75cd3b5f886376a56902c4b86c97e091.ppt
- Количество слайдов: 30
 Patient Care Device Domain Update (PCD) Planning Committee Ken Fuchs / Steve Merritt Technical Committee John Garguilo / John Rhoads www. ihe. net
Patient Care Device Domain Update (PCD) Planning Committee Ken Fuchs / Steve Merritt Technical Committee John Garguilo / John Rhoads www. ihe. net
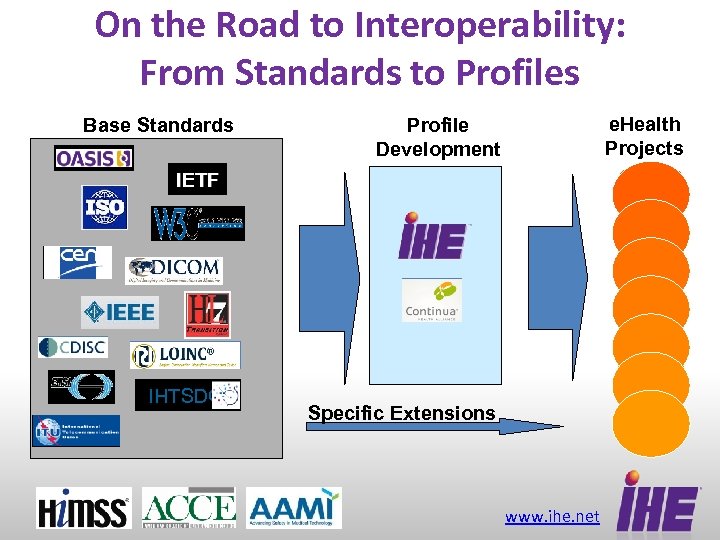 On the Road to Interoperability: From Standards to Profiles Base Standards e. Health Projects Profile Development IETF IHTSDO Specific Extensions www. ihe. net
On the Road to Interoperability: From Standards to Profiles Base Standards e. Health Projects Profile Development IETF IHTSDO Specific Extensions www. ihe. net
 Role of IHE PCD • IHE PCD was formed in 2005 to address issues related to integration of Point-of-Care medical devices: – With each other – With enterprise systems • IHE PCD wants to “raise the bar” from the current state of integration projects to out of the box interoperable solutions. rs so Spon PCD www. ihe. net
Role of IHE PCD • IHE PCD was formed in 2005 to address issues related to integration of Point-of-Care medical devices: – With each other – With enterprise systems • IHE PCD wants to “raise the bar” from the current state of integration projects to out of the box interoperable solutions. rs so Spon PCD www. ihe. net
 IHE PCD Charter The Patient Care Device Domain is concerned with use cases in which at least one actor is a patient-centric point-of-care medical device. We coordinate with other IHE clinical specialty based domains such as medical imaging and lab to ensure consistency of medical device integration solutions across all IHE technical frameworks. www. ihe. net
IHE PCD Charter The Patient Care Device Domain is concerned with use cases in which at least one actor is a patient-centric point-of-care medical device. We coordinate with other IHE clinical specialty based domains such as medical imaging and lab to ensure consistency of medical device integration solutions across all IHE technical frameworks. www. ihe. net
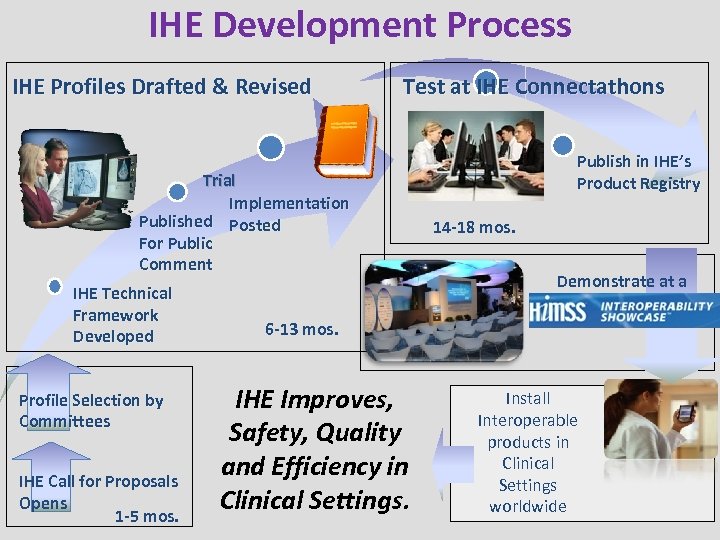 IHE Development Process IHE Profiles Drafted & Revised Test at IHE Connectathons Trial Implementation Published Posted For Public Comment IHE Technical Framework Developed Profile Selection by Committees IHE Call for Proposals Opens 1 -5 mos. Publish in IHE’s Product Registry 14 -18 mos. Demonstrate at a 6 -13 mos. IHE Improves, Safety, Quality and Efficiency in Clinical Settings. Install Interoperable products in Clinical Settings worldwide www. ihe. net
IHE Development Process IHE Profiles Drafted & Revised Test at IHE Connectathons Trial Implementation Published Posted For Public Comment IHE Technical Framework Developed Profile Selection by Committees IHE Call for Proposals Opens 1 -5 mos. Publish in IHE’s Product Registry 14 -18 mos. Demonstrate at a 6 -13 mos. IHE Improves, Safety, Quality and Efficiency in Clinical Settings. Install Interoperable products in Clinical Settings worldwide www. ihe. net
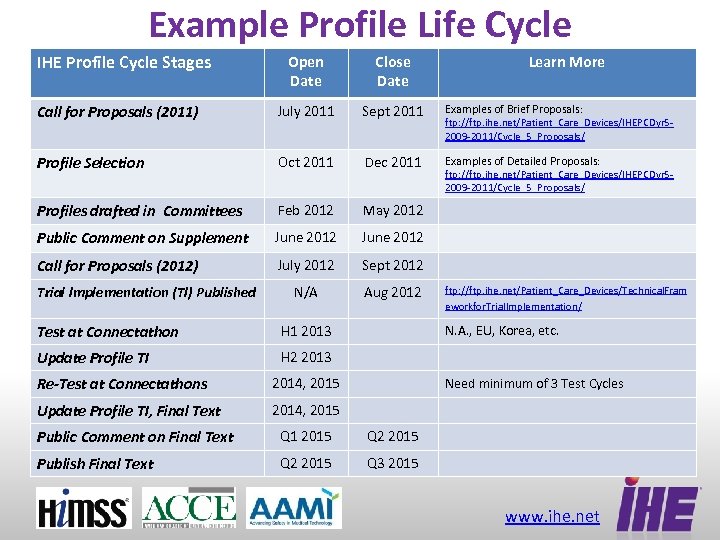 Example Profile Life Cycle IHE Profile Cycle Stages Open Date Close Date Call for Proposals (2011) July 2011 Sept 2011 Examples of Brief Proposals: Profile Selection Oct 2011 Dec 2011 Examples of Detailed Proposals: Profiles drafted in Committees Feb 2012 May 2012 Public Comment on Supplement June 2012 Call for Proposals (2012) July 2012 Sept 2012 N/A Aug 2012 Trial Implementation (TI) Published Test at Connectathon H 1 2013 Update Profile TI Learn More ftp: //ftp. ihe. net/Patient_Care_Devices/IHEPCDyr 52009 -2011/Cycle_5_Proposals/ ftp: //ftp. ihe. net/Patient_Care_Devices/Technical. Fram eworkfor. Trial. Implementation/ H 2 2013 Re-Test at Connectathons 2014, 2015 Update Profile TI, Final Text N. A. , EU, Korea, etc. 2014, 2015 Need minimum of 3 Test Cycles Public Comment on Final Text Q 1 2015 Q 2 2015 Publish Final Text Q 2 2015 Q 3 2015 www. ihe. net
Example Profile Life Cycle IHE Profile Cycle Stages Open Date Close Date Call for Proposals (2011) July 2011 Sept 2011 Examples of Brief Proposals: Profile Selection Oct 2011 Dec 2011 Examples of Detailed Proposals: Profiles drafted in Committees Feb 2012 May 2012 Public Comment on Supplement June 2012 Call for Proposals (2012) July 2012 Sept 2012 N/A Aug 2012 Trial Implementation (TI) Published Test at Connectathon H 1 2013 Update Profile TI Learn More ftp: //ftp. ihe. net/Patient_Care_Devices/IHEPCDyr 52009 -2011/Cycle_5_Proposals/ ftp: //ftp. ihe. net/Patient_Care_Devices/Technical. Fram eworkfor. Trial. Implementation/ H 2 2013 Re-Test at Connectathons 2014, 2015 Update Profile TI, Final Text N. A. , EU, Korea, etc. 2014, 2015 Need minimum of 3 Test Cycles Public Comment on Final Text Q 1 2015 Q 2 2015 Publish Final Text Q 2 2015 Q 3 2015 www. ihe. net
 Domain Profiles Developed www. ihe. net
Domain Profiles Developed www. ihe. net
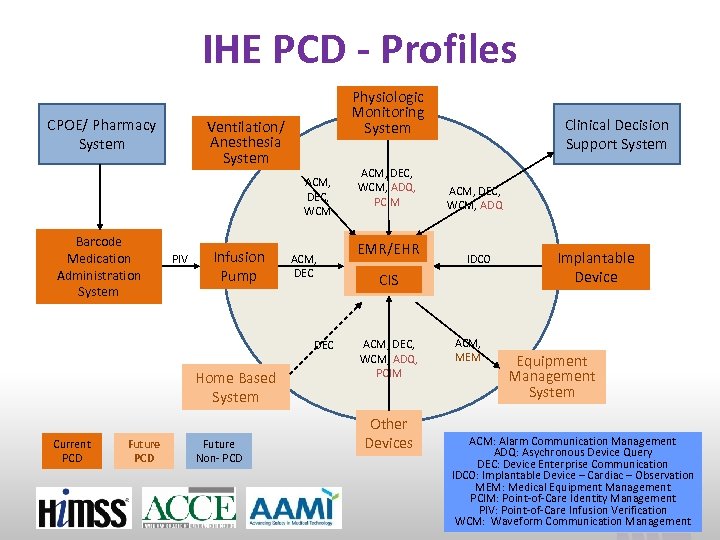 IHE PCD - Profiles CPOE/ Pharmacy System Physiologic Monitoring System Ventilation/ Anesthesia System ACM, DEC, WCM Barcode Medication Administration System PIV Infusion Pump ACM, DEC Home Based System Current PCD Future Non- PCD ACM, DEC, WCM, ADQ, PCIM EMR/EHR Clinical Decision Support System ACM, DEC, WCM, ADQ IDCO CIS ACM, DEC, WCM, ADQ, PCIM Other Devices ACM, MEM Implantable Device Equipment Management System ACM: Alarm Communication Management ADQ: Asychronous Device Query DEC: Device Enterprise Communication IDCO: Implantable Device – Cardiac – Observation MEM: Medical Equipment Management PCIM: Point-of-Care Identity Management PIV: Point-of-Care Infusion Verification www. ihe. net WCM: Waveform Communication Management
IHE PCD - Profiles CPOE/ Pharmacy System Physiologic Monitoring System Ventilation/ Anesthesia System ACM, DEC, WCM Barcode Medication Administration System PIV Infusion Pump ACM, DEC Home Based System Current PCD Future Non- PCD ACM, DEC, WCM, ADQ, PCIM EMR/EHR Clinical Decision Support System ACM, DEC, WCM, ADQ IDCO CIS ACM, DEC, WCM, ADQ, PCIM Other Devices ACM, MEM Implantable Device Equipment Management System ACM: Alarm Communication Management ADQ: Asychronous Device Query DEC: Device Enterprise Communication IDCO: Implantable Device – Cardiac – Observation MEM: Medical Equipment Management PCIM: Point-of-Care Identity Management PIV: Point-of-Care Infusion Verification www. ihe. net WCM: Waveform Communication Management
![[RTM] Rosetta Terminology Mgmnt. www. ihe. net [RTM] Rosetta Terminology Mgmnt. www. ihe. net](https://present5.com/presentation/75cd3b5f886376a56902c4b86c97e091/image-9.jpg) [RTM] Rosetta Terminology Mgmnt. www. ihe. net
[RTM] Rosetta Terminology Mgmnt. www. ihe. net
![[DEC] Device to Enterprise Communication www. ihe. net [DEC] Device to Enterprise Communication www. ihe. net](https://present5.com/presentation/75cd3b5f886376a56902c4b86c97e091/image-10.jpg) [DEC] Device to Enterprise Communication www. ihe. net
[DEC] Device to Enterprise Communication www. ihe. net
![[WCM] Waveform Content Message www. ihe. net [WCM] Waveform Content Message www. ihe. net](https://present5.com/presentation/75cd3b5f886376a56902c4b86c97e091/image-11.jpg) [WCM] Waveform Content Message www. ihe. net
[WCM] Waveform Content Message www. ihe. net
![[ACM] Alarm Communication Management HL 7 Messages per ACM and WCM profiles Alarm Source [ACM] Alarm Communication Management HL 7 Messages per ACM and WCM profiles Alarm Source](https://present5.com/presentation/75cd3b5f886376a56902c4b86c97e091/image-12.jpg) [ACM] Alarm Communication Management HL 7 Messages per ACM and WCM profiles Alarm Source Alarm Reporter AR Device Specific graphics Parameters, waveforms, etc. as evidentiary data items Report Alarm PCD-04 → ← PCD-05 Report Alarm Status Alarm Manager AM Alarm Information Source, Phase, State, Priority Patient Location Instance Alarm text Callback Timestamp Evidentiary data Disseminate Alarm PCD-06 → ← PCD-07 Disseminate Alarm Status Alarm Communicator AC Dissemination Status Instance Accepted by AC Undeliverable Delivered Read Accepted Rejected Cancelled Callback start/stop www. ihe. net
[ACM] Alarm Communication Management HL 7 Messages per ACM and WCM profiles Alarm Source Alarm Reporter AR Device Specific graphics Parameters, waveforms, etc. as evidentiary data items Report Alarm PCD-04 → ← PCD-05 Report Alarm Status Alarm Manager AM Alarm Information Source, Phase, State, Priority Patient Location Instance Alarm text Callback Timestamp Evidentiary data Disseminate Alarm PCD-06 → ← PCD-07 Disseminate Alarm Status Alarm Communicator AC Dissemination Status Instance Accepted by AC Undeliverable Delivered Read Accepted Rejected Cancelled Callback start/stop www. ihe. net
![[PIV] Point of Care Infusion Verification www. ihe. net [PIV] Point of Care Infusion Verification www. ihe. net](https://present5.com/presentation/75cd3b5f886376a56902c4b86c97e091/image-13.jpg) [PIV] Point of Care Infusion Verification www. ihe. net
[PIV] Point of Care Infusion Verification www. ihe. net
![[IDCO] Implantable Device – Cardiac Observ. www. ihe. net [IDCO] Implantable Device – Cardiac Observ. www. ihe. net](https://present5.com/presentation/75cd3b5f886376a56902c4b86c97e091/image-14.jpg) [IDCO] Implantable Device – Cardiac Observ. www. ihe. net
[IDCO] Implantable Device – Cardiac Observ. www. ihe. net
 Domain Profiles Under Development www. ihe. net
Domain Profiles Under Development www. ihe. net
![[PCIM] Point-of-Care Identity Management www. ihe. net [PCIM] Point-of-Care Identity Management www. ihe. net](https://present5.com/presentation/75cd3b5f886376a56902c4b86c97e091/image-16.jpg) [PCIM] Point-of-Care Identity Management www. ihe. net
[PCIM] Point-of-Care Identity Management www. ihe. net
![[ADQ] Asynchronous Data Query Supports retrospective query of PCD data from databases. Supports Use [ADQ] Asynchronous Data Query Supports retrospective query of PCD data from databases. Supports Use](https://present5.com/presentation/75cd3b5f886376a56902c4b86c97e091/image-17.jpg) [ADQ] Asynchronous Data Query Supports retrospective query of PCD data from databases. Supports Use Cases such as Clinical Decision Support, backfilling of EMR databases, etc. Data Query PCD-12 Data Response PCD-13 Data Response Full PCD-13 Disclosure EMR, CDSS, CIS, Etc. Data Server and/or Data Requester Data Query PCD-12 Data Response PCD-13 Data Servers Data Requesters www. ihe. net
[ADQ] Asynchronous Data Query Supports retrospective query of PCD data from databases. Supports Use Cases such as Clinical Decision Support, backfilling of EMR databases, etc. Data Query PCD-12 Data Response PCD-13 Data Response Full PCD-13 Disclosure EMR, CDSS, CIS, Etc. Data Server and/or Data Requester Data Query PCD-12 Data Response PCD-13 Data Servers Data Requesters www. ihe. net
![[MEM] Medical Equipment Management www. ihe. net [MEM] Medical Equipment Management www. ihe. net](https://present5.com/presentation/75cd3b5f886376a56902c4b86c97e091/image-18.jpg) [MEM] Medical Equipment Management www. ihe. net
[MEM] Medical Equipment Management www. ihe. net
![[MEM] Medical Device Security & Mgmnt. HIPAA Cyber Protection HITECH IEC 80001 Access Control [MEM] Medical Device Security & Mgmnt. HIPAA Cyber Protection HITECH IEC 80001 Access Control](https://present5.com/presentation/75cd3b5f886376a56902c4b86c97e091/image-19.jpg) [MEM] Medical Device Security & Mgmnt. HIPAA Cyber Protection HITECH IEC 80001 Access Control MDS 2 Authentication Lifecycle Mgmt. Medical Device Network Access Control IHE PCD Asset Discovery CE/IT Manage ment Security FDA: • 21 CFR 801, 803, 807, 812, 814, 820 • MDDS Configuration Management e. PHI Breach Risk Privacy Key Management Encryption www. ihe. net
[MEM] Medical Device Security & Mgmnt. HIPAA Cyber Protection HITECH IEC 80001 Access Control MDS 2 Authentication Lifecycle Mgmt. Medical Device Network Access Control IHE PCD Asset Discovery CE/IT Manage ment Security FDA: • 21 CFR 801, 803, 807, 812, 814, 820 • MDDS Configuration Management e. PHI Breach Risk Privacy Key Management Encryption www. ihe. net
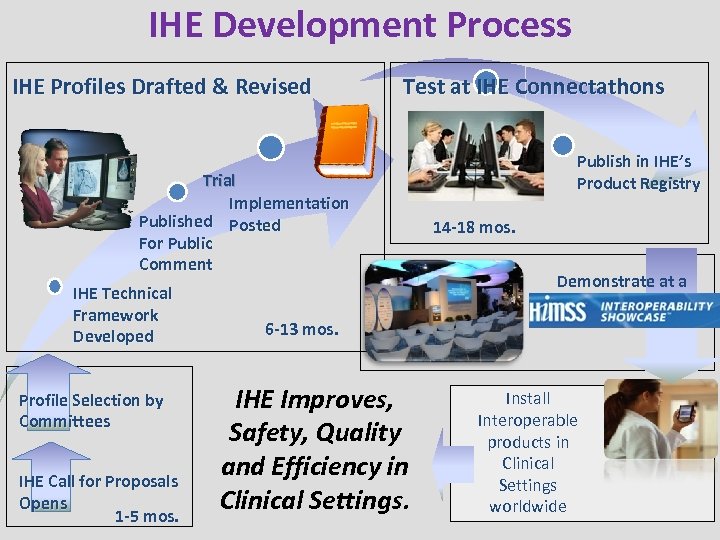 IHE Development Process IHE Profiles Drafted & Revised Test at IHE Connectathons Trial Implementation Published Posted For Public Comment IHE Technical Framework Developed Profile Selection by Committees IHE Call for Proposals Opens 1 -5 mos. Publish in IHE’s Product Registry 14 -18 mos. Demonstrate at a 6 -13 mos. IHE Improves, Safety, Quality and Efficiency in Clinical Settings. Install Interoperable products in Clinical Settings worldwide www. ihe. net
IHE Development Process IHE Profiles Drafted & Revised Test at IHE Connectathons Trial Implementation Published Posted For Public Comment IHE Technical Framework Developed Profile Selection by Committees IHE Call for Proposals Opens 1 -5 mos. Publish in IHE’s Product Registry 14 -18 mos. Demonstrate at a 6 -13 mos. IHE Improves, Safety, Quality and Efficiency in Clinical Settings. Install Interoperable products in Clinical Settings worldwide www. ihe. net
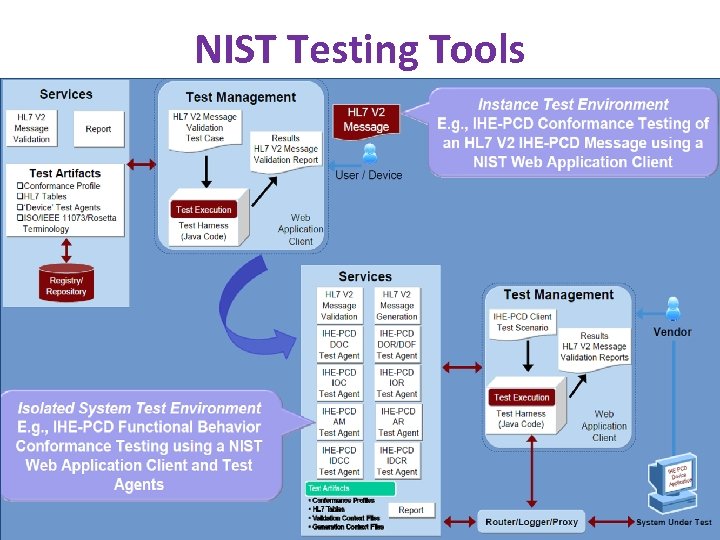 NIST Testing Tools www. ihe. net
NIST Testing Tools www. ihe. net
 Connectathon – in the dungeon… www. ihe. net
Connectathon – in the dungeon… www. ihe. net
 PCD @ Interoperability PCD – HIMSS 2010 Showcase 2011 www. ihe. net
PCD @ Interoperability PCD – HIMSS 2010 Showcase 2011 www. ihe. net
 How to Participate www. ihe. net
How to Participate www. ihe. net
 PCD Planning Committee • Recruitment • Education • Presentations • White Papers • Review IHE Profile Proposals • Identifies committee priorities and problems • Meets every 2 weeks alternating with TC Contact Information • Project Mgr: Manny Furst – pcd@accenet. org • Co-Chair: Ken Fuchs – Mindray North America • Co-Chair: Steve Merritt – Baystate Health • Google Group – ihepcdplan@googlegroups. com • Committee’s Wiki – http: //wiki. ihe. net/index. php? title =PCD_Planning_Committee www. ihe. net
PCD Planning Committee • Recruitment • Education • Presentations • White Papers • Review IHE Profile Proposals • Identifies committee priorities and problems • Meets every 2 weeks alternating with TC Contact Information • Project Mgr: Manny Furst – pcd@accenet. org • Co-Chair: Ken Fuchs – Mindray North America • Co-Chair: Steve Merritt – Baystate Health • Google Group – ihepcdplan@googlegroups. com • Committee’s Wiki – http: //wiki. ihe. net/index. php? title =PCD_Planning_Committee www. ihe. net
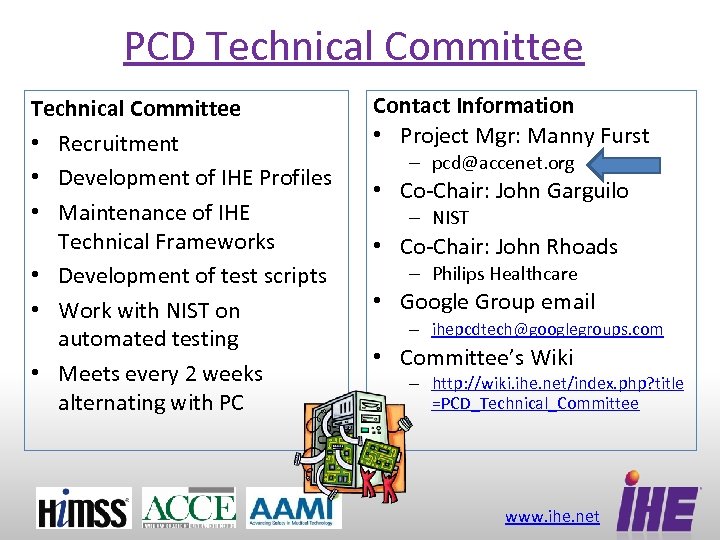 PCD Technical Committee • Recruitment • Development of IHE Profiles • Maintenance of IHE Technical Frameworks • Development of test scripts • Work with NIST on automated testing • Meets every 2 weeks alternating with PC Contact Information • Project Mgr: Manny Furst – pcd@accenet. org • Co-Chair: John Garguilo – NIST • Co-Chair: John Rhoads – Philips Healthcare • Google Group email – ihepcdtech@googlegroups. com • Committee’s Wiki – http: //wiki. ihe. net/index. php? title =PCD_Technical_Committee www. ihe. net
PCD Technical Committee • Recruitment • Development of IHE Profiles • Maintenance of IHE Technical Frameworks • Development of test scripts • Work with NIST on automated testing • Meets every 2 weeks alternating with PC Contact Information • Project Mgr: Manny Furst – pcd@accenet. org • Co-Chair: John Garguilo – NIST • Co-Chair: John Rhoads – Philips Healthcare • Google Group email – ihepcdtech@googlegroups. com • Committee’s Wiki – http: //wiki. ihe. net/index. php? title =PCD_Technical_Committee www. ihe. net
 PCD User Handbook 2011 Edition 1 Overview of IHE-PCD Value Propositions for medical device interoperability Advice on how to specify IHE in technology assessment Tools to find IHE-PCD compliant products Guidance on installation testing to confirm that IHE capabilities are functioning properly • Issues to consider when installing and configuring IHEcompliant system • Identifying and addressing potential problems in order to maximize your benefit despite existing “legacy” systems • • • 1 http: //wiki. ihe. net/index. php? title=PCD_User_Handbook www. ihe. net
PCD User Handbook 2011 Edition 1 Overview of IHE-PCD Value Propositions for medical device interoperability Advice on how to specify IHE in technology assessment Tools to find IHE-PCD compliant products Guidance on installation testing to confirm that IHE capabilities are functioning properly • Issues to consider when installing and configuring IHEcompliant system • Identifying and addressing potential problems in order to maximize your benefit despite existing “legacy” systems • • • 1 http: //wiki. ihe. net/index. php? title=PCD_User_Handbook www. ihe. net
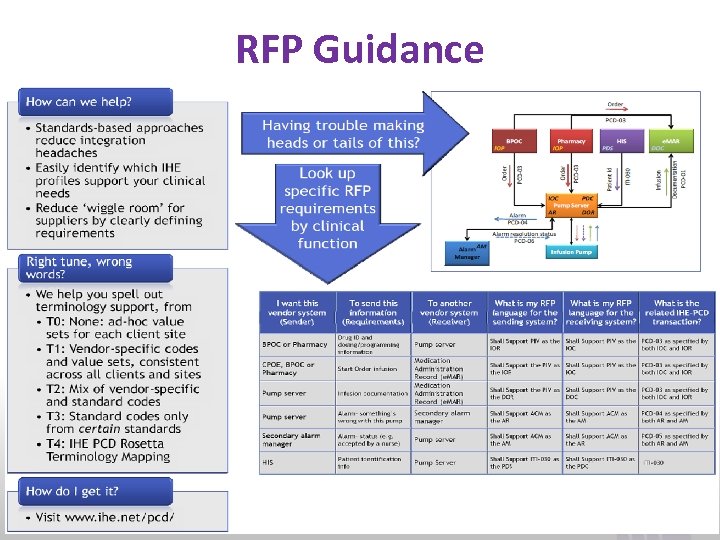 RFP Guidance www. ihe. net
RFP Guidance www. ihe. net
 Go Shopping!! • • • Accent on Integration Amcom Biotronik B Braun Carefusion Cerner Epic GE Healthcare Hospira i. MDSoft i. Sirona • • • Mindray Nuvon OZ Systems Philips Emergin Philips Healthcare Scott. Care St. Jude Medical Surgical Info. Systems Vocera Welch Allyn www. ihe. net
Go Shopping!! • • • Accent on Integration Amcom Biotronik B Braun Carefusion Cerner Epic GE Healthcare Hospira i. MDSoft i. Sirona • • • Mindray Nuvon OZ Systems Philips Emergin Philips Healthcare Scott. Care St. Jude Medical Surgical Info. Systems Vocera Welch Allyn www. ihe. net
 Thank you for your attention Questions? www. ihe. net
Thank you for your attention Questions? www. ihe. net


