitrogen molecule is very strong. As a result, N 2 doesn’t give any reaction with acids, bases, water and halogens at ordinary conditions. But at very high temperature, (or electrical arc with 2500 o C ) nitrogen reacts with oxygen directly and produces nitrogen oxides. N 2 (g)+ O 2 (g) 2 NO (g)
The triple bond in nitrogen molecule is very strong. As a result, N 2 doesn’t give any reaction with acids, bases, water and halogens at ordinary conditions. But at very high temperature, (or electrical arc with 2500 o C ) nitrogen reacts with oxygen directly and produces nitrogen oxides. N 2 (g)+ O 2 (g) 2 NO (g)
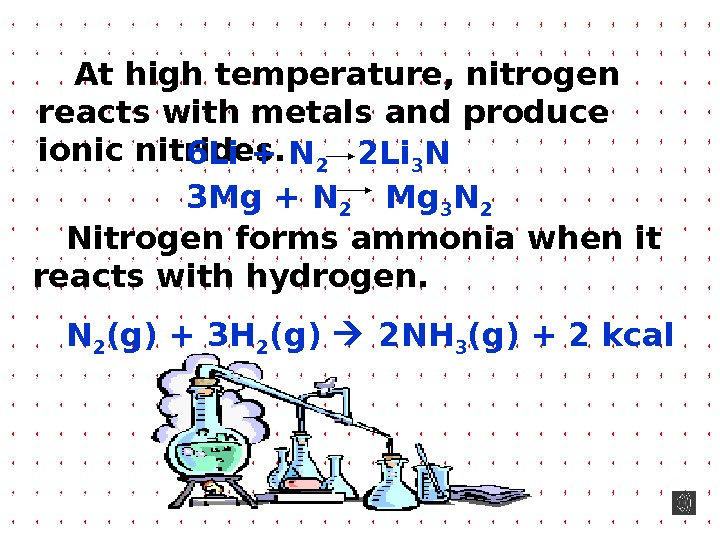
 At high temperature, nitrogen reacts with metals and produce ionic nitrides. Nitrogen forms ammonia when it reacts with hydrogen. N 2 (g) + 3 H 2 (g) 2 NH 3 (g) + 2 kcal 6 Li + N 2 2 Li 3 N 3 Mg + N 2 Mg 3 N
At high temperature, nitrogen reacts with metals and produce ionic nitrides. Nitrogen forms ammonia when it reacts with hydrogen. N 2 (g) + 3 H 2 (g) 2 NH 3 (g) + 2 kcal 6 Li + N 2 2 Li 3 N 3 Mg + N 2 Mg 3 N
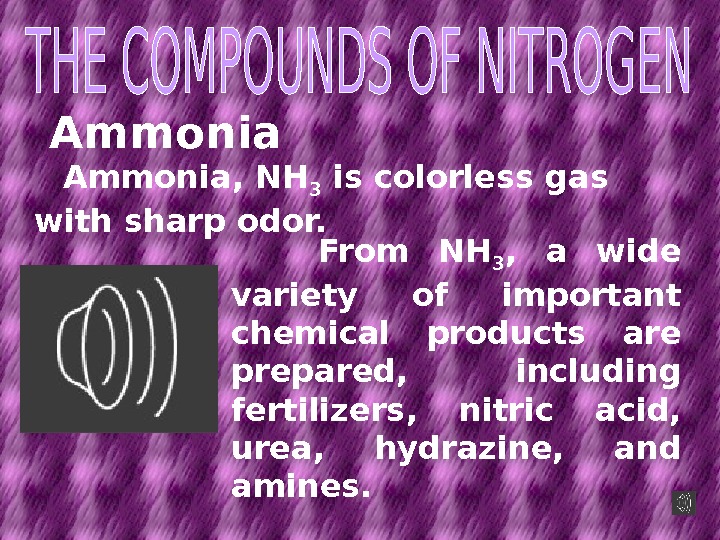
 Ammonia, NH 3 is colorless gas with sharp odor. From NH 3 , a wide variety of important chemical products are prepared, including fertilizers, nitric acid, urea, hydrazine, and amines.
Ammonia, NH 3 is colorless gas with sharp odor. From NH 3 , a wide variety of important chemical products are prepared, including fertilizers, nitric acid, urea, hydrazine, and amines.
 Oxides of nitrogen is called NO X , and are produced in motor engines , because of the high temperature. The N 2 and O 2 in air reacts and form NO and in air NO turns to NO 2. Both of them are pollutants to air, they form smog (smoke +fog ) . They irritate eyes and skin , produce lung cancer.
Oxides of nitrogen is called NO X , and are produced in motor engines , because of the high temperature. The N 2 and O 2 in air reacts and form NO and in air NO turns to NO 2. Both of them are pollutants to air, they form smog (smoke +fog ) . They irritate eyes and skin , produce lung cancer.
 SMOG IS A BIG PROBLEM IN BIG CITIES.
SMOG IS A BIG PROBLEM IN BIG CITIES.
 Nitrous oxide (N 2 O) a colorless gas popularly known as laughing gas. NH 4 NO 3 (s) N 2 O(g)+30 kcal
Nitrous oxide (N 2 O) a colorless gas popularly known as laughing gas. NH 4 NO 3 (s) N 2 O(g)+30 kcal
 In air NO 2 reacts with water droplets and forms acidic rain. NO + ½O 2 NO 2 + H 2 O HNO 3 (nitric acid)
In air NO 2 reacts with water droplets and forms acidic rain. NO + ½O 2 NO 2 + H 2 O HNO 3 (nitric acid)
 It’s a very strong acid, ionizes completely. HNO 3 (aq) H + (aq) + NO 3 — (aq) In industry HNO 3 is consumed for manufacturing explosives, dyes and nitrogen containing fertilizes. The salts of HNO 3 are known as “nitrates”. They are highly soluble in water. : HNO
It’s a very strong acid, ionizes completely. HNO 3 (aq) H + (aq) + NO 3 — (aq) In industry HNO 3 is consumed for manufacturing explosives, dyes and nitrogen containing fertilizes. The salts of HNO 3 are known as “nitrates”. They are highly soluble in water. : HNO
 Nitrogen N
Nitrogen N
 Phosphorus exists in three main allotropic forms: ordinary (or white) phosphorus, red phosphorus, and black phosphorus. Of these, only white and red phosphorus are of commercial importance.
Phosphorus exists in three main allotropic forms: ordinary (or white) phosphorus, red phosphorus, and black phosphorus. Of these, only white and red phosphorus are of commercial importance.
 When freshly prepared, ordinary phosphorus is white, but it turns light yellow when exposed to sunlight. It is a crystalline, translucent, waxy solid, which glows faintly in moist air and is extremely poisonous. It ignites spontaneously in air and must be stored under water. It is insoluble in water, slightly soluble in organic solvents, and very soluble in carbon disulfide. VIDEO
When freshly prepared, ordinary phosphorus is white, but it turns light yellow when exposed to sunlight. It is a crystalline, translucent, waxy solid, which glows faintly in moist air and is extremely poisonous. It ignites spontaneously in air and must be stored under water. It is insoluble in water, slightly soluble in organic solvents, and very soluble in carbon disulfide. VIDEO
 Red phosphorus is a microcrystalline, nonpoisonous powder. It does not occur in the free state but is found mostly as a phosphate, as in phosphate rock and apatite. It is also found in the combined state in all fertile soil and in many natural waters. The element is important in plant and animal physiology and is a constituent of all animal bones, in the form of calcium phosphate VIDEO
Red phosphorus is a microcrystalline, nonpoisonous powder. It does not occur in the free state but is found mostly as a phosphate, as in phosphate rock and apatite. It is also found in the combined state in all fertile soil and in many natural waters. The element is important in plant and animal physiology and is a constituent of all animal bones, in the form of calcium phosphate VIDEO
 Two important oxides of phosphorus are ; Phosphorus oxide, P 4 O 6 Phosphoric oxide, P 4 O 10 Phosphoric oxide reacts with water to form phosphoric acid, H 3 PO 4 and is used as a drying agent. Phosphorus forms hydrides with hydrogen; the important hydride of phosphorus is PH 3 , phosphine.
Two important oxides of phosphorus are ; Phosphorus oxide, P 4 O 6 Phosphoric oxide, P 4 O 10 Phosphoric oxide reacts with water to form phosphoric acid, H 3 PO 4 and is used as a drying agent. Phosphorus forms hydrides with hydrogen; the important hydride of phosphorus is PH 3 , phosphine.
 USAGE OF PHOSPHOROUS : Scientists use phosphorus to make baking soda. Phosphorus is also used to make dishes. Fine china is very expensive because a lot of special procedures go into making it. Phosphorus is one of the special elements that are used to make that fine china.
USAGE OF PHOSPHOROUS : Scientists use phosphorus to make baking soda. Phosphorus is also used to make dishes. Fine china is very expensive because a lot of special procedures go into making it. Phosphorus is one of the special elements that are used to make that fine china.
 You can find lots of phosphorus in fireworks. When phosphorus gets hot it burns really brightly. The bright sparks and flashes are usually because of that phosphorus.
You can find lots of phosphorus in fireworks. When phosphorus gets hot it burns really brightly. The bright sparks and flashes are usually because of that phosphorus.
 Phosphorus is a very important element in fertilizers. Plants need small amounts of phosphorus to grow up healthy. People also need phosphorus and they get it by eating plants.
Phosphorus is a very important element in fertilizers. Plants need small amounts of phosphorus to grow up healthy. People also need phosphorus and they get it by eating plants.
 Scientists use phosphorus when they make glass. If you look at your computer or television they have glass monitors. So much is made of glass. It’s everywhere you look. A lot of that glass was made with help from phosphorus.
Scientists use phosphorus when they make glass. If you look at your computer or television they have glass monitors. So much is made of glass. It’s everywhere you look. A lot of that glass was made with help from phosphorus.
 Phosphorus-containing compounds are used as fertilizers. Phosphorous compounds are also used in clarifying sugar solutions, weighing silk, and fireproofing, , detergent industry, toothpaste and in such alloys as phosphor bronze and phosphor copper. White phosphorus is used in the making of rat poison and red phosphorus is used in matches.
Phosphorus-containing compounds are used as fertilizers. Phosphorous compounds are also used in clarifying sugar solutions, weighing silk, and fireproofing, , detergent industry, toothpaste and in such alloys as phosphor bronze and phosphor copper. White phosphorus is used in the making of rat poison and red phosphorus is used in matches.
 P
P
 ALLAH ! BU KADAR İLGİNÇ KİMYAYI SEVMEMEK OLMAZ! OLMAZ ARKADAŞ, OLMAZ! KİMYAYI SEVELİM!
ALLAH ! BU KADAR İLGİNÇ KİMYAYI SEVMEMEK OLMAZ! OLMAZ ARKADAŞ, OLMAZ! KİMYAYI SEVELİM!

