Professional English Language Prof. Dr. Yüksel KÖSEOĞLU Suleyman

















22790-week-2.ppt
- Количество слайдов: 48
 Professional English Language Prof. Dr. Yüksel KÖSEOĞLU Suleyman Demirel University Almaty-Kazakhstan
Professional English Language Prof. Dr. Yüksel KÖSEOĞLU Suleyman Demirel University Almaty-Kazakhstan
 Objectives Label the objectives in the box with the right modules. One has been done for you. 1. Academic Reading 2. Speaking for Academic Purposes 3. Listening and Note-taking 4. Academic Writing
Objectives Label the objectives in the box with the right modules. One has been done for you. 1. Academic Reading 2. Speaking for Academic Purposes 3. Listening and Note-taking 4. Academic Writing
 By the end of the course You will be able to….. Identify the main idea – 1&2 Develop an argument Understand important points Take notes Write an essay Participate in a discussion Quote correctly Describe a process and developments Understand supporting ideas Use sources Use abbreviations Understand an author’s point of view Use graphs when giving a speech Cite references Read efficiently Get the general idea of a lecture Differentiate between facts and opinions Make a presentation
By the end of the course You will be able to….. Identify the main idea – 1&2 Develop an argument Understand important points Take notes Write an essay Participate in a discussion Quote correctly Describe a process and developments Understand supporting ideas Use sources Use abbreviations Understand an author’s point of view Use graphs when giving a speech Cite references Read efficiently Get the general idea of a lecture Differentiate between facts and opinions Make a presentation
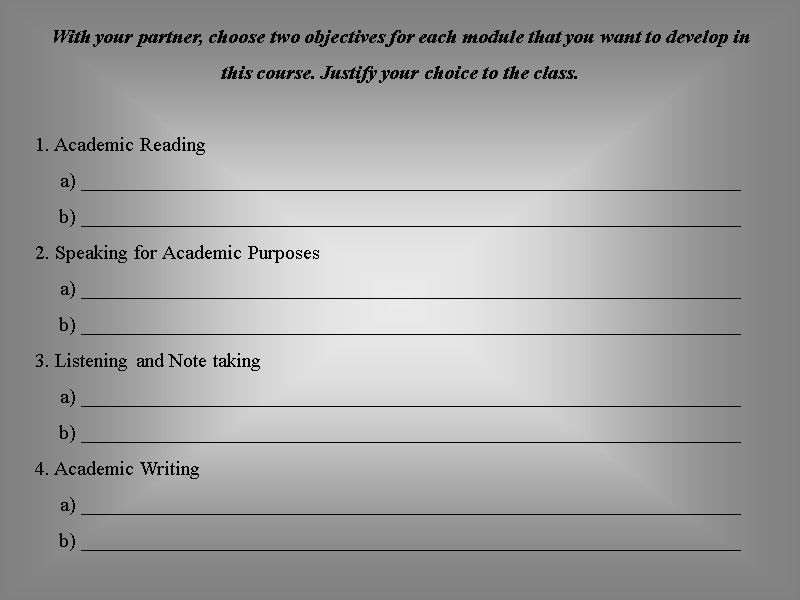
 With your partner, choose two objectives for each module that you want to develop in this course. Justify your choice to the class. 1. Academic Reading a) __________________________________________________________________ b) __________________________________________________________________ 2. Speaking for Academic Purposes a) __________________________________________________________________ b) __________________________________________________________________ 3. Listening and Note taking a) __________________________________________________________________ b) __________________________________________________________________ 4. Academic Writing a) __________________________________________________________________ b) __________________________________________________________________
With your partner, choose two objectives for each module that you want to develop in this course. Justify your choice to the class. 1. Academic Reading a) __________________________________________________________________ b) __________________________________________________________________ 2. Speaking for Academic Purposes a) __________________________________________________________________ b) __________________________________________________________________ 3. Listening and Note taking a) __________________________________________________________________ b) __________________________________________________________________ 4. Academic Writing a) __________________________________________________________________ b) __________________________________________________________________
 Part C: SPEAKING In groups, prepare a short introduction to the Academic Skills in English course for students who may know nothing about the course. Make sure you include the following information: Differences between school and university Modules Input/Output
Part C: SPEAKING In groups, prepare a short introduction to the Academic Skills in English course for students who may know nothing about the course. Make sure you include the following information: Differences between school and university Modules Input/Output
 PART D: WRITING Answer the following questions: What do you hope to improve by the end of the course? During your university life, how do you think this course will help you? ___________________________________________________________________________ ___________________________________________________________________________ ___________________________________________________________________________ ___________________________________________________________________________ ___________________________________________________________________________ ___________________________________________________________________________ ___________________________________________________________________________ ___________________________________________________________________________ ___________________________________________________________________________
PART D: WRITING Answer the following questions: What do you hope to improve by the end of the course? During your university life, how do you think this course will help you? ___________________________________________________________________________ ___________________________________________________________________________ ___________________________________________________________________________ ___________________________________________________________________________ ___________________________________________________________________________ ___________________________________________________________________________ ___________________________________________________________________________ ___________________________________________________________________________ ___________________________________________________________________________
 UNIT I: STARTING POINT By the end of this unit, I will be able to: · identify the purpose of a spoken text · use a suggested note-taking method · take notes while listening · summarize spoken texts and lectures
UNIT I: STARTING POINT By the end of this unit, I will be able to: · identify the purpose of a spoken text · use a suggested note-taking method · take notes while listening · summarize spoken texts and lectures
 TARGET OUTPUT At the end of this unit, You will be asked to: ‘write a summary on one aspect of note-taking’
TARGET OUTPUT At the end of this unit, You will be asked to: ‘write a summary on one aspect of note-taking’
 Part A: DISCUSSION 1. Consider the following questions alone: a) What reasons do people have for listening to others? ___________________________________________ ___________________________________________ ___________________________________________ b) Why do they listen at university? ____________________________________ ____________________________________ c) What is the difference between academic and non-academic listening? _________________________________________________ _________________________________________________
Part A: DISCUSSION 1. Consider the following questions alone: a) What reasons do people have for listening to others? ___________________________________________ ___________________________________________ ___________________________________________ b) Why do they listen at university? ____________________________________ ____________________________________ c) What is the difference between academic and non-academic listening? _________________________________________________ _________________________________________________
 ACADEMIC SKILL TIP: CHOOSING A NOTE-TAKING METHOD In order to get the most from your lessons, you must develop a note-taking method that works for you. You can do this by looking at different ways of note-taking and deciding which one is suitable for your learning style. Work on a method that will gradually help you to structure and organize your notes to make your note-taking speed and comprehension better. · Start each lecture on a new page; date and number each page. · Write on one side of the paper only. You can set them out side-by-side for easier reviewing when studying for an exam. · Leave blank spaces. This allows you to add comments or note questions later. · Make your notes as brief as possible. · Develop a system of abbreviations and symbols you can use wherever possible. · Note all unfamiliar vocabulary or concepts you don't understand. This reminds you to look them up later.
ACADEMIC SKILL TIP: CHOOSING A NOTE-TAKING METHOD In order to get the most from your lessons, you must develop a note-taking method that works for you. You can do this by looking at different ways of note-taking and deciding which one is suitable for your learning style. Work on a method that will gradually help you to structure and organize your notes to make your note-taking speed and comprehension better. · Start each lecture on a new page; date and number each page. · Write on one side of the paper only. You can set them out side-by-side for easier reviewing when studying for an exam. · Leave blank spaces. This allows you to add comments or note questions later. · Make your notes as brief as possible. · Develop a system of abbreviations and symbols you can use wherever possible. · Note all unfamiliar vocabulary or concepts you don't understand. This reminds you to look them up later.
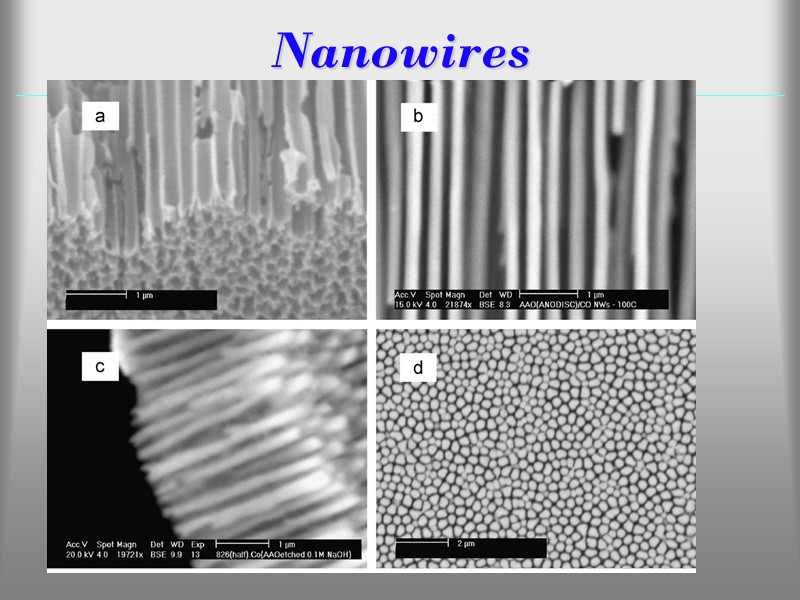
 Nanowires
Nanowires
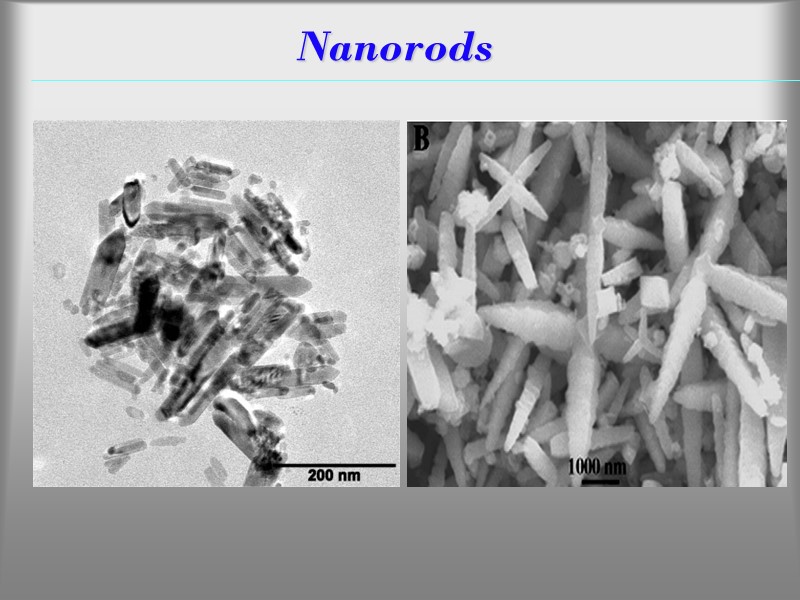
 Nanorods
Nanorods
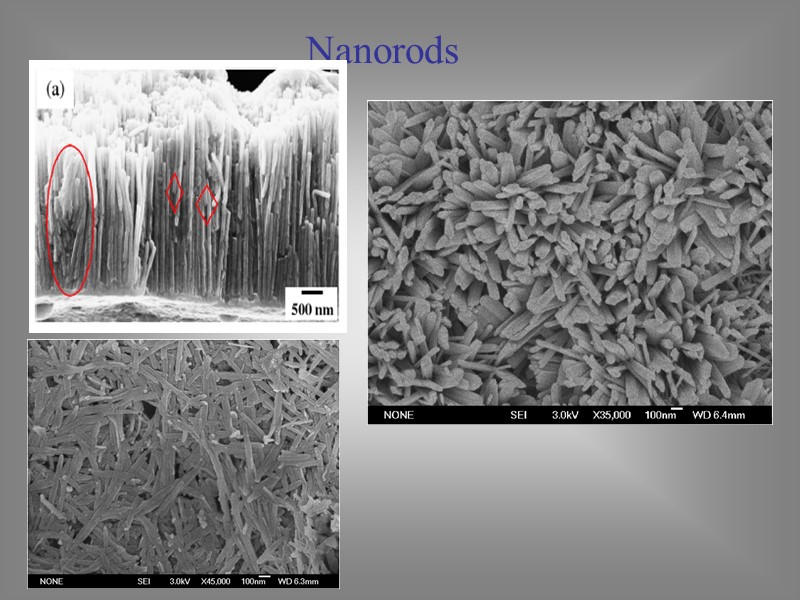
 Nanorods
Nanorods
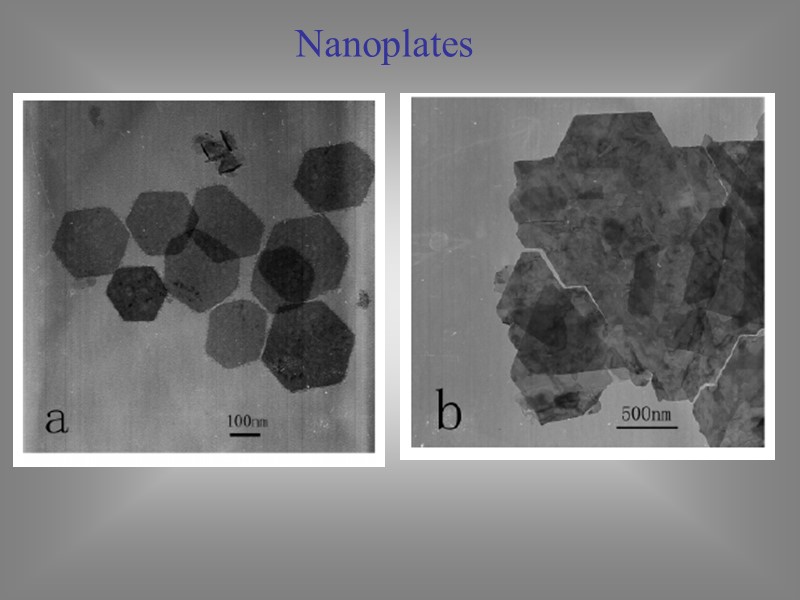
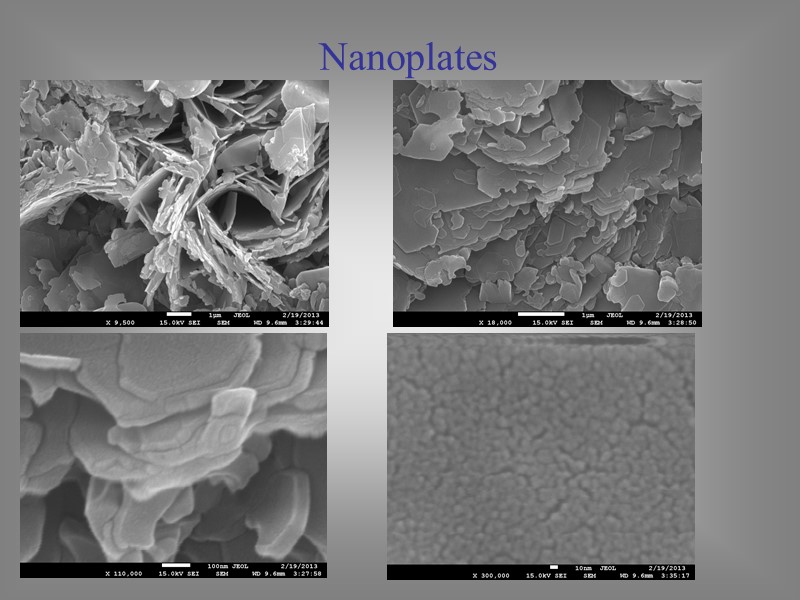
 Nanoplates
Nanoplates
 Nanoplates
Nanoplates
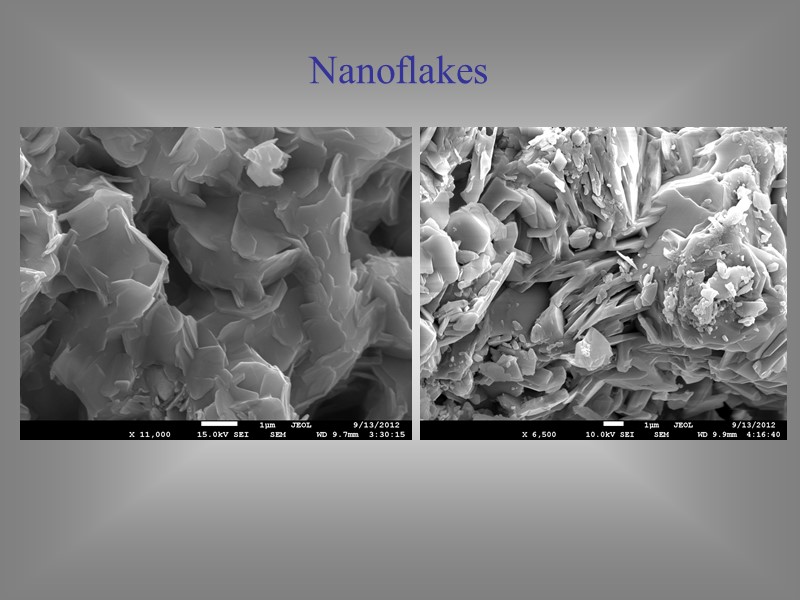
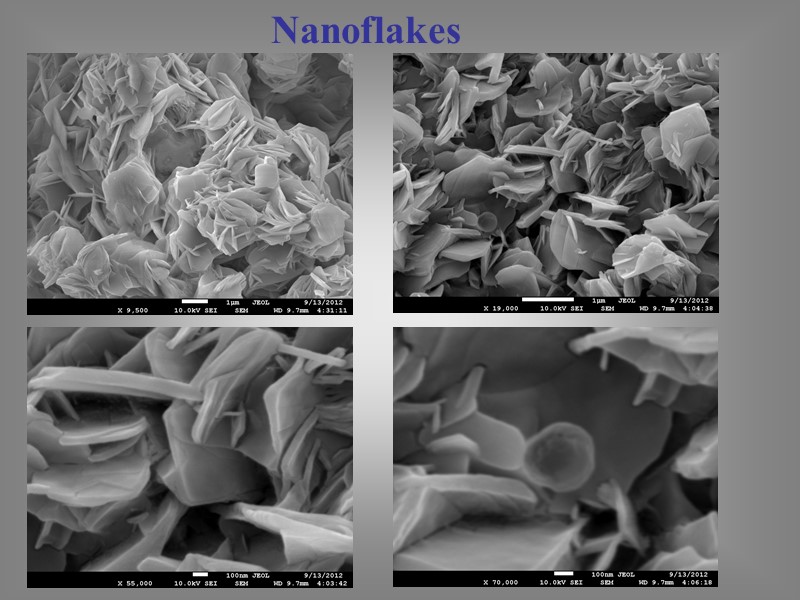
 Nanoflakes
Nanoflakes
 Nanoflakes
Nanoflakes
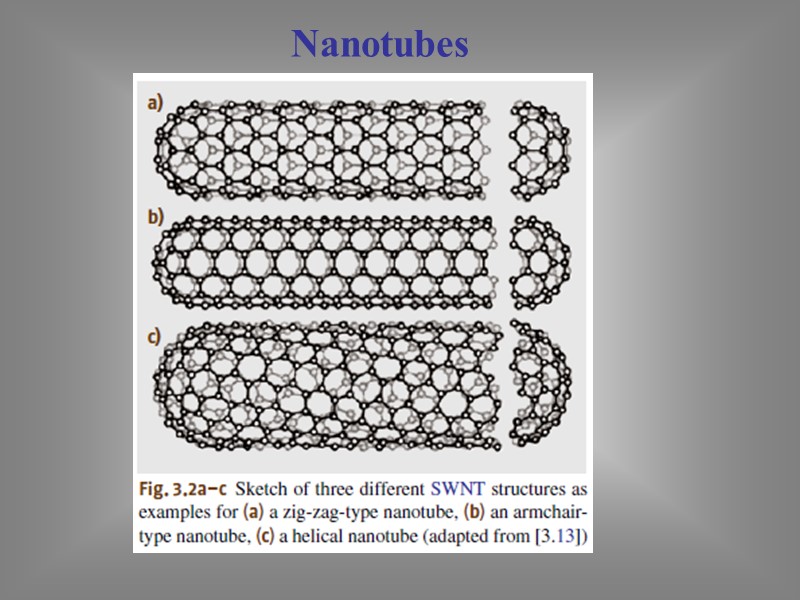
 Nanotubes
Nanotubes
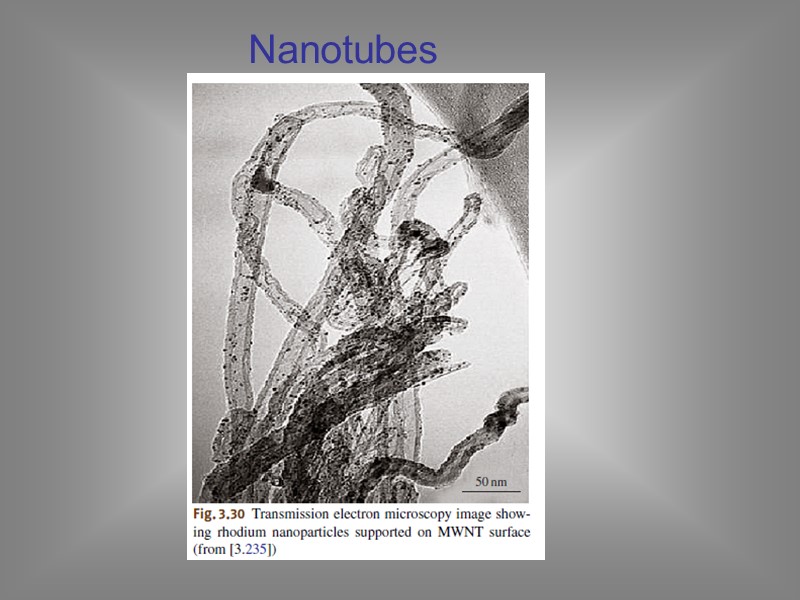
 Nanotubes
Nanotubes
 Nanotubes
Nanotubes
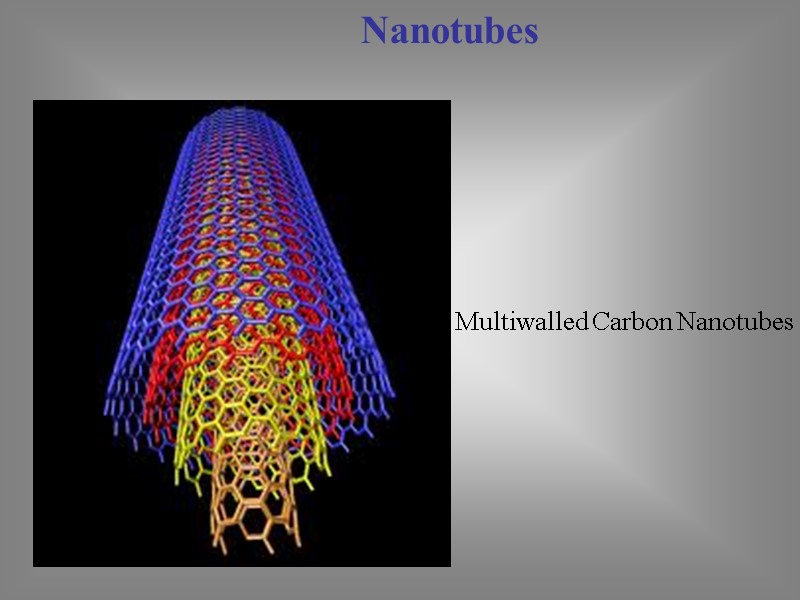
 Nanotubes Multiwalled Carbon Nanotubes
Nanotubes Multiwalled Carbon Nanotubes
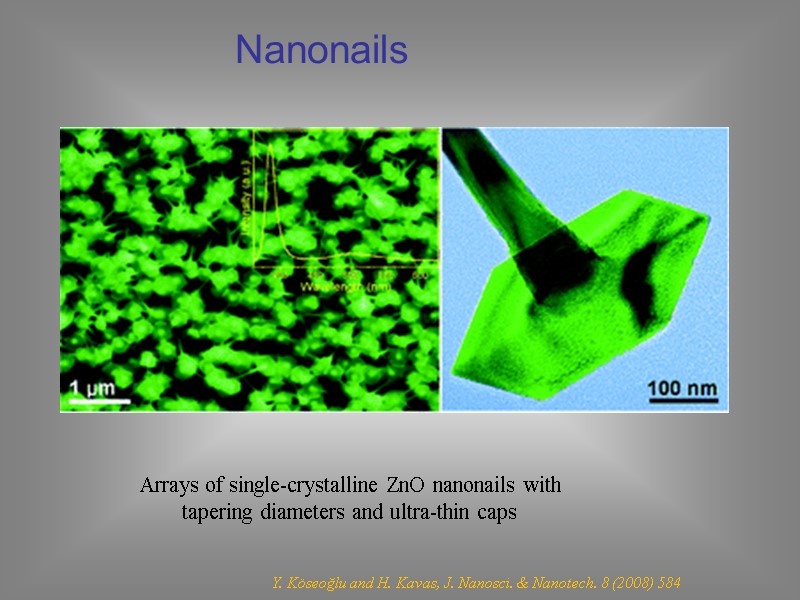
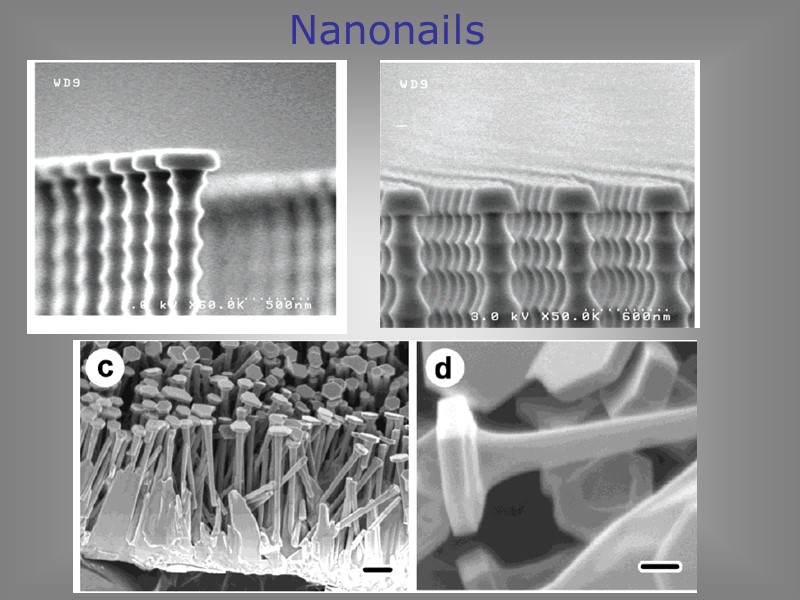
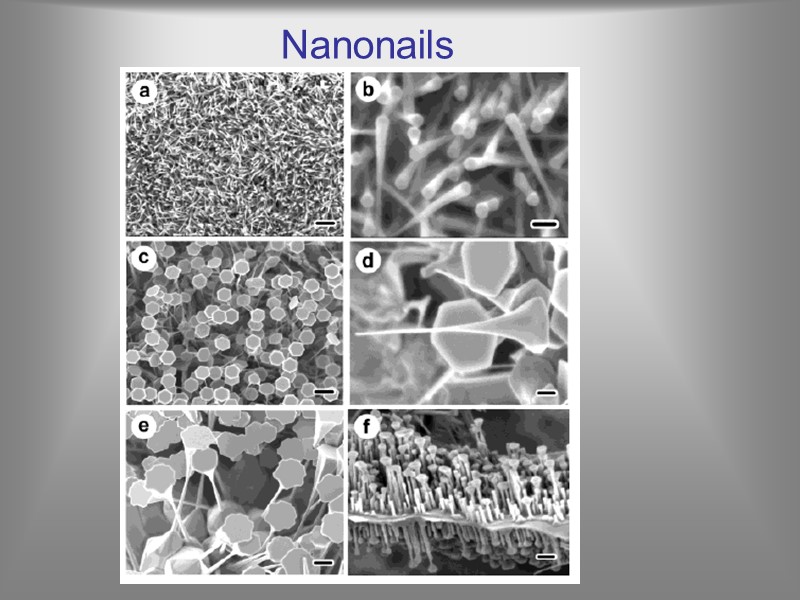
 Y. Köseoğlu and H. Kavas, J. Nanosci. & Nanotech. 8 (2008) 584 Nanonails Arrays of single-crystalline ZnO nanonails with tapering diameters and ultra-thin caps
Y. Köseoğlu and H. Kavas, J. Nanosci. & Nanotech. 8 (2008) 584 Nanonails Arrays of single-crystalline ZnO nanonails with tapering diameters and ultra-thin caps
 Nanonails
Nanonails
 Nanonails
Nanonails
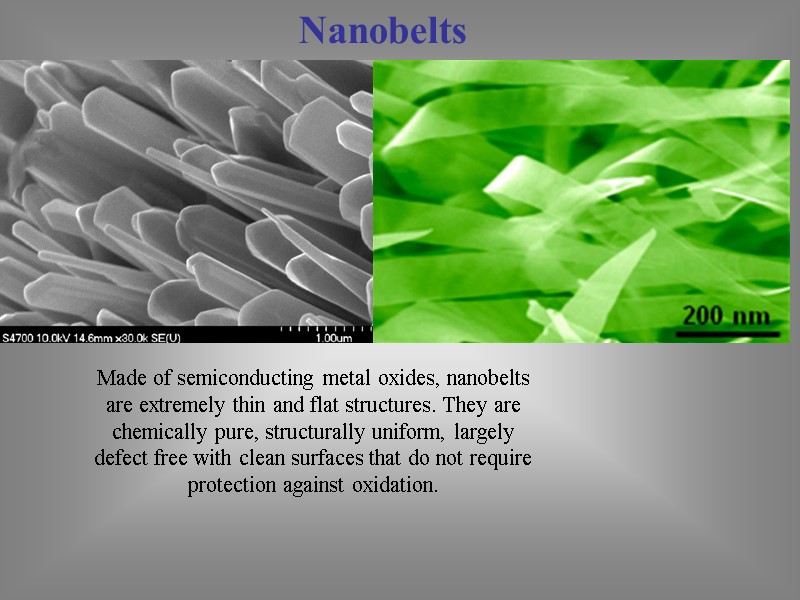
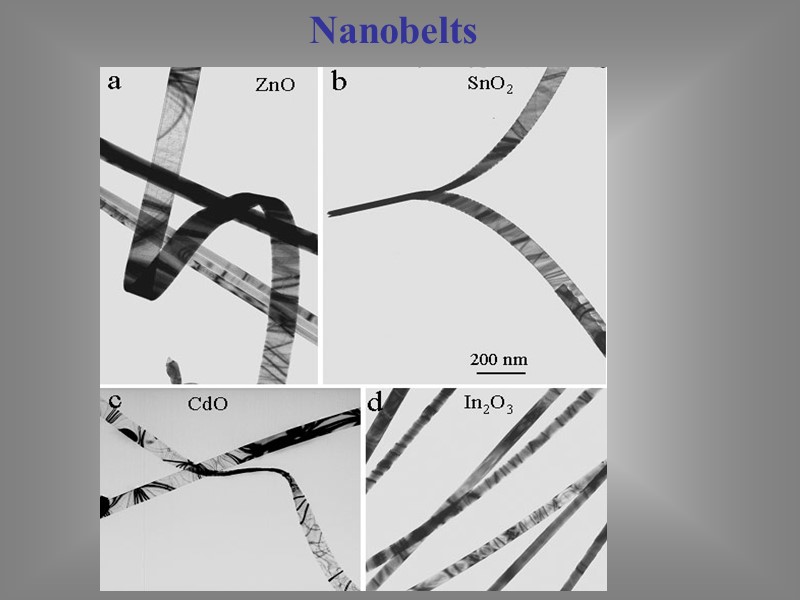
 Nanobelts Made of semiconducting metal oxides, nanobelts are extremely thin and flat structures. They are chemically pure, structurally uniform, largely defect free with clean surfaces that do not require protection against oxidation.
Nanobelts Made of semiconducting metal oxides, nanobelts are extremely thin and flat structures. They are chemically pure, structurally uniform, largely defect free with clean surfaces that do not require protection against oxidation.
 Nanobelts
Nanobelts
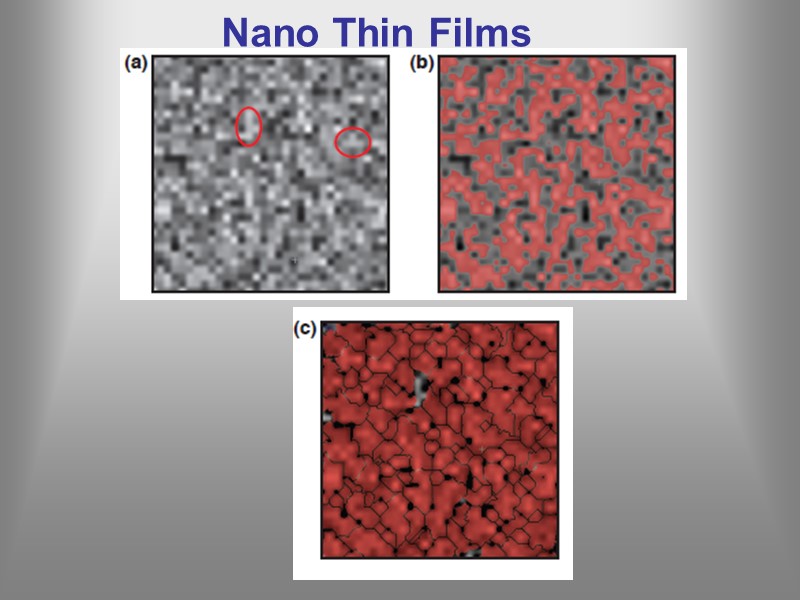
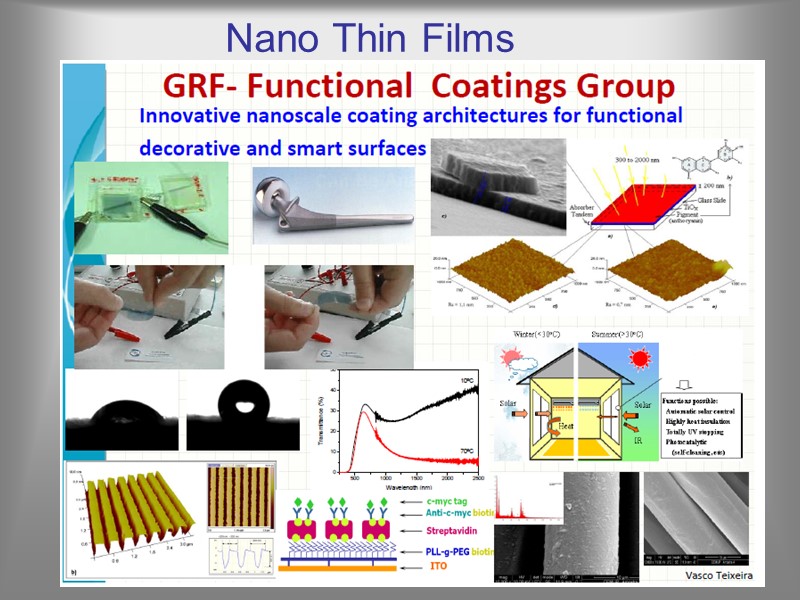
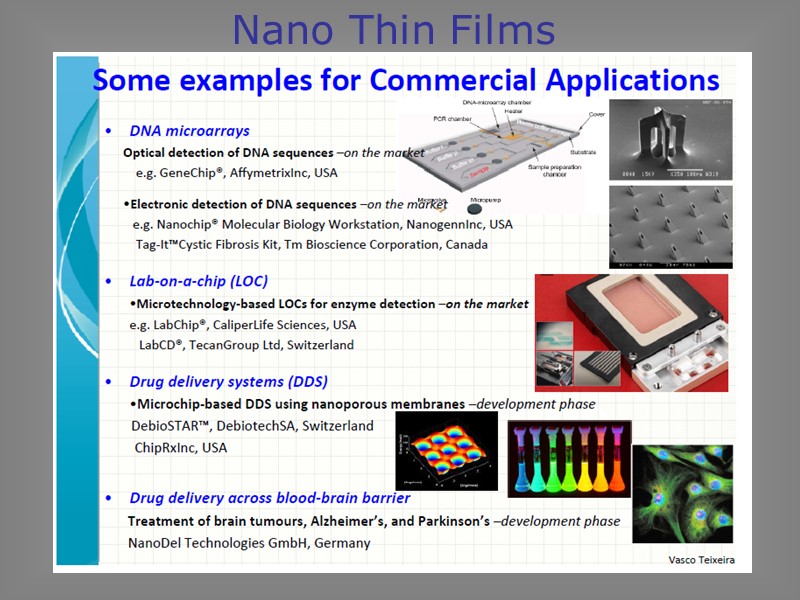
 Nano Thin Films
Nano Thin Films
 Nano Thin Films
Nano Thin Films
 Nano Thin Films
Nano Thin Films
 Nano Thin Films
Nano Thin Films
 Nano Thin Films
Nano Thin Films
 Water treatment or Water Purification Arsenic is a colorless, odorless, tasteless element that can lead to skin discoloration, sickness, cancer and death. Arsenic contamination in drinking water is a global problem, and while there are ways to remove arsenic, they require extensive hardware and high-pressure pumps that run on electricity, Our approach is simple and requires no electricity. While the magnetic nanoparticles used in the publication are expensive, we are working on new approaches to their production and reusage.
Water treatment or Water Purification Arsenic is a colorless, odorless, tasteless element that can lead to skin discoloration, sickness, cancer and death. Arsenic contamination in drinking water is a global problem, and while there are ways to remove arsenic, they require extensive hardware and high-pressure pumps that run on electricity, Our approach is simple and requires no electricity. While the magnetic nanoparticles used in the publication are expensive, we are working on new approaches to their production and reusage.
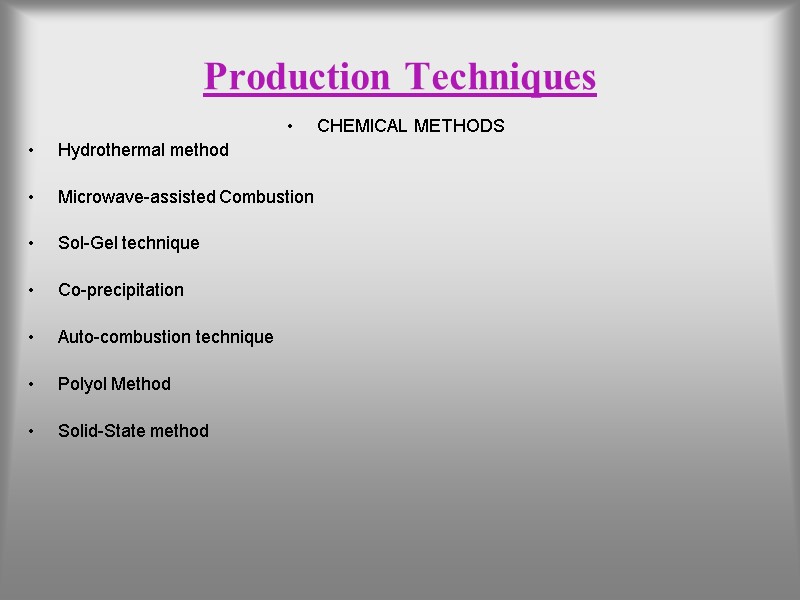
 Production Techniques CHEMICAL METHODS Hydrothermal method Microwave-assisted Combustion Sol-Gel technique Co-precipitation Auto-combustion technique Polyol Method Solid-State method
Production Techniques CHEMICAL METHODS Hydrothermal method Microwave-assisted Combustion Sol-Gel technique Co-precipitation Auto-combustion technique Polyol Method Solid-State method
 PHYSICAL METHODS Pulsed Laser Ablation (PLA) Chemical Vapor deposition (CVD) Molecular Beam Epitaxy (MBE) Etc.
PHYSICAL METHODS Pulsed Laser Ablation (PLA) Chemical Vapor deposition (CVD) Molecular Beam Epitaxy (MBE) Etc.
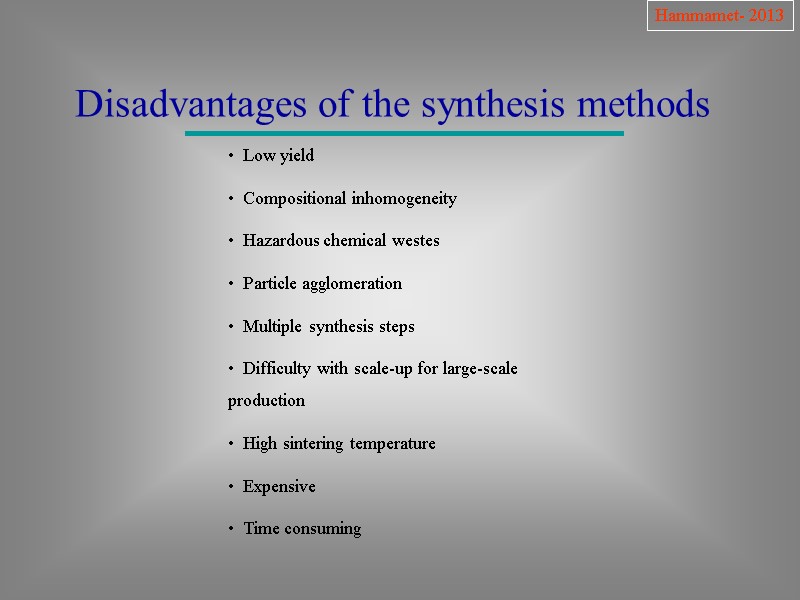
 Hammamet- 2013 Disadvantages of the synthesis methods Low yield Compositional inhomogeneity Hazardous chemical westes Particle agglomeration Multiple synthesis steps Difficulty with scale-up for large-scale production High sintering temperature Expensive Time consuming
Hammamet- 2013 Disadvantages of the synthesis methods Low yield Compositional inhomogeneity Hazardous chemical westes Particle agglomeration Multiple synthesis steps Difficulty with scale-up for large-scale production High sintering temperature Expensive Time consuming
 Hammamet- 2013
Hammamet- 2013
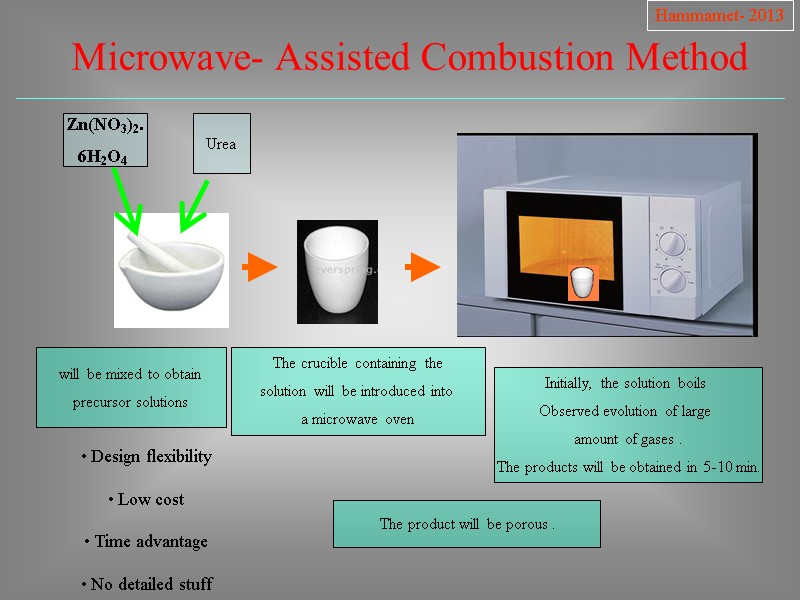
 Zn(NO3)2. 6H2O4 will be mixed to obtain precursor solutions The crucible containing the solution will be introduced into a microwave oven Initially, the solution boils Observed evolution of large amount of gases . The products will be obtained in 5-10 min. Urea The product will be porous . Design flexibility Low cost Time advantage No detailed stuff Hammamet- 2013 Microwave- Assisted Combustion Method
Zn(NO3)2. 6H2O4 will be mixed to obtain precursor solutions The crucible containing the solution will be introduced into a microwave oven Initially, the solution boils Observed evolution of large amount of gases . The products will be obtained in 5-10 min. Urea The product will be porous . Design flexibility Low cost Time advantage No detailed stuff Hammamet- 2013 Microwave- Assisted Combustion Method
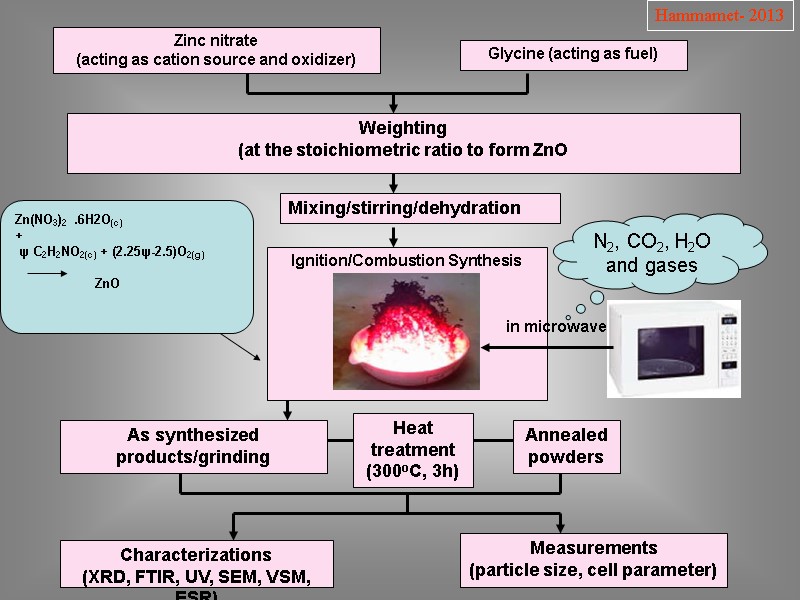
 Zinc nitrate (acting as cation source and oxidizer) Glycine (acting as fuel) Weighting (at the stoichiometric ratio to form ZnO Mixing/stirring/dehydration Ignition/Combustion Synthesis As synthesized products/grinding Heat treatment (300oC, 3h) Annealed powders Characterizations (XRD, FTIR, UV, SEM, VSM, ESR) Measurements (particle size, cell parameter) N2, CO2, H2O and gases in microwave Hammamet- 2013
Zinc nitrate (acting as cation source and oxidizer) Glycine (acting as fuel) Weighting (at the stoichiometric ratio to form ZnO Mixing/stirring/dehydration Ignition/Combustion Synthesis As synthesized products/grinding Heat treatment (300oC, 3h) Annealed powders Characterizations (XRD, FTIR, UV, SEM, VSM, ESR) Measurements (particle size, cell parameter) N2, CO2, H2O and gases in microwave Hammamet- 2013
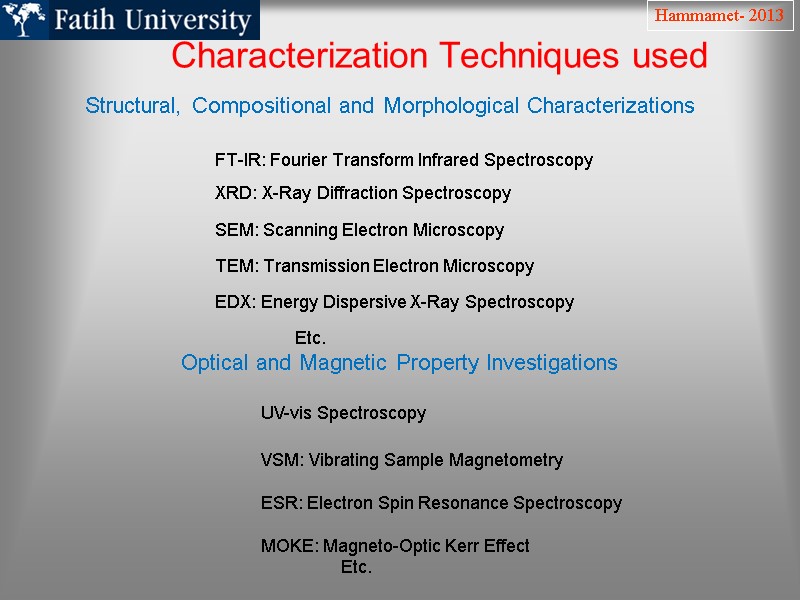
 Characterization Techniques used Structural, Compositional and Morphological Characterizations FT-IR: Fourier Transform Infrared Spectroscopy XRD: X-Ray Diffraction Spectroscopy SEM: Scanning Electron Microscopy TEM: Transmission Electron Microscopy EDX: Energy Dispersive X-Ray Spectroscopy Etc. Optical and Magnetic Property Investigations UV-vis Spectroscopy VSM: Vibrating Sample Magnetometry ESR: Electron Spin Resonance Spectroscopy MOKE: Magneto-Optic Kerr Effect Etc. Hammamet- 2013
Characterization Techniques used Structural, Compositional and Morphological Characterizations FT-IR: Fourier Transform Infrared Spectroscopy XRD: X-Ray Diffraction Spectroscopy SEM: Scanning Electron Microscopy TEM: Transmission Electron Microscopy EDX: Energy Dispersive X-Ray Spectroscopy Etc. Optical and Magnetic Property Investigations UV-vis Spectroscopy VSM: Vibrating Sample Magnetometry ESR: Electron Spin Resonance Spectroscopy MOKE: Magneto-Optic Kerr Effect Etc. Hammamet- 2013
 ELECTRICAL and DIELECTRIC CHARACTERIZATION Conductivity Measurements Dielectric Measurements Etc.
ELECTRICAL and DIELECTRIC CHARACTERIZATION Conductivity Measurements Dielectric Measurements Etc.
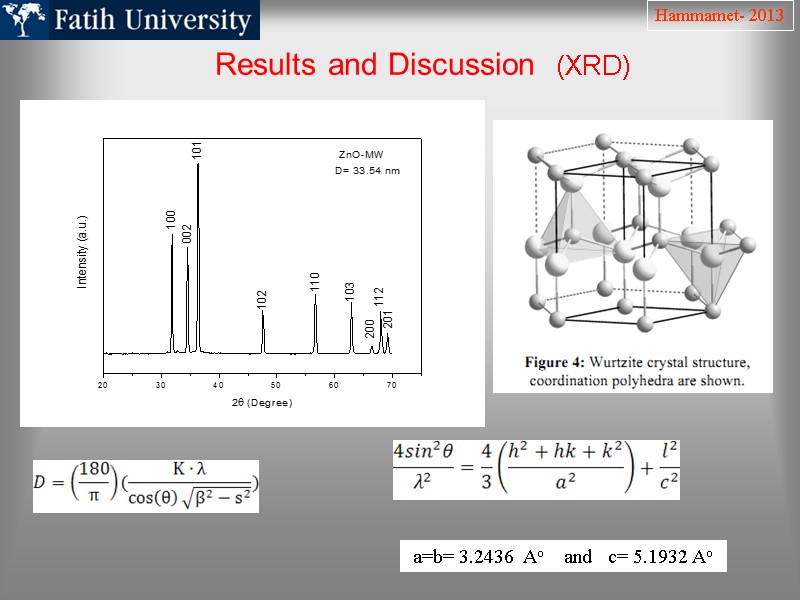
 Results and Discussion (XRD) Hammamet- 2013 a=b= 3.2436 Ao and c= 5.1932 Ao
Results and Discussion (XRD) Hammamet- 2013 a=b= 3.2436 Ao and c= 5.1932 Ao
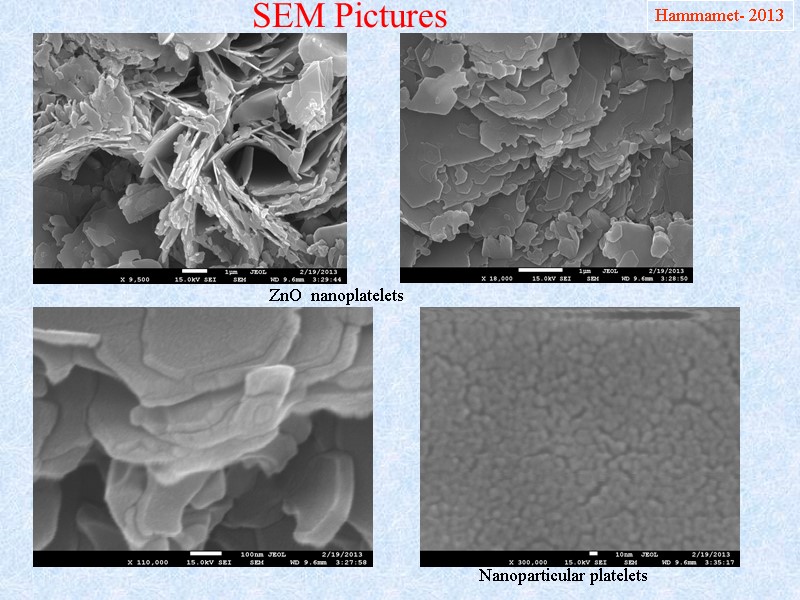
 SEM Pictures Hammamet- 2013 ZnO nanoplatelets Nanoparticular platelets
SEM Pictures Hammamet- 2013 ZnO nanoplatelets Nanoparticular platelets
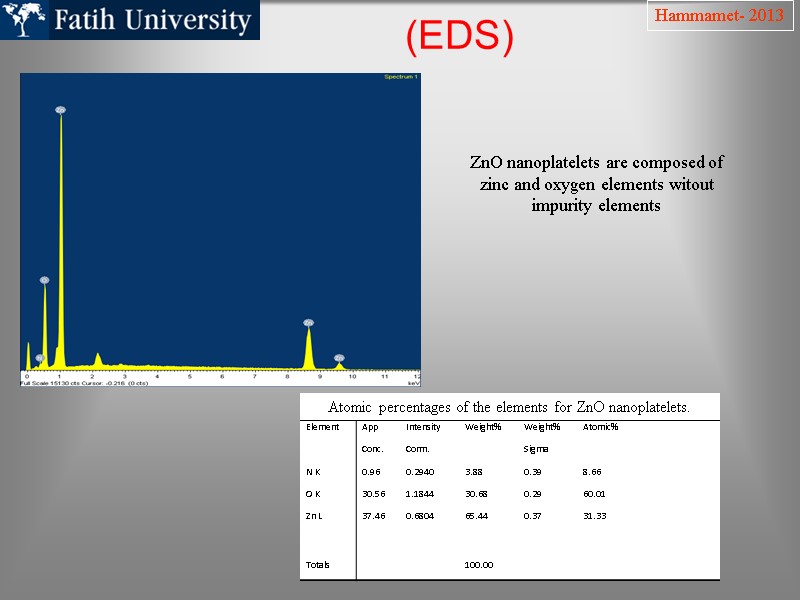
 (EDS) Hammamet- 2013 Atomic percentages of the elements for ZnO nanoplatelets. ZnO nanoplatelets are composed of zinc and oxygen elements witout impurity elements
(EDS) Hammamet- 2013 Atomic percentages of the elements for ZnO nanoplatelets. ZnO nanoplatelets are composed of zinc and oxygen elements witout impurity elements
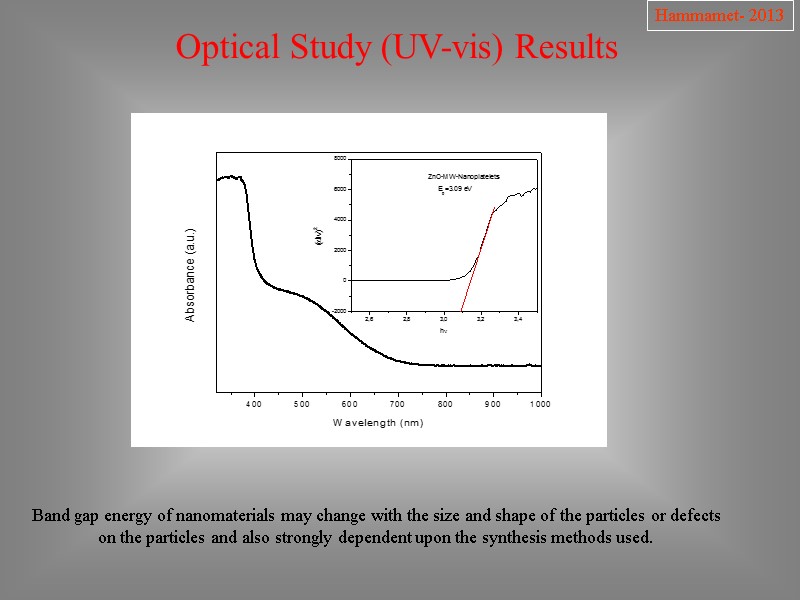
 Optical Study (UV-vis) Results Hammamet- 2013 Band gap energy of nanomaterials may change with the size and shape of the particles or defects on the particles and also strongly dependent upon the synthesis methods used.
Optical Study (UV-vis) Results Hammamet- 2013 Band gap energy of nanomaterials may change with the size and shape of the particles or defects on the particles and also strongly dependent upon the synthesis methods used.
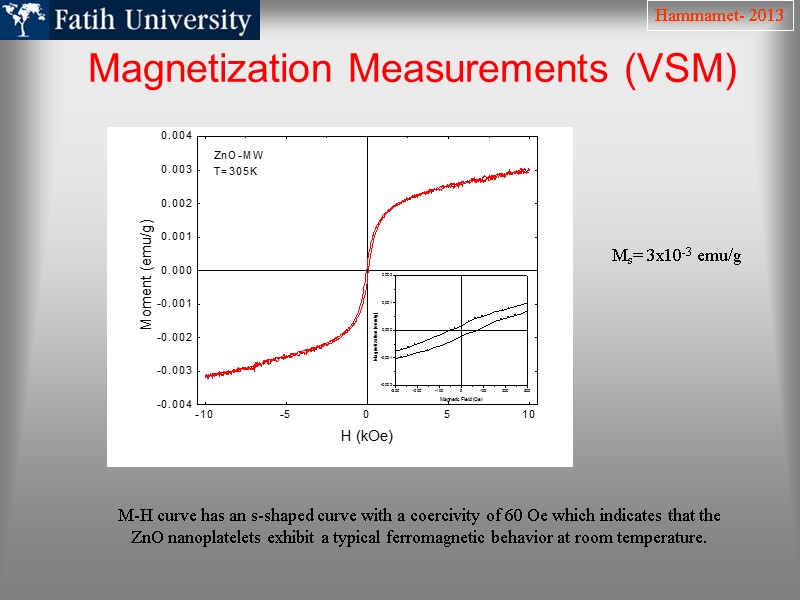
 Magnetization Measurements (VSM) Hammamet- 2013 Ms= 3x10-3 emu/g M-H curve has an s-shaped curve with a coercivity of 60 Oe which indicates that the ZnO nanoplatelets exhibit a typical ferromagnetic behavior at room temperature.
Magnetization Measurements (VSM) Hammamet- 2013 Ms= 3x10-3 emu/g M-H curve has an s-shaped curve with a coercivity of 60 Oe which indicates that the ZnO nanoplatelets exhibit a typical ferromagnetic behavior at room temperature.
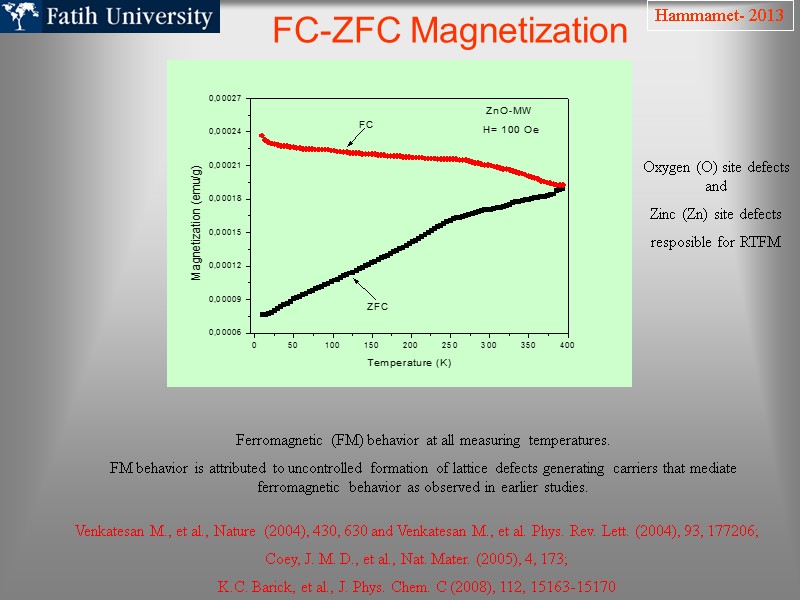
 FC-ZFC Magnetization Ferromagnetic (FM) behavior at all measuring temperatures. FM behavior is attributed to uncontrolled formation of lattice defects generating carriers that mediate ferromagnetic behavior as observed in earlier studies. Hammamet- 2013 Venkatesan M., et al., Nature (2004), 430, 630 and Venkatesan M., et al. Phys. Rev. Lett. (2004), 93, 177206; Coey, J. M. D., et al., Nat. Mater. (2005), 4, 173; K.C. Barick, et al., J. Phys. Chem. C (2008), 112, 15163-15170 Oxygen (O) site defects and Zinc (Zn) site defects resposible for RTFM
FC-ZFC Magnetization Ferromagnetic (FM) behavior at all measuring temperatures. FM behavior is attributed to uncontrolled formation of lattice defects generating carriers that mediate ferromagnetic behavior as observed in earlier studies. Hammamet- 2013 Venkatesan M., et al., Nature (2004), 430, 630 and Venkatesan M., et al. Phys. Rev. Lett. (2004), 93, 177206; Coey, J. M. D., et al., Nat. Mater. (2005), 4, 173; K.C. Barick, et al., J. Phys. Chem. C (2008), 112, 15163-15170 Oxygen (O) site defects and Zinc (Zn) site defects resposible for RTFM
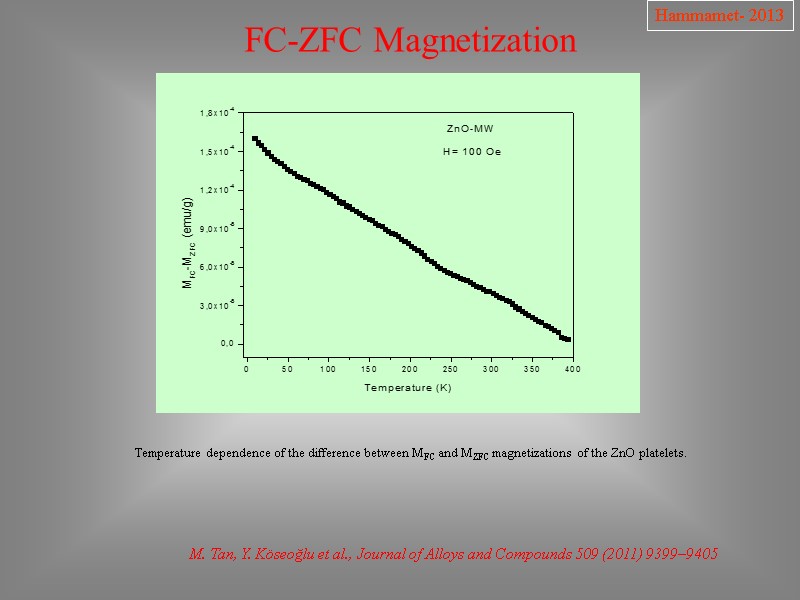
 FC-ZFC Magnetization M. Tan, Y. Köseoğlu et al., Journal of Alloys and Compounds 509 (2011) 9399–9405 Hammamet- 2013 Temperature dependence of the difference between MFC and MZFC magnetizations of the ZnO platelets.
FC-ZFC Magnetization M. Tan, Y. Köseoğlu et al., Journal of Alloys and Compounds 509 (2011) 9399–9405 Hammamet- 2013 Temperature dependence of the difference between MFC and MZFC magnetizations of the ZnO platelets.
 Thank You For Your Attention !!! Hammamet- 2013
Thank You For Your Attention !!! Hammamet- 2013
