Invasive Cardiology.ppt
- Количество слайдов: 69
 Right Heart Catheterization: Swan-Ganz Catheter
Right Heart Catheterization: Swan-Ganz Catheter
 Swan-Ganz Catheter: History Right Heart Catheterization l Jeremy Swan (1922 -2005), an Irish cardiologist, worked in the Mayo Clinic, Rochester, and later moved to Cedars. Sinai Medical Center in Los Angeles. l His invention of the catheter is said to have derived from watching the wind playing with sails in Santa Monica.
Swan-Ganz Catheter: History Right Heart Catheterization l Jeremy Swan (1922 -2005), an Irish cardiologist, worked in the Mayo Clinic, Rochester, and later moved to Cedars. Sinai Medical Center in Los Angeles. l His invention of the catheter is said to have derived from watching the wind playing with sails in Santa Monica.
 Swan-Ganz Catheter: History Jeremy Swan (19222005), an Irish cardiologist, worked in the Mayo Clinic, Rochester, and later moved to Cedars -Sinai Medical Center in Los Angeles. l His description of the invention of the catheter is said to have derived from watching the wind playing with sails in Santa Monica. l l l William Ganz (born 1919), an American cardiologist, at Cedars. Sinai Medical Center, Los Angeles, a Professor of Medicine, University of California, Los Angeles, CA. The work of Ganz on thermodilution method of measuring cardiac output was incorporated into the catheter's use. Swan HJ, Ganz W, Forrester J, Marcus H, Diamond G, Chonette D. Catheterization of the heart in man with use of a flow-directed balloon-tipped catheter. N Engl J Med 1970; 283: 447 -51.
Swan-Ganz Catheter: History Jeremy Swan (19222005), an Irish cardiologist, worked in the Mayo Clinic, Rochester, and later moved to Cedars -Sinai Medical Center in Los Angeles. l His description of the invention of the catheter is said to have derived from watching the wind playing with sails in Santa Monica. l l l William Ganz (born 1919), an American cardiologist, at Cedars. Sinai Medical Center, Los Angeles, a Professor of Medicine, University of California, Los Angeles, CA. The work of Ganz on thermodilution method of measuring cardiac output was incorporated into the catheter's use. Swan HJ, Ganz W, Forrester J, Marcus H, Diamond G, Chonette D. Catheterization of the heart in man with use of a flow-directed balloon-tipped catheter. N Engl J Med 1970; 283: 447 -51.
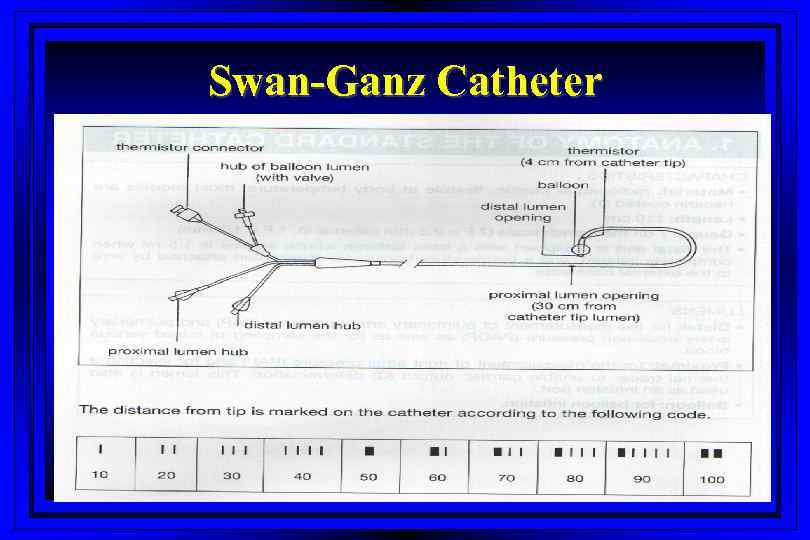 Swan-Ganz Catheter
Swan-Ganz Catheter
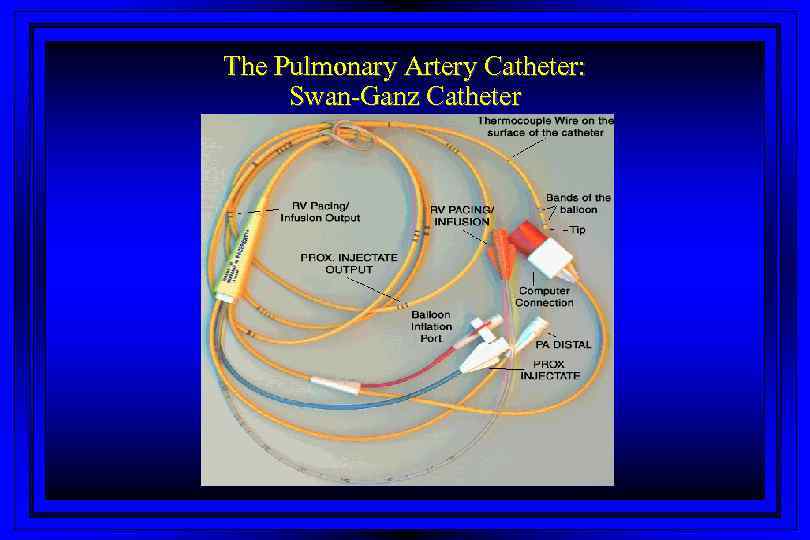 The Pulmonary Artery Catheter: Swan-Ganz Catheter
The Pulmonary Artery Catheter: Swan-Ganz Catheter
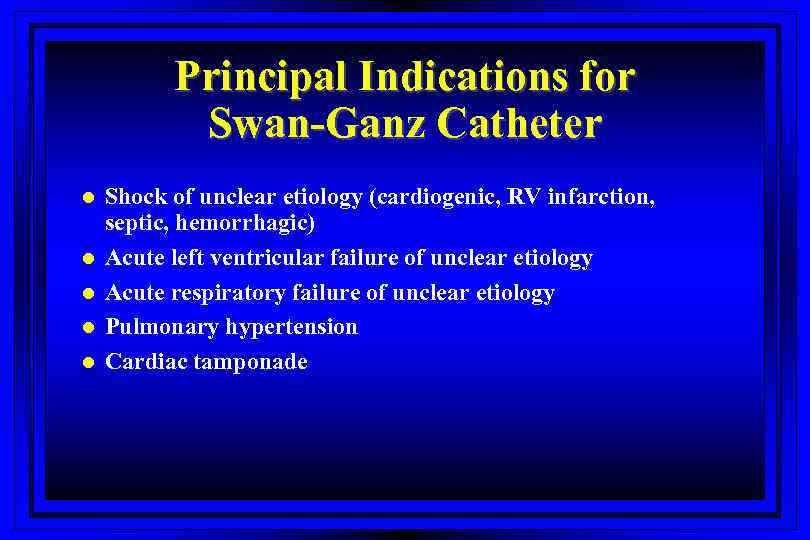 Principal Indications for Swan-Ganz Catheter l l l Shock of unclear etiology (cardiogenic, RV infarction, septic, hemorrhagic) Acute left ventricular failure of unclear etiology Acute respiratory failure of unclear etiology Pulmonary hypertension Cardiac tamponade
Principal Indications for Swan-Ganz Catheter l l l Shock of unclear etiology (cardiogenic, RV infarction, septic, hemorrhagic) Acute left ventricular failure of unclear etiology Acute respiratory failure of unclear etiology Pulmonary hypertension Cardiac tamponade
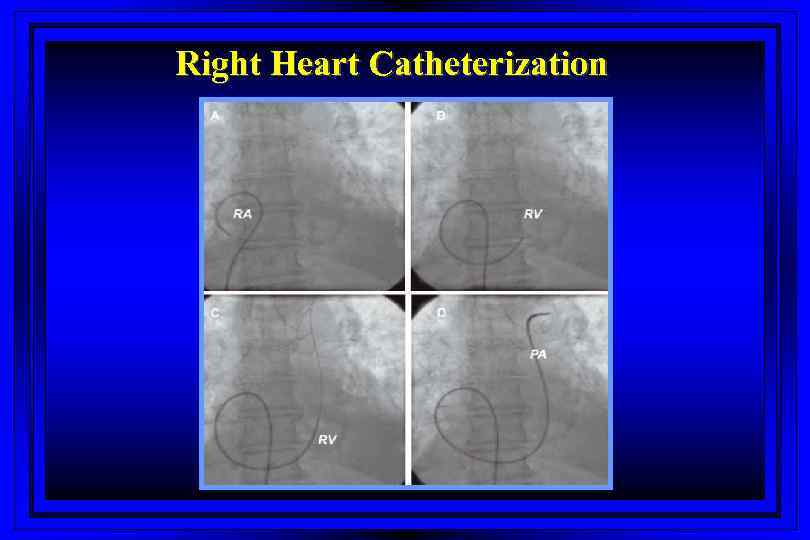 Right Heart Catheterization
Right Heart Catheterization
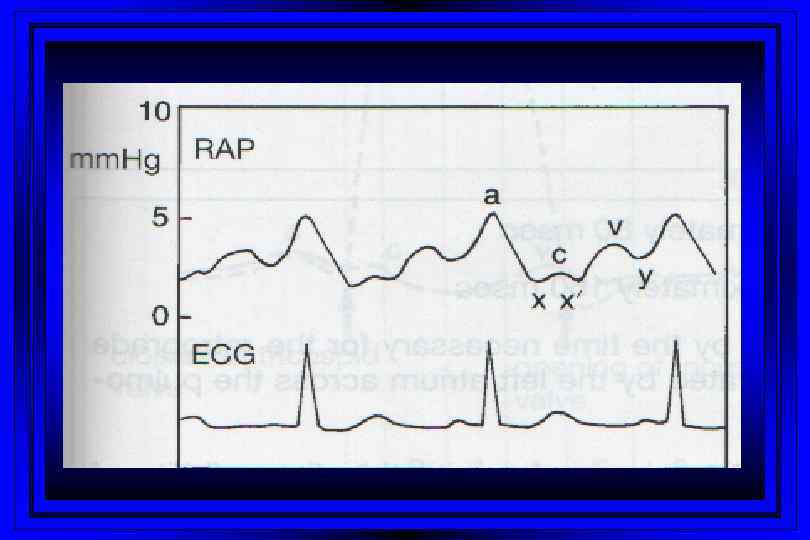
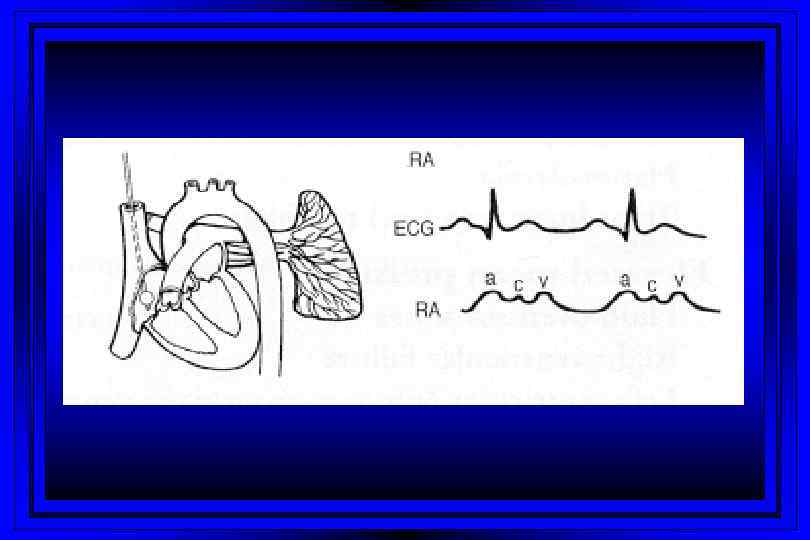
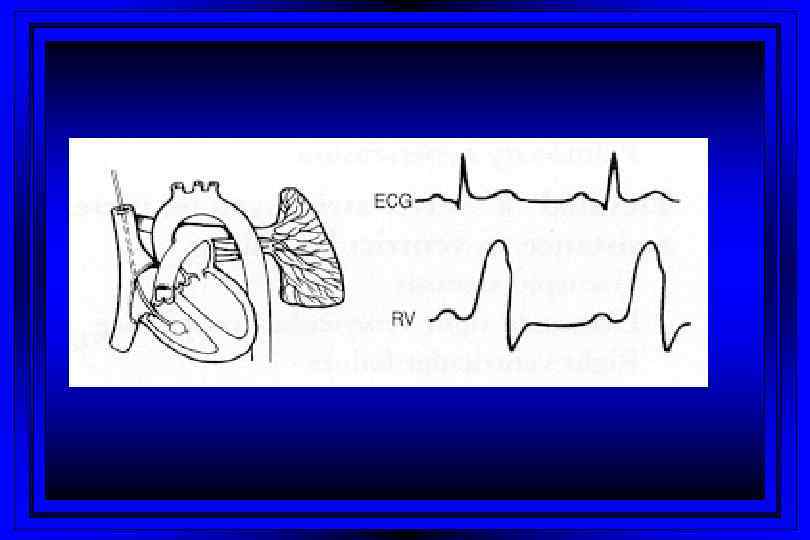
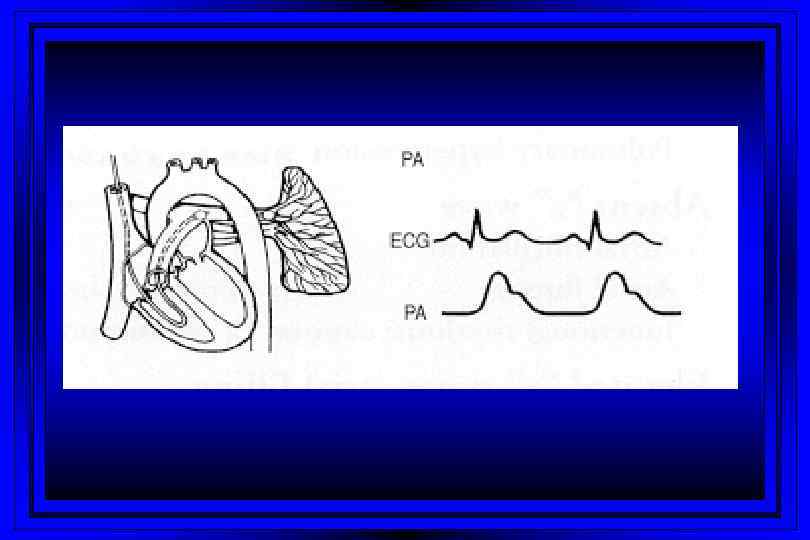
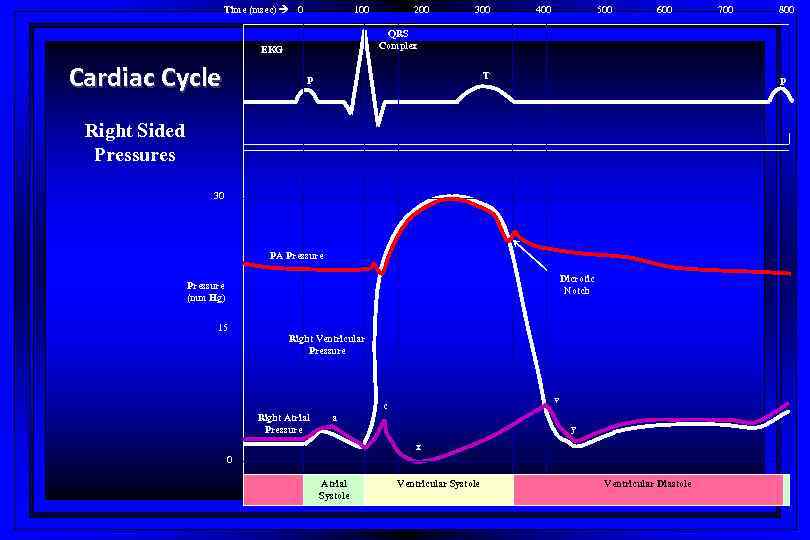 Time (msec) 0 100 300 400 500 600 T P 800 P Right Sided Pressures 30 PA Pressure Dicrotic Notch Pressure (mm Hg) 15 700 QRS Complex EKG Cardiac Cycle 200 Right Ventricular Pressure v c Right Atrial Pressure a y x 0 Atrial Systole Ventricular Diastole
Time (msec) 0 100 300 400 500 600 T P 800 P Right Sided Pressures 30 PA Pressure Dicrotic Notch Pressure (mm Hg) 15 700 QRS Complex EKG Cardiac Cycle 200 Right Ventricular Pressure v c Right Atrial Pressure a y x 0 Atrial Systole Ventricular Diastole
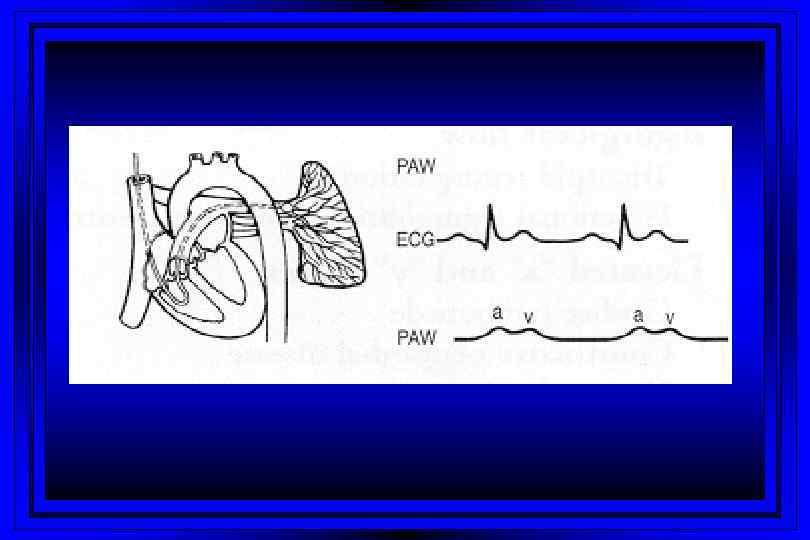
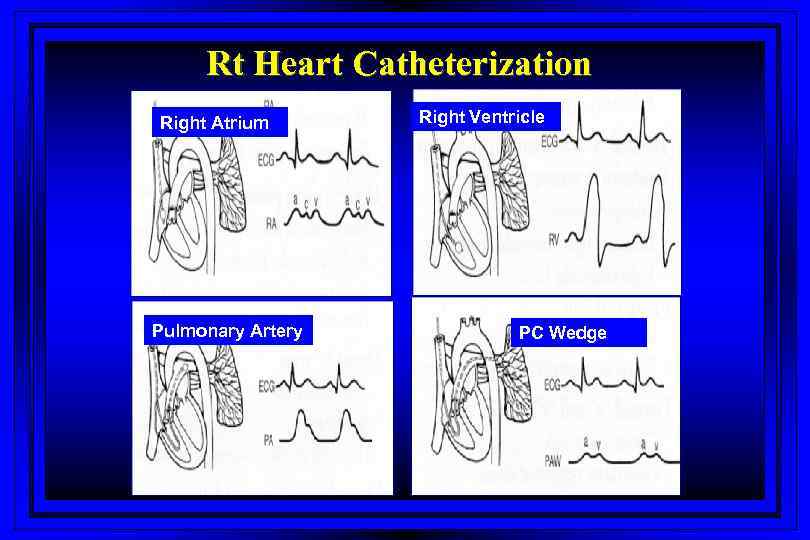 Rt Heart Catheterization Right Atrium Pulmonary Artery Right Ventricle PC Wedge
Rt Heart Catheterization Right Atrium Pulmonary Artery Right Ventricle PC Wedge
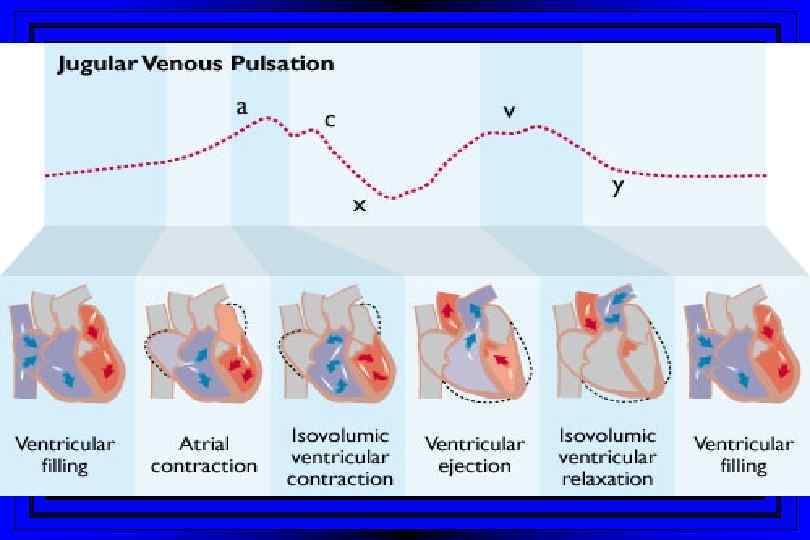
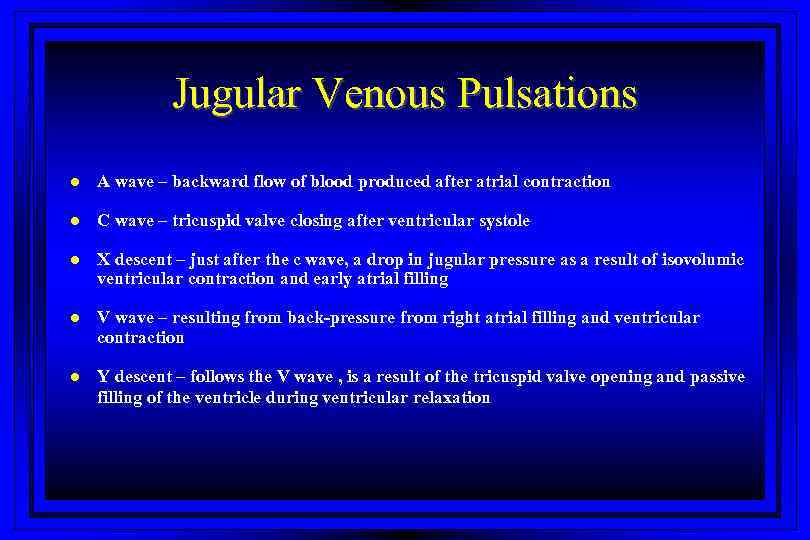 Jugular Venous Pulsations l A wave – backward flow of blood produced after atrial contraction l C wave – tricuspid valve closing after ventricular systole l X descent – just after the c wave, a drop in jugular pressure as a result of isovolumic ventricular contraction and early atrial filling l V wave – resulting from back-pressure from right atrial filling and ventricular contraction l Y descent – follows the V wave , is a result of the tricuspid valve opening and passive filling of the ventricle during ventricular relaxation
Jugular Venous Pulsations l A wave – backward flow of blood produced after atrial contraction l C wave – tricuspid valve closing after ventricular systole l X descent – just after the c wave, a drop in jugular pressure as a result of isovolumic ventricular contraction and early atrial filling l V wave – resulting from back-pressure from right atrial filling and ventricular contraction l Y descent – follows the V wave , is a result of the tricuspid valve opening and passive filling of the ventricle during ventricular relaxation
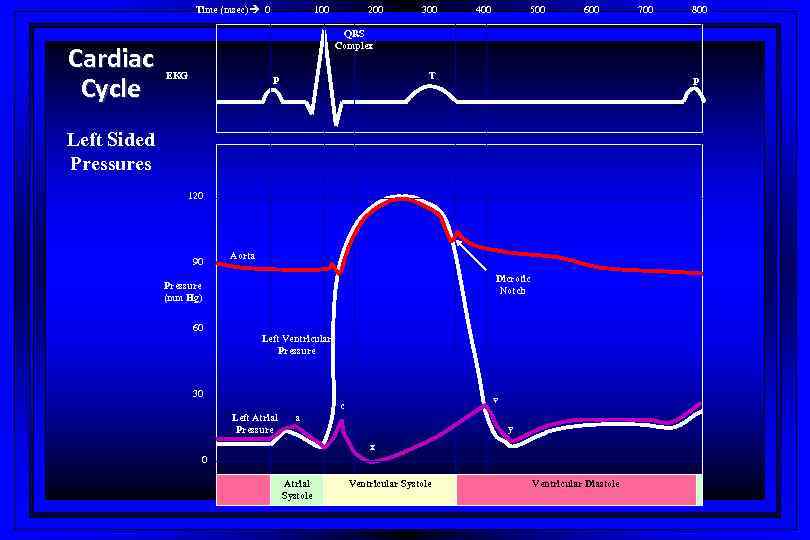 Time (msec) 0 Cardiac Cycle 100 200 300 400 500 600 700 800 QRS Complex EKG T P P Left Sided Pressures 120 90 Aorta Dicrotic Notch Pressure (mm Hg) 60 Left Ventricular Pressure 30 v c Left Atrial Pressure a y x 0 Atrial Systole Ventricular Diastole
Time (msec) 0 Cardiac Cycle 100 200 300 400 500 600 700 800 QRS Complex EKG T P P Left Sided Pressures 120 90 Aorta Dicrotic Notch Pressure (mm Hg) 60 Left Ventricular Pressure 30 v c Left Atrial Pressure a y x 0 Atrial Systole Ventricular Diastole
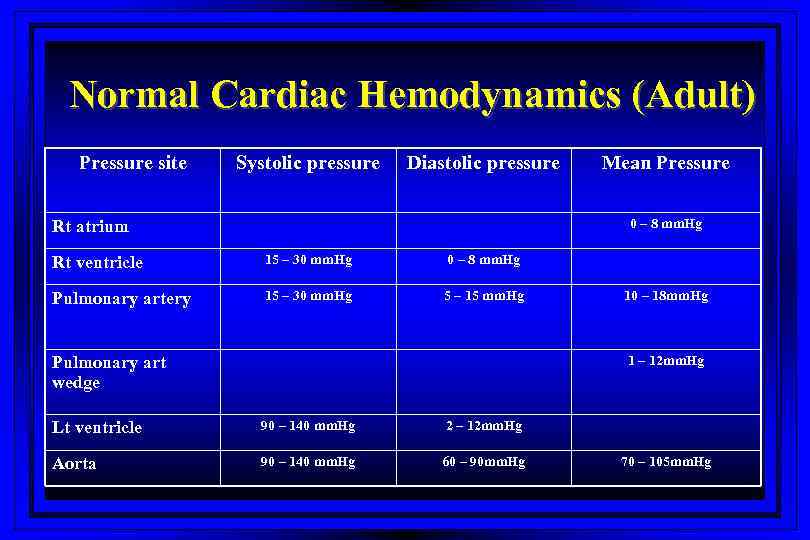 Normal Cardiac Hemodynamics (Adult) Pressure site Systolic pressure Diastolic pressure Rt atrium Mean Pressure 0 – 8 mm. Hg Rt ventricle 15 – 30 mm. Hg 0 – 8 mm. Hg Pulmonary artery 15 – 30 mm. Hg 5 – 15 mm. Hg Pulmonary art wedge 10 – 18 mm. Hg 1 – 12 mm. Hg Lt ventricle 90 – 140 mm. Hg 2 – 12 mm. Hg Aorta 90 – 140 mm. Hg 60 – 90 mm. Hg 70 – 105 mm. Hg
Normal Cardiac Hemodynamics (Adult) Pressure site Systolic pressure Diastolic pressure Rt atrium Mean Pressure 0 – 8 mm. Hg Rt ventricle 15 – 30 mm. Hg 0 – 8 mm. Hg Pulmonary artery 15 – 30 mm. Hg 5 – 15 mm. Hg Pulmonary art wedge 10 – 18 mm. Hg 1 – 12 mm. Hg Lt ventricle 90 – 140 mm. Hg 2 – 12 mm. Hg Aorta 90 – 140 mm. Hg 60 – 90 mm. Hg 70 – 105 mm. Hg
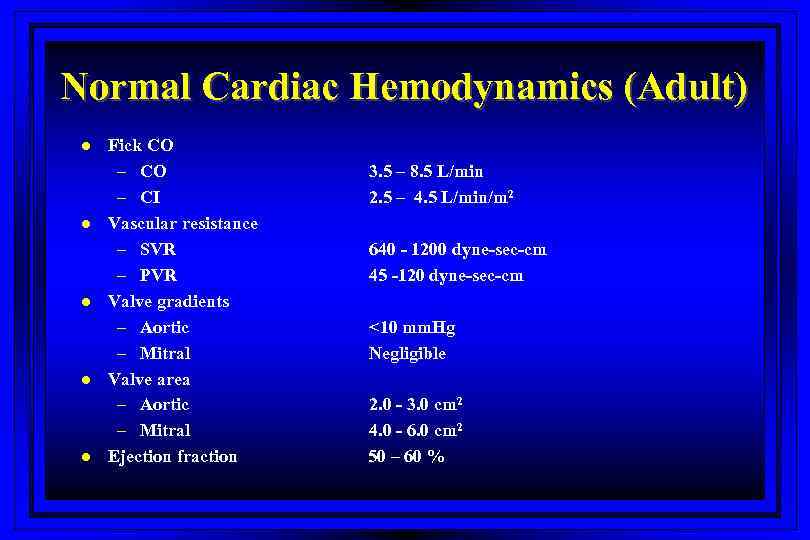 Normal Cardiac Hemodynamics (Adult) l l l Fick CO – CI Vascular resistance – SVR – PVR Valve gradients – Aortic – Mitral Valve area – Aortic – Mitral Ejection fraction 3. 5 – 8. 5 L/min 2. 5 – 4. 5 L/min/m 2 640 - 1200 dyne-sec-cm 45 -120 dyne-sec-cm <10 mm. Hg Negligible 2. 0 - 3. 0 cm 2 4. 0 - 6. 0 cm 2 50 – 60 %
Normal Cardiac Hemodynamics (Adult) l l l Fick CO – CI Vascular resistance – SVR – PVR Valve gradients – Aortic – Mitral Valve area – Aortic – Mitral Ejection fraction 3. 5 – 8. 5 L/min 2. 5 – 4. 5 L/min/m 2 640 - 1200 dyne-sec-cm 45 -120 dyne-sec-cm <10 mm. Hg Negligible 2. 0 - 3. 0 cm 2 4. 0 - 6. 0 cm 2 50 – 60 %
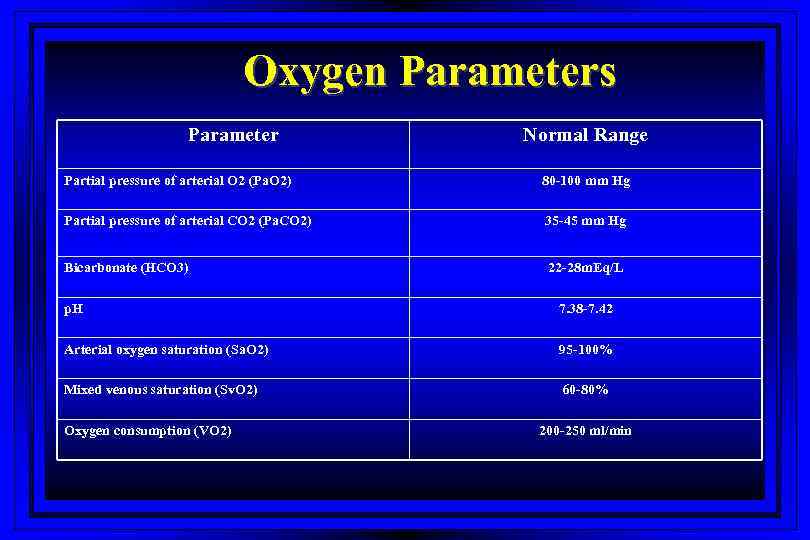 Oxygen Parameters Parameter Normal Range Partial pressure of arterial O 2 (Pa. O 2) 80 -100 mm Hg Partial pressure of arterial CO 2 (Pa. CO 2) 35 -45 mm Hg Bicarbonate (HCO 3) 22 -28 m. Eq/L p. H 7. 38 -7. 42 Arterial oxygen saturation (Sa. O 2) 95 -100% Mixed venous saturation (Sv. O 2) 60 -80% Oxygen consumption (VO 2) 200 -250 ml/min
Oxygen Parameters Parameter Normal Range Partial pressure of arterial O 2 (Pa. O 2) 80 -100 mm Hg Partial pressure of arterial CO 2 (Pa. CO 2) 35 -45 mm Hg Bicarbonate (HCO 3) 22 -28 m. Eq/L p. H 7. 38 -7. 42 Arterial oxygen saturation (Sa. O 2) 95 -100% Mixed venous saturation (Sv. O 2) 60 -80% Oxygen consumption (VO 2) 200 -250 ml/min
 Calculated Variables l l l l Cardiac index Stroke index Systemic vascular resistance Pulmonary vascular resistance Shunts Ventricular function Valvular stenosis or regurgitation
Calculated Variables l l l l Cardiac index Stroke index Systemic vascular resistance Pulmonary vascular resistance Shunts Ventricular function Valvular stenosis or regurgitation
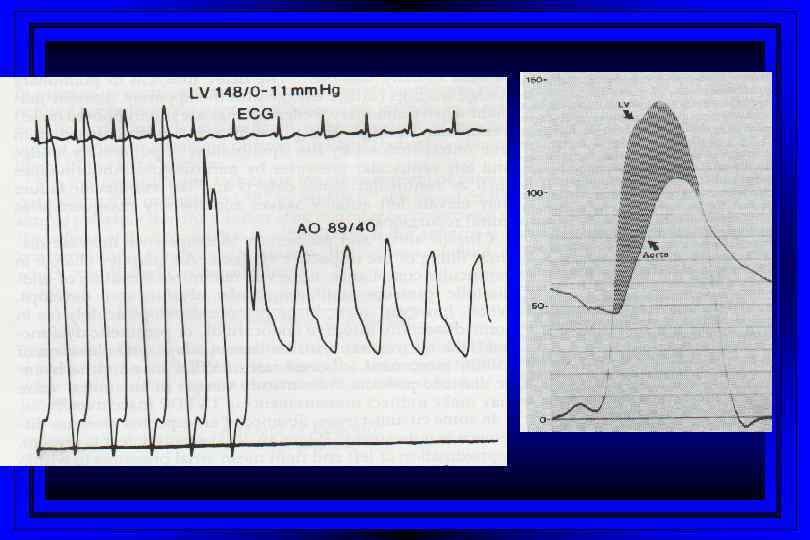
 Stenotic Orifices l l l Gradients Valve orifice cross-sectional areas Measurements assist in making decisions regarding surgical intervention
Stenotic Orifices l l l Gradients Valve orifice cross-sectional areas Measurements assist in making decisions regarding surgical intervention
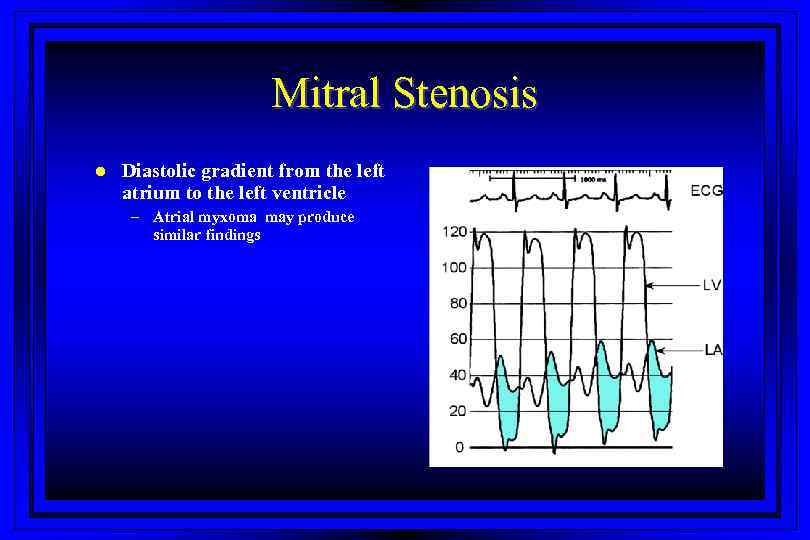 Mitral Stenosis l Diastolic gradient from the left atrium to the left ventricle – Atrial myxoma may produce similar findings
Mitral Stenosis l Diastolic gradient from the left atrium to the left ventricle – Atrial myxoma may produce similar findings
 Cardiac Output l Three main invasive methods of measurement – Flick method – Indicator-dilution method – Angiographic method
Cardiac Output l Three main invasive methods of measurement – Flick method – Indicator-dilution method – Angiographic method
 Fick Method The amount of oxygen extracted by the lungs from air = The amount taken up by blood in its passage through the lungs • rate of lung oxygen extraction (estimated) • oxygen content of the pulmonary arterial and pulmonary venous blood • the rate of pulmonary blood flow can be calculated • pulmonary blood flow=cardiac output (Unless there is a shunt) • CO=O 2 consumption/AVO 2 difference x 1. 36 x Hgb x 10 (L/min)
Fick Method The amount of oxygen extracted by the lungs from air = The amount taken up by blood in its passage through the lungs • rate of lung oxygen extraction (estimated) • oxygen content of the pulmonary arterial and pulmonary venous blood • the rate of pulmonary blood flow can be calculated • pulmonary blood flow=cardiac output (Unless there is a shunt) • CO=O 2 consumption/AVO 2 difference x 1. 36 x Hgb x 10 (L/min)
 The Indicator-dilution Technique and Thermodilution Technique • Dilution of an indicator is proportional to the volume of fluid to which it is added • If the amount and concentration (Temperature) of an indicator is known the volume of fluid in which it is diluted can be calculated • The most common is thermodilution method
The Indicator-dilution Technique and Thermodilution Technique • Dilution of an indicator is proportional to the volume of fluid to which it is added • If the amount and concentration (Temperature) of an indicator is known the volume of fluid in which it is diluted can be calculated • The most common is thermodilution method
 Cardiac Output (High) l Acute – – – – Acute hypervolemia ARDS, severe pneumonia Septic shock Acute intoxications Fever, heat stress, malignant hyperthermia Anxiety, emotional stress Delirium tremens
Cardiac Output (High) l Acute – – – – Acute hypervolemia ARDS, severe pneumonia Septic shock Acute intoxications Fever, heat stress, malignant hyperthermia Anxiety, emotional stress Delirium tremens
 Cardiac Output (High) l Chronic – – – – Severe chronic anemia Cirrhosis Chronic renal failure Pregnancy Thyrotoxicosis Polycythemia vera Labile hypertension Congenital heart disease (PDA)
Cardiac Output (High) l Chronic – – – – Severe chronic anemia Cirrhosis Chronic renal failure Pregnancy Thyrotoxicosis Polycythemia vera Labile hypertension Congenital heart disease (PDA)
 Cardiac Output (Low) l Acute – Acute hypovolemia (absolute or relative) – Acute severe pulmonary hypertension – Acute myocardial pump failure (cardiogenic shock) • extensive MI • myocardial toxic injury (ethanol, CO poisoning, septic shock) • following cardiopulmonary bypass – Acute impairment of ventricular filling • Increased intrathoracic pressure • Cardiac tamponade • Stunned myocardium • Acute ischemia
Cardiac Output (Low) l Acute – Acute hypovolemia (absolute or relative) – Acute severe pulmonary hypertension – Acute myocardial pump failure (cardiogenic shock) • extensive MI • myocardial toxic injury (ethanol, CO poisoning, septic shock) • following cardiopulmonary bypass – Acute impairment of ventricular filling • Increased intrathoracic pressure • Cardiac tamponade • Stunned myocardium • Acute ischemia
 Cardiac Output (Low) l Acute – Arrhythmias • Sustained VT • Extreme bradycardia – Acute inotropic changes in a failing myocardium • Beta-blockers • Ischemia • Acidosis
Cardiac Output (Low) l Acute – Arrhythmias • Sustained VT • Extreme bradycardia – Acute inotropic changes in a failing myocardium • Beta-blockers • Ischemia • Acidosis
 Cardiac Output (Low) l Chronic – Chronic severe pulmonary hypertension – Chronic myocardial pump failure • Ischemia • Hypertensive or dilated cardiomyopathy • Severe valvular heart disease – Chronic impairment of ventricular filling • Constrictive pericarditis • Restrictive cardiomyopathy • Mitral or tricuspid stenosis • Atrial myxoma
Cardiac Output (Low) l Chronic – Chronic severe pulmonary hypertension – Chronic myocardial pump failure • Ischemia • Hypertensive or dilated cardiomyopathy • Severe valvular heart disease – Chronic impairment of ventricular filling • Constrictive pericarditis • Restrictive cardiomyopathy • Mitral or tricuspid stenosis • Atrial myxoma
 Shunts l l l Demonstrated by an absence of an expected pressure difference With a significant ASD the left and right mean atrial pressures are within 5 mm. Hg With VSD’s the ventricular pressures may also equilibrate
Shunts l l l Demonstrated by an absence of an expected pressure difference With a significant ASD the left and right mean atrial pressures are within 5 mm. Hg With VSD’s the ventricular pressures may also equilibrate
 Shunts l Evaluation of shunts requires: – – Detection Classification Localization Quantitation
Shunts l Evaluation of shunts requires: – – Detection Classification Localization Quantitation
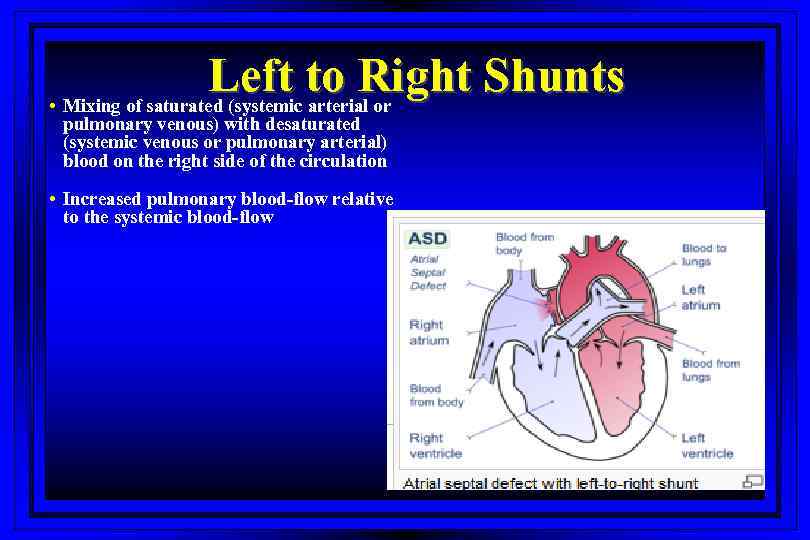 Left to Right Shunts • Mixing of saturated (systemic arterial or pulmonary venous) with desaturated (systemic venous or pulmonary arterial) blood on the right side of the circulation • Increased pulmonary blood-flow relative to the systemic blood-flow
Left to Right Shunts • Mixing of saturated (systemic arterial or pulmonary venous) with desaturated (systemic venous or pulmonary arterial) blood on the right side of the circulation • Increased pulmonary blood-flow relative to the systemic blood-flow
 Right to Left Shunts • Mixing of desaturated (systemic venous or pulmonary arterial) with saturated (systemic arterial or pulmonary venous) blood on the left side of the circulation, thus creating a oxygen step-down • Decreased pulmonary blood flow relative to systemic blood flow
Right to Left Shunts • Mixing of desaturated (systemic venous or pulmonary arterial) with saturated (systemic arterial or pulmonary venous) blood on the left side of the circulation, thus creating a oxygen step-down • Decreased pulmonary blood flow relative to systemic blood flow
 Pulmonary Hypertension: Role of Right Heart Catheterization l For diagnosis l For evaluating acute vasodilator response l For evaluating progression l For treatment selection l Lung vs. heart-lung transplantation
Pulmonary Hypertension: Role of Right Heart Catheterization l For diagnosis l For evaluating acute vasodilator response l For evaluating progression l For treatment selection l Lung vs. heart-lung transplantation
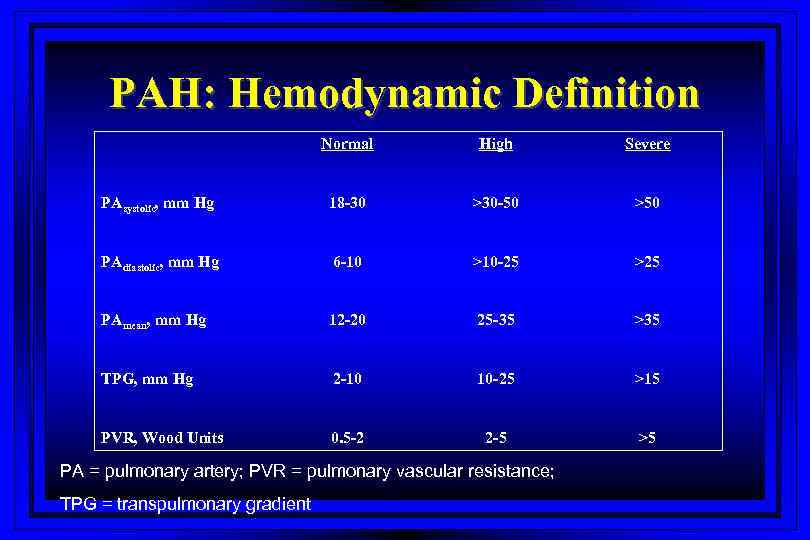 PAH: Hemodynamic Definition Normal High Severe PAsystolic, mm Hg 18 -30 >30 -50 >50 PAdiastolic, mm Hg 6 -10 >10 -25 >25 PAmean, mm Hg 12 -20 25 -35 >35 TPG, mm Hg 2 -10 10 -25 >15 PVR, Wood Units 0. 5 -2 2 -5 >5 PA = pulmonary artery; PVR = pulmonary vascular resistance; TPG = transpulmonary gradient
PAH: Hemodynamic Definition Normal High Severe PAsystolic, mm Hg 18 -30 >30 -50 >50 PAdiastolic, mm Hg 6 -10 >10 -25 >25 PAmean, mm Hg 12 -20 25 -35 >35 TPG, mm Hg 2 -10 10 -25 >15 PVR, Wood Units 0. 5 -2 2 -5 >5 PA = pulmonary artery; PVR = pulmonary vascular resistance; TPG = transpulmonary gradient
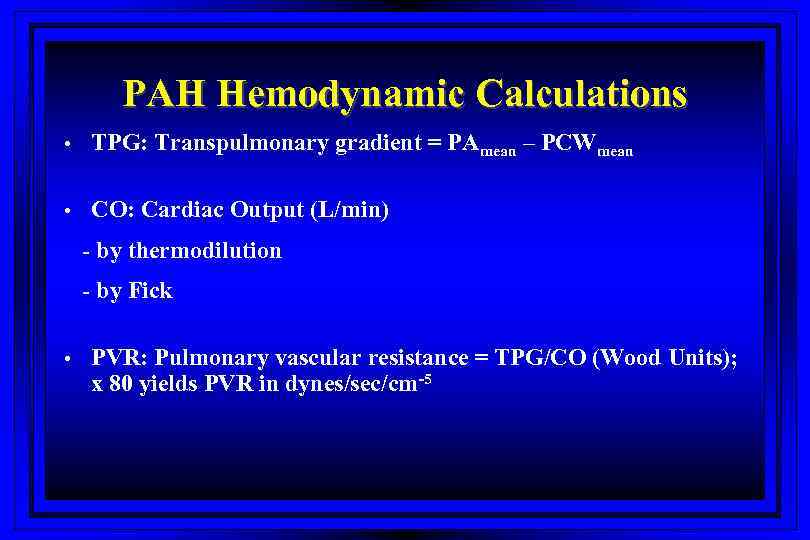 PAH Hemodynamic Calculations • TPG: Transpulmonary gradient = PAmean – PCWmean • CO: Cardiac Output (L/min) - by thermodilution - by Fick • PVR: Pulmonary vascular resistance = TPG/CO (Wood Units); x 80 yields PVR in dynes/sec/cm-5
PAH Hemodynamic Calculations • TPG: Transpulmonary gradient = PAmean – PCWmean • CO: Cardiac Output (L/min) - by thermodilution - by Fick • PVR: Pulmonary vascular resistance = TPG/CO (Wood Units); x 80 yields PVR in dynes/sec/cm-5
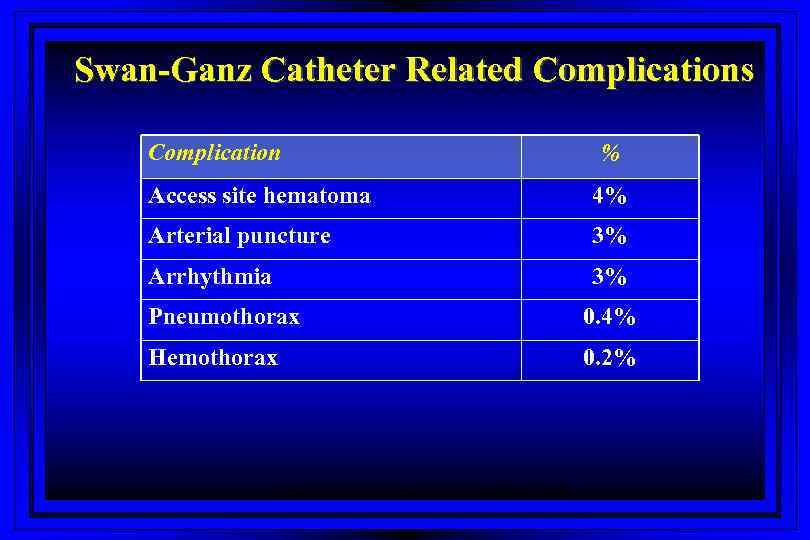 Swan-Ganz Catheter Related Complications Complication % Access site hematoma 4% Arterial puncture 3% Arrhythmia 3% Pneumothorax 0. 4% Hemothorax 0. 2% Harvey S et al. The Lancet 2005; 366: 472 -477
Swan-Ganz Catheter Related Complications Complication % Access site hematoma 4% Arterial puncture 3% Arrhythmia 3% Pneumothorax 0. 4% Hemothorax 0. 2% Harvey S et al. The Lancet 2005; 366: 472 -477
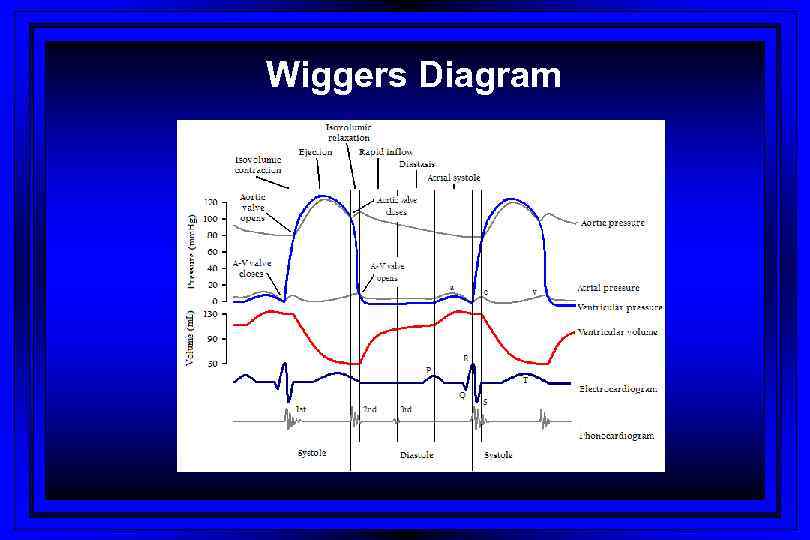 Wiggers Diagram
Wiggers Diagram
 Left Heart Catheterization: History l l l First human catheterization by Werner Forssmann: 1929 His work was not recognized until after World War II, when André Cournand Dickinson W. Richards, working in the US, demonstrated the importance of catheterization to the diagnosis of heart and lung diseases. Forssmann and the two Americans shared the 1956 Nobel Prize in Physiology or Medicine for their work. Selective coronary angiography by Mason Sones, working at the Cleveland Clinic: 1958 Melvin P. Judkins introduced the method he developed for transfemoral selective coronary angiography, known as the Judkins technique: 1966 Andreas Gruentzig in Zurich, Switzerland performed the first angioplasty on an awake patient, which was the first case to be entered into a worldwide percutaneous transluminal coronary angioplasty (PTCA) registry: 1977 Jacques Puel and Ulrich Sigwart inserted the first stent in a human coronary artery
Left Heart Catheterization: History l l l First human catheterization by Werner Forssmann: 1929 His work was not recognized until after World War II, when André Cournand Dickinson W. Richards, working in the US, demonstrated the importance of catheterization to the diagnosis of heart and lung diseases. Forssmann and the two Americans shared the 1956 Nobel Prize in Physiology or Medicine for their work. Selective coronary angiography by Mason Sones, working at the Cleveland Clinic: 1958 Melvin P. Judkins introduced the method he developed for transfemoral selective coronary angiography, known as the Judkins technique: 1966 Andreas Gruentzig in Zurich, Switzerland performed the first angioplasty on an awake patient, which was the first case to be entered into a worldwide percutaneous transluminal coronary angioplasty (PTCA) registry: 1977 Jacques Puel and Ulrich Sigwart inserted the first stent in a human coronary artery
 Vascular Access: Left Heart Cath l l l Sones’ technique (brachial approach) Judkin’s technique (femoral approach) Radial approach
Vascular Access: Left Heart Cath l l l Sones’ technique (brachial approach) Judkin’s technique (femoral approach) Radial approach
 Left Heart Catheterization l l Coronary angiography Left ventriculogram Ascending aortogram Pressure measurements in LV/aorta
Left Heart Catheterization l l Coronary angiography Left ventriculogram Ascending aortogram Pressure measurements in LV/aorta
 Cardiac Angiography: Ventriculography l A contrast roadmap of the left ventricle allows for evaluation of: – – – Ventricular chamber dimensions Global and segmental systolic function Presence and severity of mitral regurgitation Congenital defects (VSD) LVH Mitral valve prolapse
Cardiac Angiography: Ventriculography l A contrast roadmap of the left ventricle allows for evaluation of: – – – Ventricular chamber dimensions Global and segmental systolic function Presence and severity of mitral regurgitation Congenital defects (VSD) LVH Mitral valve prolapse
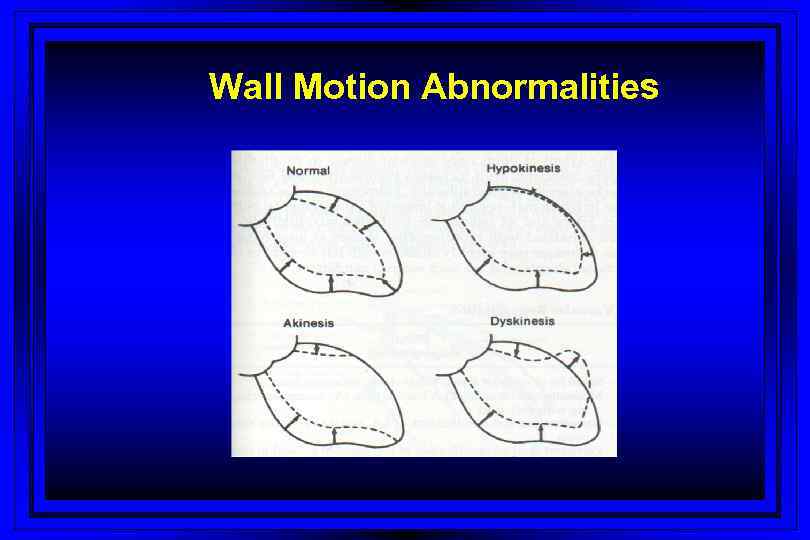 Wall Motion Abnormalities
Wall Motion Abnormalities
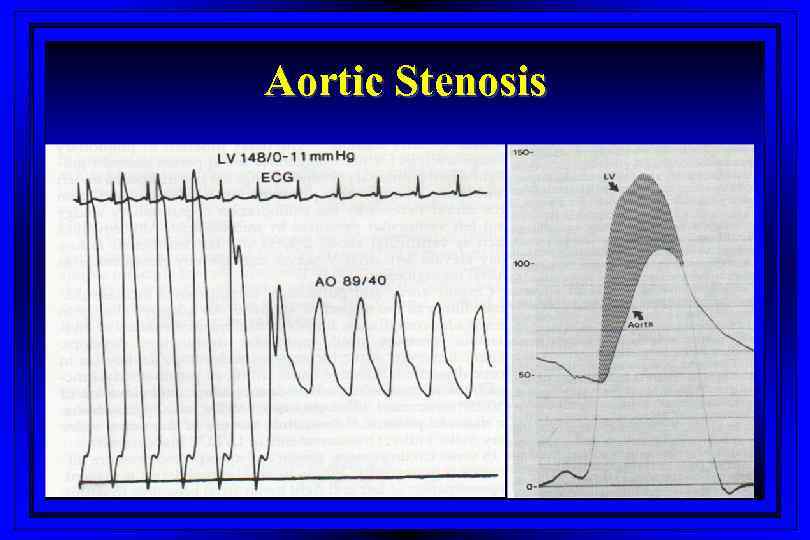 Aortic Stenosis
Aortic Stenosis
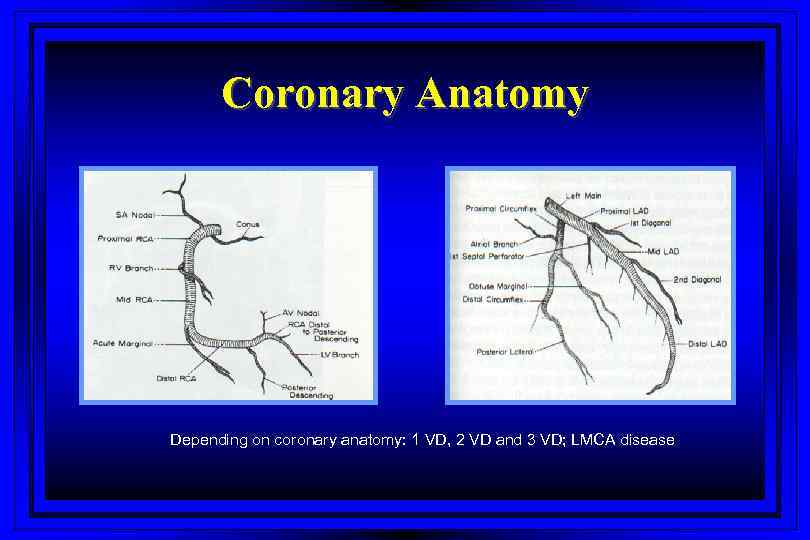 Coronary Anatomy Depending on coronary anatomy: 1 VD, 2 VD and 3 VD; LMCA disease
Coronary Anatomy Depending on coronary anatomy: 1 VD, 2 VD and 3 VD; LMCA disease
 Treatment Strategies of CAD Medical treatment, PCI or CABG - for pts with distal CAD; risk factors modification, ASA, b-blockers, Ca-channel antagonists, nitrates l PCI: for pts with treatable lesions in coronary arteries l CABG: for pts with 3 VD, LMCA- disease and lesions that can not be treated with PCI l
Treatment Strategies of CAD Medical treatment, PCI or CABG - for pts with distal CAD; risk factors modification, ASA, b-blockers, Ca-channel antagonists, nitrates l PCI: for pts with treatable lesions in coronary arteries l CABG: for pts with 3 VD, LMCA- disease and lesions that can not be treated with PCI l
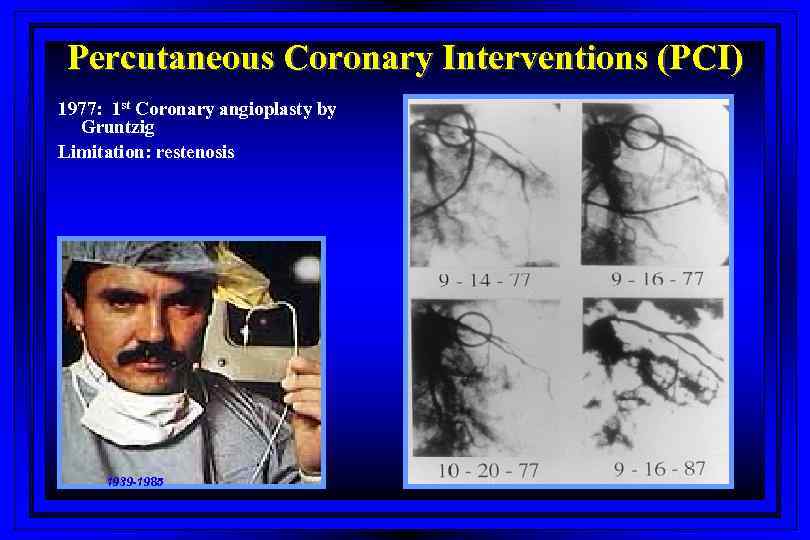 Percutaneous Coronary Interventions (PCI) 1977: 1 st Coronary angioplasty by Gruntzig Limitation: restenosis 1939 -1985
Percutaneous Coronary Interventions (PCI) 1977: 1 st Coronary angioplasty by Gruntzig Limitation: restenosis 1939 -1985
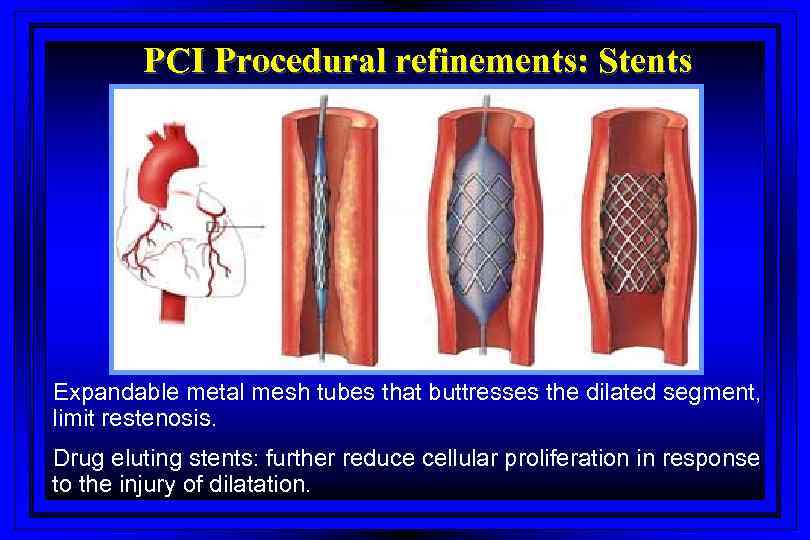 PCI Procedural refinements: Stents Expandable metal mesh tubes that buttresses the dilated segment, limit restenosis. Drug eluting stents: further reduce cellular proliferation in response to the injury of dilatation.
PCI Procedural refinements: Stents Expandable metal mesh tubes that buttresses the dilated segment, limit restenosis. Drug eluting stents: further reduce cellular proliferation in response to the injury of dilatation.
 Treatment Strategies of CAD l l Stable angina Unstable angina/non ST-elevation MI - Risk stratification; high-risk patients: elderly, history of CAD/MI, ST-T changes and positive cardiac markers (CK -MB and/or Troponin) - Early invasive approach including coronary angiography within 72 hours followed by medical management (30%), PCI (60%) or CABG (10%)
Treatment Strategies of CAD l l Stable angina Unstable angina/non ST-elevation MI - Risk stratification; high-risk patients: elderly, history of CAD/MI, ST-T changes and positive cardiac markers (CK -MB and/or Troponin) - Early invasive approach including coronary angiography within 72 hours followed by medical management (30%), PCI (60%) or CABG (10%)
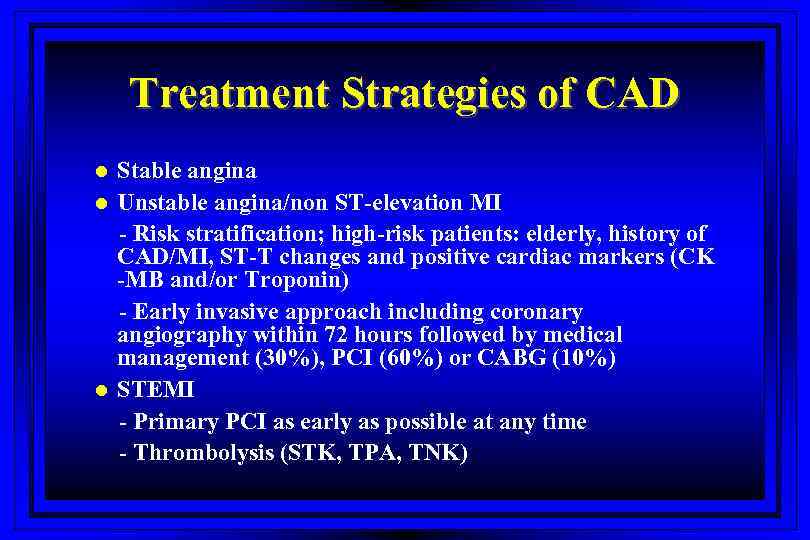 Treatment Strategies of CAD l l l Stable angina Unstable angina/non ST-elevation MI - Risk stratification; high-risk patients: elderly, history of CAD/MI, ST-T changes and positive cardiac markers (CK -MB and/or Troponin) - Early invasive approach including coronary angiography within 72 hours followed by medical management (30%), PCI (60%) or CABG (10%) STEMI - Primary PCI as early as possible at any time - Thrombolysis (STK, TPA, TNK)
Treatment Strategies of CAD l l l Stable angina Unstable angina/non ST-elevation MI - Risk stratification; high-risk patients: elderly, history of CAD/MI, ST-T changes and positive cardiac markers (CK -MB and/or Troponin) - Early invasive approach including coronary angiography within 72 hours followed by medical management (30%), PCI (60%) or CABG (10%) STEMI - Primary PCI as early as possible at any time - Thrombolysis (STK, TPA, TNK)
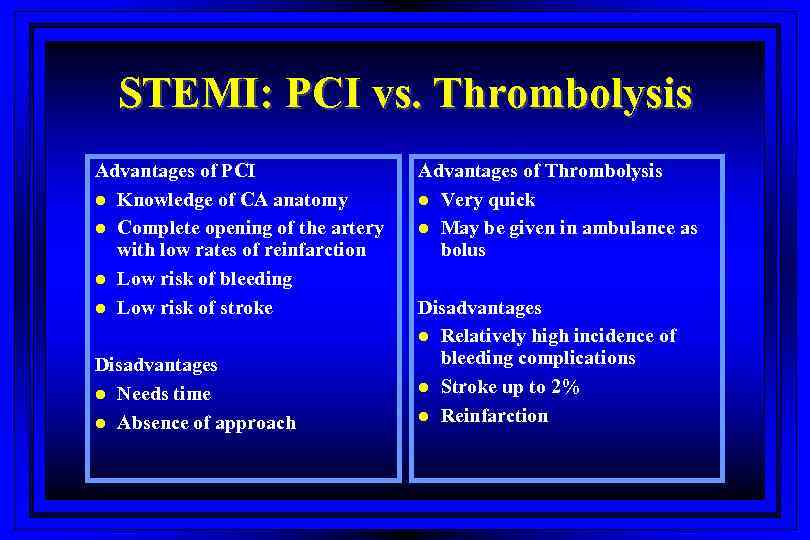 STEMI: PCI vs. Thrombolysis Advantages of PCI l Knowledge of CA anatomy l Complete opening of the artery with low rates of reinfarction l Low risk of bleeding l Low risk of stroke Disadvantages l Needs time l Absence of approach Advantages of Thrombolysis l Very quick l May be given in ambulance as bolus Disadvantages l Relatively high incidence of bleeding complications l Stroke up to 2% l Reinfarction
STEMI: PCI vs. Thrombolysis Advantages of PCI l Knowledge of CA anatomy l Complete opening of the artery with low rates of reinfarction l Low risk of bleeding l Low risk of stroke Disadvantages l Needs time l Absence of approach Advantages of Thrombolysis l Very quick l May be given in ambulance as bolus Disadvantages l Relatively high incidence of bleeding complications l Stroke up to 2% l Reinfarction
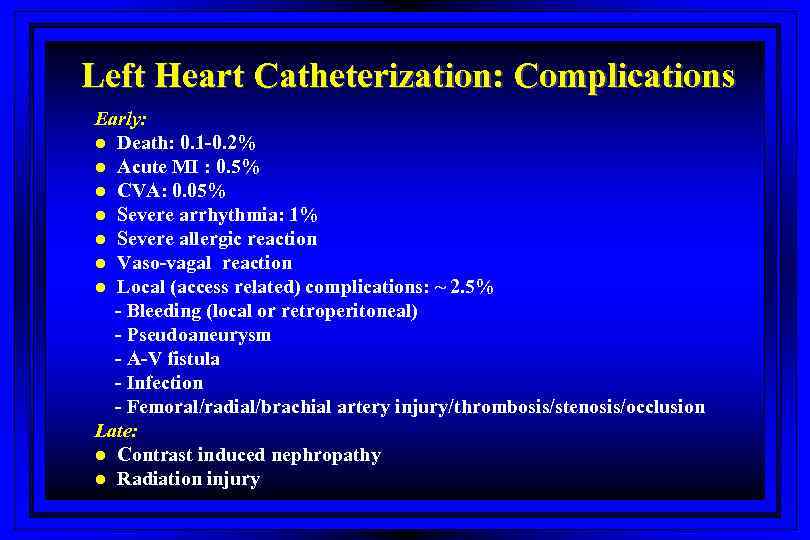 Left Heart Catheterization: Complications Early: l Death: 0. 1 -0. 2% l Acute MI : 0. 5% l CVA: 0. 05% l Severe arrhythmia: 1% l Severe allergic reaction l Vaso-vagal reaction l Local (access related) complications: ~ 2. 5% - Bleeding (local or retroperitoneal) - Pseudoaneurysm - A-V fistula - Infection - Femoral/radial/brachial artery injury/thrombosis/stenosis/occlusion Late: l Contrast induced nephropathy l Radiation injury
Left Heart Catheterization: Complications Early: l Death: 0. 1 -0. 2% l Acute MI : 0. 5% l CVA: 0. 05% l Severe arrhythmia: 1% l Severe allergic reaction l Vaso-vagal reaction l Local (access related) complications: ~ 2. 5% - Bleeding (local or retroperitoneal) - Pseudoaneurysm - A-V fistula - Infection - Femoral/radial/brachial artery injury/thrombosis/stenosis/occlusion Late: l Contrast induced nephropathy l Radiation injury
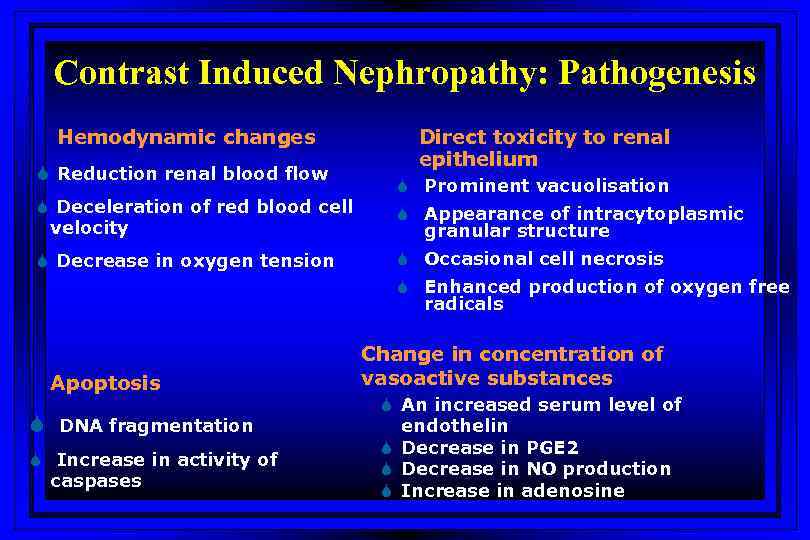 Contrast Induced Nephropathy: Pathogenesis Hemodynamic changes S Reduction renal blood flow S Deceleration of red blood cell velocity S Decrease in oxygen tension Direct toxicity to renal epithelium S Prominent vacuolisation S Appearance of intracytoplasmic granular structure S Occasional cell necrosis S Enhanced production of oxygen free radicals Apoptosis S DNA fragmentation S Increase in activity of caspases Change in concentration of vasoactive substances S An increased serum level of endothelin S Decrease in PGE 2 S Decrease in NO production S Increase in adenosine
Contrast Induced Nephropathy: Pathogenesis Hemodynamic changes S Reduction renal blood flow S Deceleration of red blood cell velocity S Decrease in oxygen tension Direct toxicity to renal epithelium S Prominent vacuolisation S Appearance of intracytoplasmic granular structure S Occasional cell necrosis S Enhanced production of oxygen free radicals Apoptosis S DNA fragmentation S Increase in activity of caspases Change in concentration of vasoactive substances S An increased serum level of endothelin S Decrease in PGE 2 S Decrease in NO production S Increase in adenosine
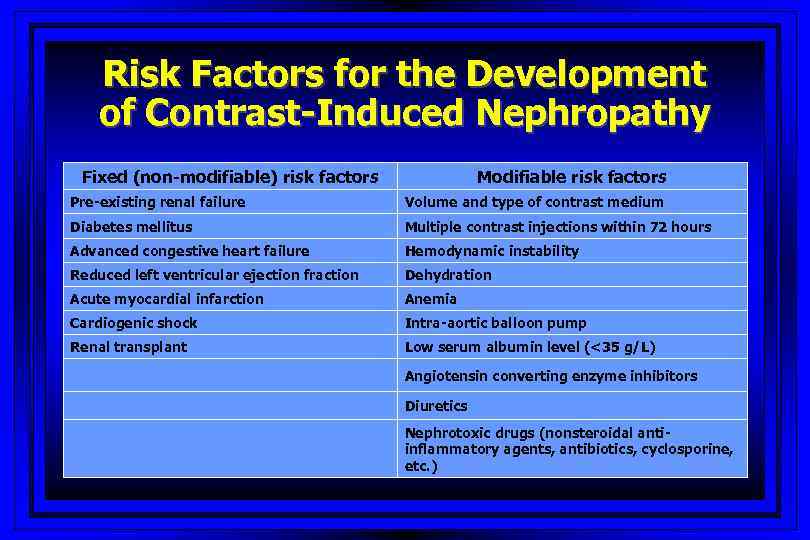 Risk Factors for the Development of Contrast-Induced Nephropathy Fixed (non-modifiable) risk factors Modifiable risk factors Pre-existing renal failure Volume and type of contrast medium Diabetes mellitus Multiple contrast injections within 72 hours Advanced congestive heart failure Hemodynamic instability Reduced left ventricular ejection fraction Dehydration Acute myocardial infarction Anemia Cardiogenic shock Intra-aortic balloon pump Renal transplant Low serum albumin level (<35 g/L) Angiotensin converting enzyme inhibitors Diuretics Nephrotoxic drugs (nonsteroidal antiinflammatory agents, antibiotics, cyclosporine, etc. )
Risk Factors for the Development of Contrast-Induced Nephropathy Fixed (non-modifiable) risk factors Modifiable risk factors Pre-existing renal failure Volume and type of contrast medium Diabetes mellitus Multiple contrast injections within 72 hours Advanced congestive heart failure Hemodynamic instability Reduced left ventricular ejection fraction Dehydration Acute myocardial infarction Anemia Cardiogenic shock Intra-aortic balloon pump Renal transplant Low serum albumin level (<35 g/L) Angiotensin converting enzyme inhibitors Diuretics Nephrotoxic drugs (nonsteroidal antiinflammatory agents, antibiotics, cyclosporine, etc. )
 Treatment Modalities Assessed in Randomized Trials on Prevention of CIN Treatment Effect Hydration + Sodium bicarbonate + Hemofiltration + Prostaglandin E 1 + N-acetyl-l-cysteine +/– Dopamine +/– Fenoldopam +/– Theophylline +/– Calcium channel blockers +/– Atrial natriuretic peptide – Hemodialysis Deleterious effect + positive effect; – no effect; +/– conflicting data
Treatment Modalities Assessed in Randomized Trials on Prevention of CIN Treatment Effect Hydration + Sodium bicarbonate + Hemofiltration + Prostaglandin E 1 + N-acetyl-l-cysteine +/– Dopamine +/– Fenoldopam +/– Theophylline +/– Calcium channel blockers +/– Atrial natriuretic peptide – Hemodialysis Deleterious effect + positive effect; – no effect; +/– conflicting data
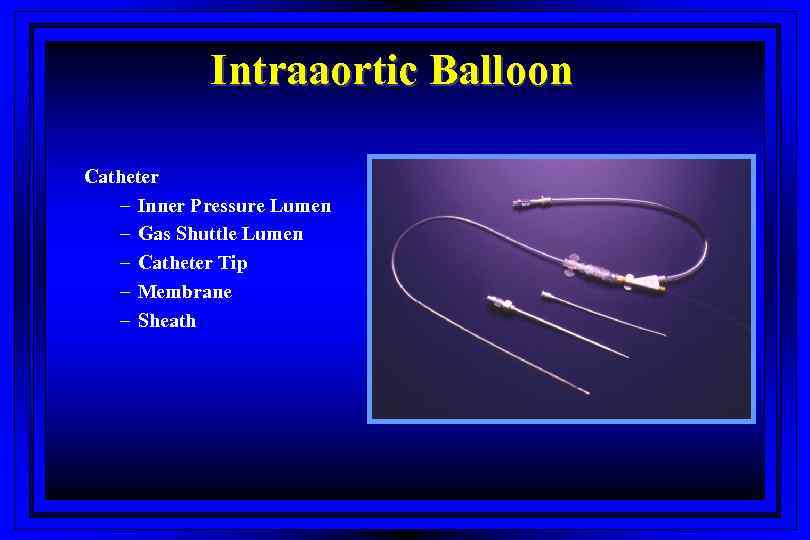 Intraaortic Balloon Catheter – Inner Pressure Lumen – Gas Shuttle Lumen – Catheter Tip – Membrane – Sheath
Intraaortic Balloon Catheter – Inner Pressure Lumen – Gas Shuttle Lumen – Catheter Tip – Membrane – Sheath
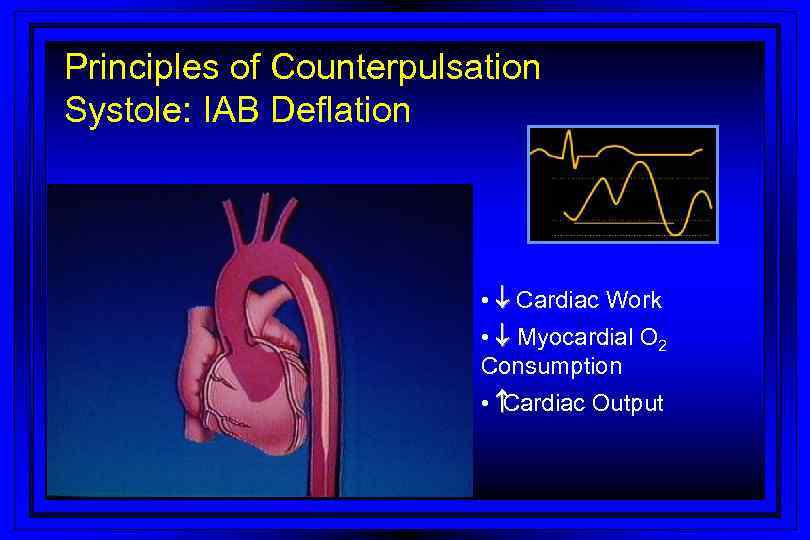 Principles of Counterpulsation Systole: IAB Deflation • ¯ Cardiac Work • ¯ Myocardial O 2 Consumption • Cardiac Output
Principles of Counterpulsation Systole: IAB Deflation • ¯ Cardiac Work • ¯ Myocardial O 2 Consumption • Cardiac Output
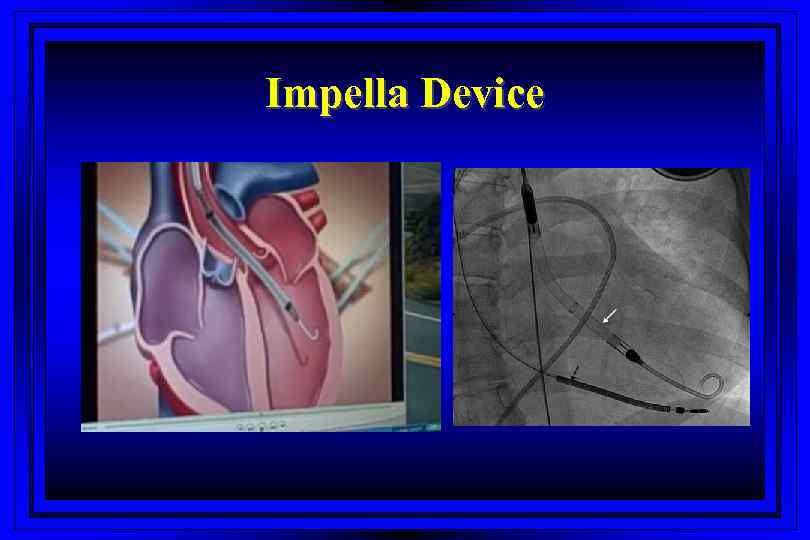 Impella Device
Impella Device
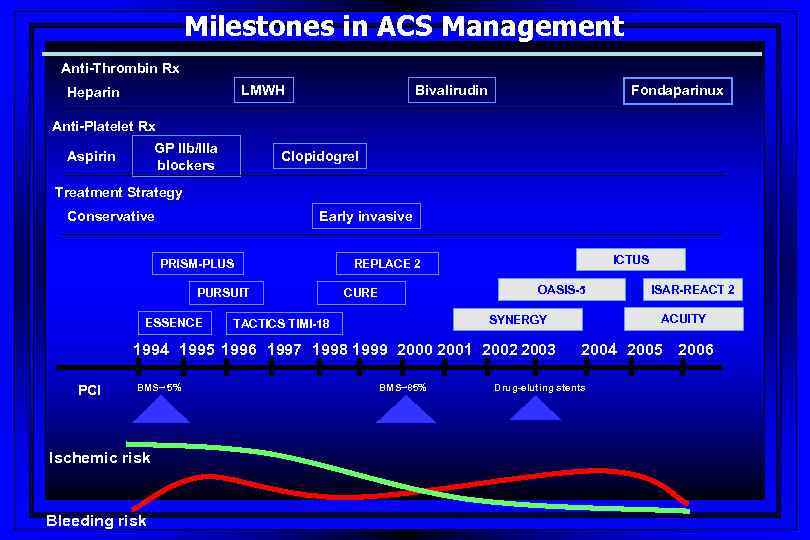 Milestones in ACS Management Anti-Thrombin Rx LMWH Heparin Bivalirudin Fondaparinux Anti-Platelet Rx GP IIb/IIIa blockers Aspirin Clopidogrel Treatment Strategy Early invasive Conservative PRISM-PLUS PURSUIT ESSENCE ICTUS REPLACE 2 OASIS-5 CURE 1994 1995 1996 1997 1998 1999 2000 2001 2002 2003 PCI BMS~ 5% Ischemic risk Bleeding risk ACUITY SYNERGY TACTICS TIMI-18 BMS~85% ISAR-REACT 2 2004 2005 2006 Drug-eluting stents
Milestones in ACS Management Anti-Thrombin Rx LMWH Heparin Bivalirudin Fondaparinux Anti-Platelet Rx GP IIb/IIIa blockers Aspirin Clopidogrel Treatment Strategy Early invasive Conservative PRISM-PLUS PURSUIT ESSENCE ICTUS REPLACE 2 OASIS-5 CURE 1994 1995 1996 1997 1998 1999 2000 2001 2002 2003 PCI BMS~ 5% Ischemic risk Bleeding risk ACUITY SYNERGY TACTICS TIMI-18 BMS~85% ISAR-REACT 2 2004 2005 2006 Drug-eluting stents
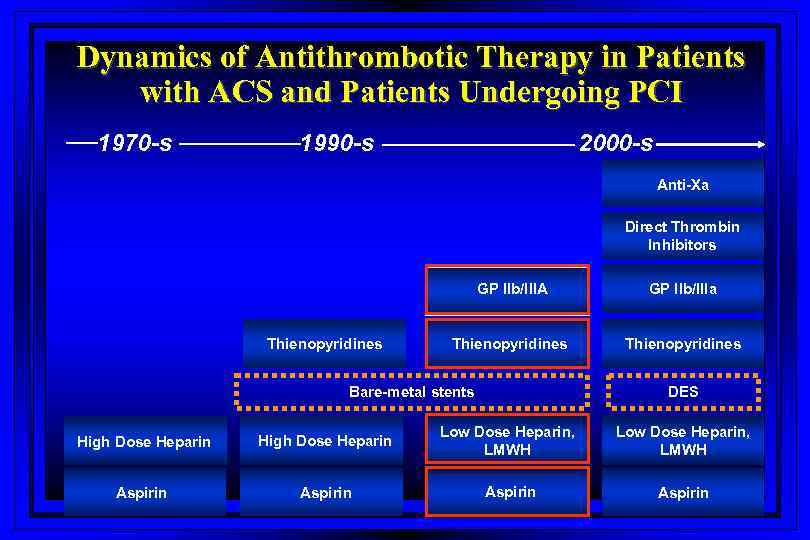 Dynamics of Antithrombotic Therapy in Patients with ACS and Patients Undergoing PCI 1970 -s 1990 -s 2000 -s Anti-Xa Direct Thrombin Inhibitors GP IIb/IIIA Thienopyridines GP IIb/IIIa Thienopyridines Bare-metal stents DES High Dose Heparin Low Dose Heparin, LMWH Aspirin
Dynamics of Antithrombotic Therapy in Patients with ACS and Patients Undergoing PCI 1970 -s 1990 -s 2000 -s Anti-Xa Direct Thrombin Inhibitors GP IIb/IIIA Thienopyridines GP IIb/IIIa Thienopyridines Bare-metal stents DES High Dose Heparin Low Dose Heparin, LMWH Aspirin
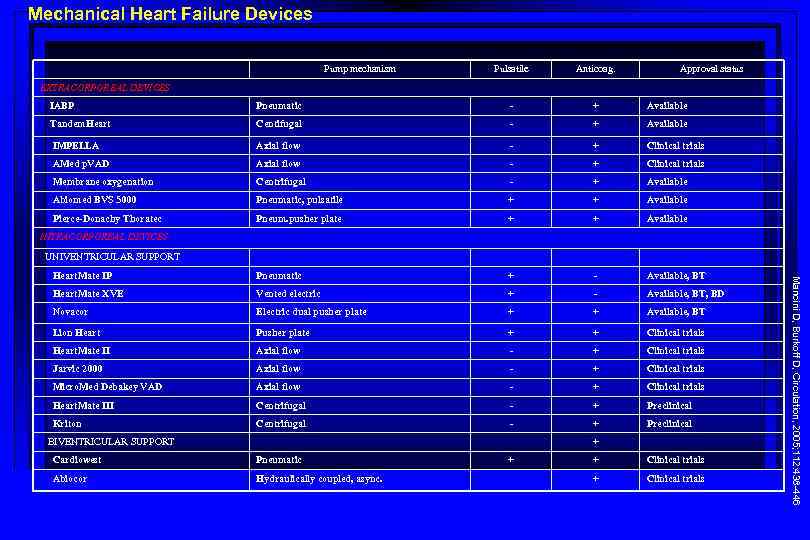 Mechanical Heart Failure Devices Pump mechanism Pulsatile Anticoag. Approval status EXTRACORPOREAL DEVICES IABP Pneumatic - + Available Tandem. Heart Centifugal - + Available IMPELLA Axial flow - + Clinical trials AMed p. VAD Axial flow - + Clinical trials Membrane oxygenation Centrifugal - + Available Abiomed BVS 5000 Pneumatic, pulsatile + + Available Pierce-Donachy Thoratec Pneum. pusher plate + + Available Heart. Mate IP Pneumatic + - Available, BT Heart. Mate XVE Vented electric + - Available, BT, BD Novacor Electric dual pusher plate + + Available, BT Lion Heart Pusher plate + + Clinical trials Heart. Mate II Axial flow - + Clinical trials Jarvic 2000 Axial flow - + Clinical trials Micro. Med Debakey VAD Axial flow - + Clinical trials Heart. Mate III Centrifugal - + Preclinical Kriton Centrifugal - + Preclinical INTRACORPOREAL DEVICES UNIVENTRICULAR SUPPORT + Cardiowest Pneumatic Abiocor Hydraulically coupled, async. + + Clinical trials Mancini D, Burkoff D, Circulation, 2005; 112: 438 -446 BIVENTRICULAR SUPPORT
Mechanical Heart Failure Devices Pump mechanism Pulsatile Anticoag. Approval status EXTRACORPOREAL DEVICES IABP Pneumatic - + Available Tandem. Heart Centifugal - + Available IMPELLA Axial flow - + Clinical trials AMed p. VAD Axial flow - + Clinical trials Membrane oxygenation Centrifugal - + Available Abiomed BVS 5000 Pneumatic, pulsatile + + Available Pierce-Donachy Thoratec Pneum. pusher plate + + Available Heart. Mate IP Pneumatic + - Available, BT Heart. Mate XVE Vented electric + - Available, BT, BD Novacor Electric dual pusher plate + + Available, BT Lion Heart Pusher plate + + Clinical trials Heart. Mate II Axial flow - + Clinical trials Jarvic 2000 Axial flow - + Clinical trials Micro. Med Debakey VAD Axial flow - + Clinical trials Heart. Mate III Centrifugal - + Preclinical Kriton Centrifugal - + Preclinical INTRACORPOREAL DEVICES UNIVENTRICULAR SUPPORT + Cardiowest Pneumatic Abiocor Hydraulically coupled, async. + + Clinical trials Mancini D, Burkoff D, Circulation, 2005; 112: 438 -446 BIVENTRICULAR SUPPORT
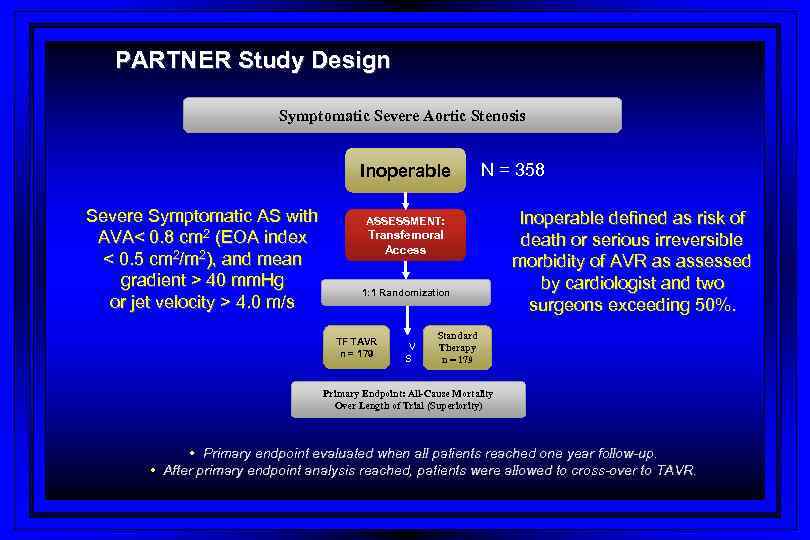 PARTNER Study Design Symptomatic Severe Aortic Stenosis Inoperable Severe Symptomatic AS with AVA< 0. 8 cm 2 (EOA index < 0. 5 cm 2/m 2), and mean gradient > 40 mm. Hg or jet velocity > 4. 0 m/s N = 358 ASSESSMENT: Transfemoral Access 1: 1 Randomization TF TAVR n = 179 V S Inoperable defined as risk of death or serious irreversible morbidity of AVR as assessed by cardiologist and two surgeons exceeding 50%. Standard Therapy n = 179 Primary Endpoint: All-Cause Mortality Over Length of Trial (Superiority) • Primary endpoint evaluated when all patients reached one year follow-up. • After primary endpoint analysis reached, patients were allowed to cross-over to TAVR.
PARTNER Study Design Symptomatic Severe Aortic Stenosis Inoperable Severe Symptomatic AS with AVA< 0. 8 cm 2 (EOA index < 0. 5 cm 2/m 2), and mean gradient > 40 mm. Hg or jet velocity > 4. 0 m/s N = 358 ASSESSMENT: Transfemoral Access 1: 1 Randomization TF TAVR n = 179 V S Inoperable defined as risk of death or serious irreversible morbidity of AVR as assessed by cardiologist and two surgeons exceeding 50%. Standard Therapy n = 179 Primary Endpoint: All-Cause Mortality Over Length of Trial (Superiority) • Primary endpoint evaluated when all patients reached one year follow-up. • After primary endpoint analysis reached, patients were allowed to cross-over to TAVR.
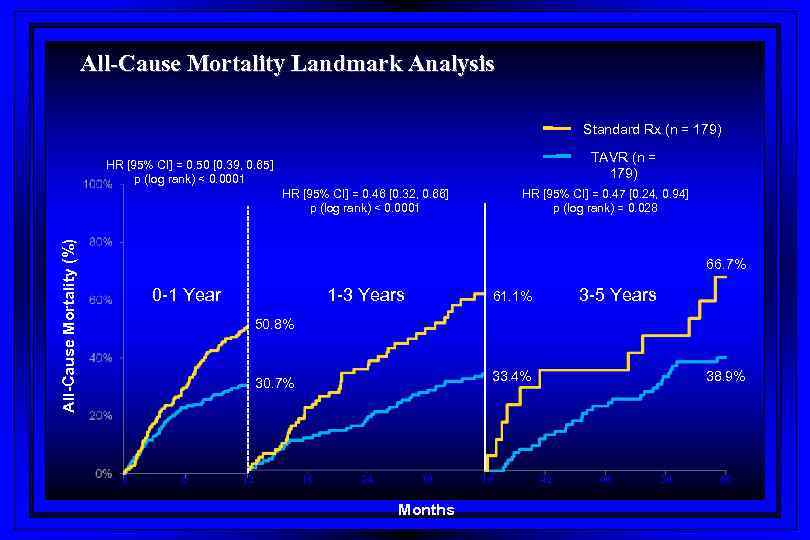 All-Cause Mortality Landmark Analysis Standard Rx (n = 179) TAVR (n = 179) HR [95% CI] = 0. 50 [0. 39, 0. 65] p (log rank) < 0. 0001 All-Cause Mortality (%) HR [95% CI] = 0. 46 [0. 32, 0. 66] p (log rank) < 0. 0001 HR [95% CI] = 0. 47 [0. 24, 0. 94] p (log rank) = 0. 028 66. 7% 0 -1 Year 1 -3 Years 3 -5 Years 61. 1% 50. 8% 0 6 12 38. 9% 33. 4% 30. 7% 18 24 30 Months 36 42 48 54 60
All-Cause Mortality Landmark Analysis Standard Rx (n = 179) TAVR (n = 179) HR [95% CI] = 0. 50 [0. 39, 0. 65] p (log rank) < 0. 0001 All-Cause Mortality (%) HR [95% CI] = 0. 46 [0. 32, 0. 66] p (log rank) < 0. 0001 HR [95% CI] = 0. 47 [0. 24, 0. 94] p (log rank) = 0. 028 66. 7% 0 -1 Year 1 -3 Years 3 -5 Years 61. 1% 50. 8% 0 6 12 38. 9% 33. 4% 30. 7% 18 24 30 Months 36 42 48 54 60
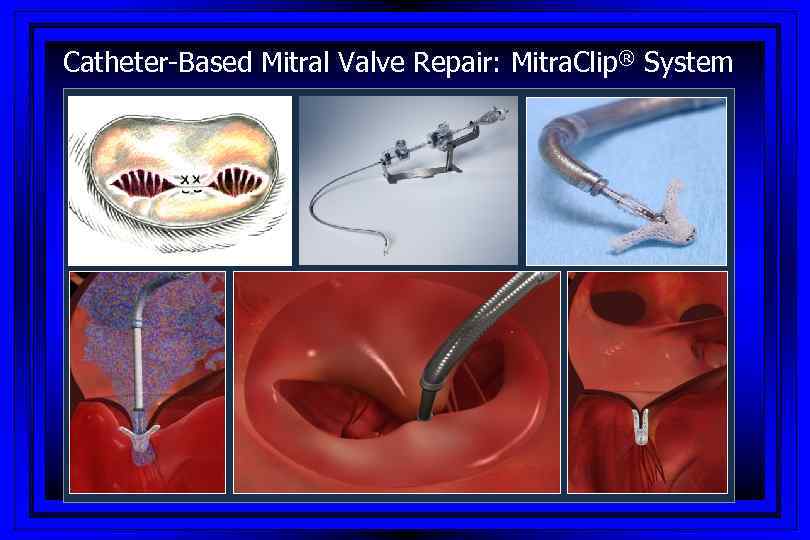 Catheter-Based Mitral Valve Repair: Mitra. Clip® System
Catheter-Based Mitral Valve Repair: Mitra. Clip® System
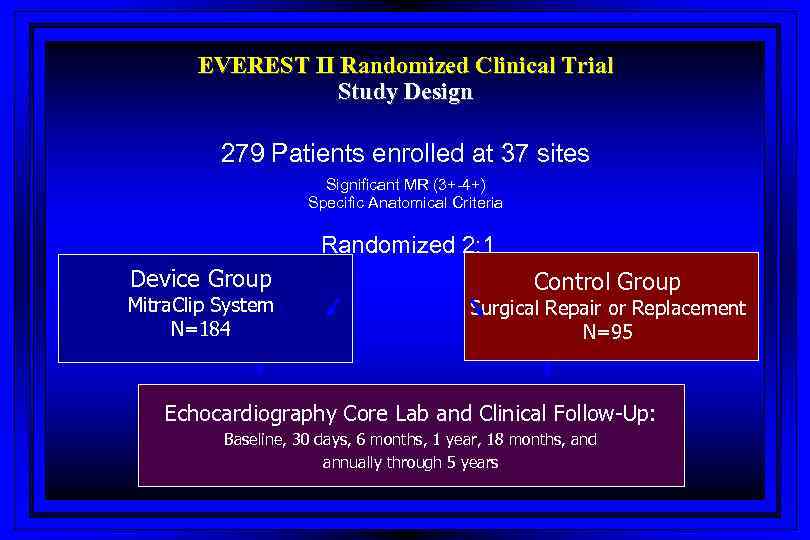 EVEREST II Randomized Clinical Trial Study Design 279 Patients enrolled at 37 sites Significant MR (3+-4+) Specific Anatomical Criteria Randomized 2: 1 Device Group Mitra. Clip System N=184 Control Group Surgical Repair or Replacement N=95 Echocardiography Core Lab and Clinical Follow-Up: Baseline, 30 days, 6 months, 1 year, 18 months, and annually through 5 years
EVEREST II Randomized Clinical Trial Study Design 279 Patients enrolled at 37 sites Significant MR (3+-4+) Specific Anatomical Criteria Randomized 2: 1 Device Group Mitra. Clip System N=184 Control Group Surgical Repair or Replacement N=95 Echocardiography Core Lab and Clinical Follow-Up: Baseline, 30 days, 6 months, 1 year, 18 months, and annually through 5 years
 EVEREST II RCT: Summary l Safety & effectiveness endpoints met – Safety: MAE rate at 30 days • Mitra. Clip device patients: 9. 6% • MV surgery patients: 57% – Effectiveness: Clinical Success Rate at 12 months • Mitra. Clip device patients: 72% • MV Surgery patients: 88% l Clinical benefit demonstrated for Mitra. Clip System and MV surgery patients through 12 months • Improved LV function • Improved NYHA Functional Class • Improved Quality of Life l Surgery remains an option after the Mitra. Clip procedure
EVEREST II RCT: Summary l Safety & effectiveness endpoints met – Safety: MAE rate at 30 days • Mitra. Clip device patients: 9. 6% • MV surgery patients: 57% – Effectiveness: Clinical Success Rate at 12 months • Mitra. Clip device patients: 72% • MV Surgery patients: 88% l Clinical benefit demonstrated for Mitra. Clip System and MV surgery patients through 12 months • Improved LV function • Improved NYHA Functional Class • Improved Quality of Life l Surgery remains an option after the Mitra. Clip procedure


