32370d0bd8e9618ce0c37f26c85a5bc4.ppt
- Количество слайдов: 68
 OPPORTUNITA’ DI FINANZIAMENTO 2012 Health VIIPQ HEALTH 25 luglio 2011 Ufficio Presìdi Ufficio Relazioni con l’Unione Europea
OPPORTUNITA’ DI FINANZIAMENTO 2012 Health VIIPQ HEALTH 25 luglio 2011 Ufficio Presìdi Ufficio Relazioni con l’Unione Europea
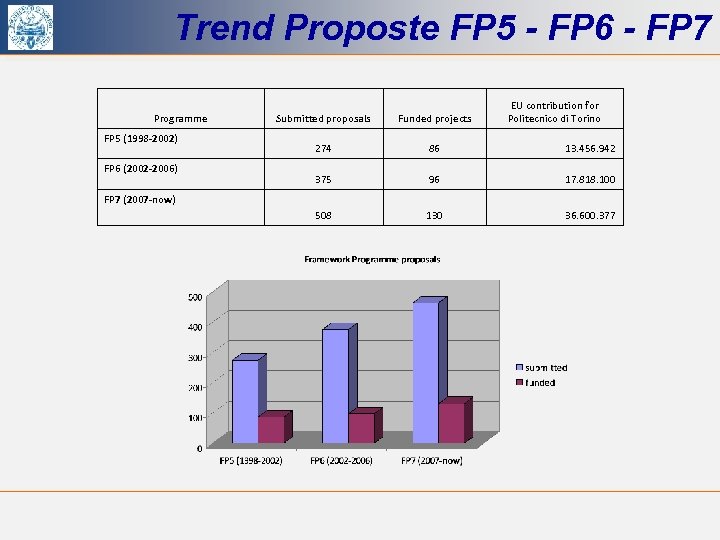 Trend Proposte FP 5 - FP 6 - FP 7 Programme FP 5 (1998 -2002) FP 6 (2002 -2006) EU contribution for Politecnico di Torino Submitted proposals Funded projects 274 86 13. 456. 942 375 96 17. 818. 100 508 130 36. 600. 377 FP 7 (2007 -now)
Trend Proposte FP 5 - FP 6 - FP 7 Programme FP 5 (1998 -2002) FP 6 (2002 -2006) EU contribution for Politecnico di Torino Submitted proposals Funded projects 274 86 13. 456. 942 375 96 17. 818. 100 508 130 36. 600. 377 FP 7 (2007 -now)
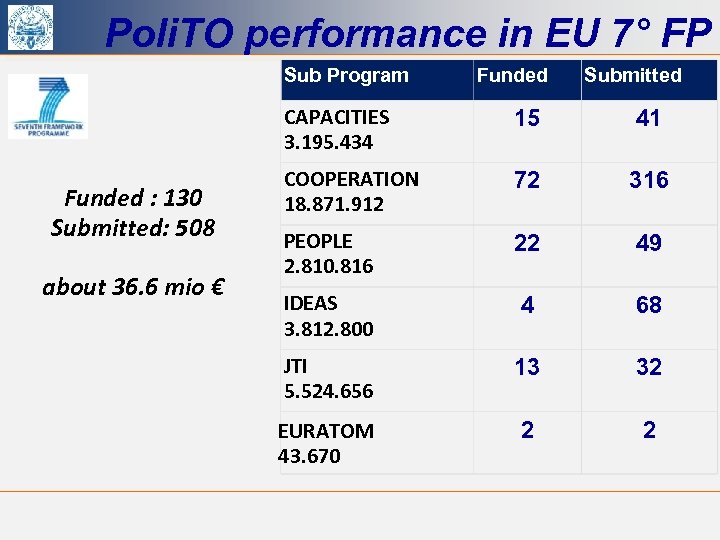 Poli. TO performance in EU 7° FP Sub Program Funded Submitted CAPACITIES 3. 195. 434 Funded : 130 Submitted: 508 about 36. 6 mio € 15 41 COOPERATION 18. 871. 912 72 316 PEOPLE 2. 810. 816 22 49 IDEAS 3. 812. 800 4 68 JTI 5. 524. 656 13 32 EURATOM 43. 670 2 2
Poli. TO performance in EU 7° FP Sub Program Funded Submitted CAPACITIES 3. 195. 434 Funded : 130 Submitted: 508 about 36. 6 mio € 15 41 COOPERATION 18. 871. 912 72 316 PEOPLE 2. 810. 816 22 49 IDEAS 3. 812. 800 4 68 JTI 5. 524. 656 13 32 EURATOM 43. 670 2 2
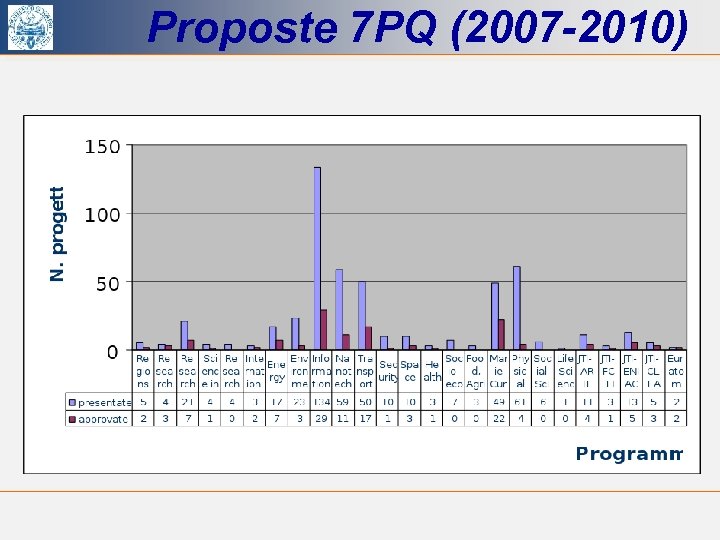 Proposte 7 PQ (2007 -2010)
Proposte 7 PQ (2007 -2010)
 Cfr VI PQ-VII PQ 1. 2. 3. 4. budget medio per progetto: da 185. 000 a 280. 000 € progetti coordinati: da 12 a 22 progetti multidisciplinari: aumentati del 15% partecipazione ad iniziative di carattere strategico come le ICT FET FLAGSHIP, progetti di larga scala e di lungo termine (10 anni), con un budget previsionale di circa 100 M€ euro all’anno 5. partecipazione ai progetti: - “ULAB-European Laboratory for Modelling the Technical Research University of Tomorrow”, non tanto per il contributo economico quanto per l’obiettivo di confrontarsi con altre università d’eccellenza in Europa (UPM di Madrid, Oxford, TUM, Paritech) 6. "Safe and green road vehicles- SAGE" - knowledge triangle (oltre il Politecnico, CRF e Regione Piemonte). Focus sullo sviluppo di una politica condivisa nell'automotive 7. coinvolgimento dell’Ateneo in reti internazionali (es Caesar, Airen)
Cfr VI PQ-VII PQ 1. 2. 3. 4. budget medio per progetto: da 185. 000 a 280. 000 € progetti coordinati: da 12 a 22 progetti multidisciplinari: aumentati del 15% partecipazione ad iniziative di carattere strategico come le ICT FET FLAGSHIP, progetti di larga scala e di lungo termine (10 anni), con un budget previsionale di circa 100 M€ euro all’anno 5. partecipazione ai progetti: - “ULAB-European Laboratory for Modelling the Technical Research University of Tomorrow”, non tanto per il contributo economico quanto per l’obiettivo di confrontarsi con altre università d’eccellenza in Europa (UPM di Madrid, Oxford, TUM, Paritech) 6. "Safe and green road vehicles- SAGE" - knowledge triangle (oltre il Politecnico, CRF e Regione Piemonte). Focus sullo sviluppo di una politica condivisa nell'automotive 7. coinvolgimento dell’Ateneo in reti internazionali (es Caesar, Airen)
 Workprogramme Health 2012 Published the 20 th of July 2011
Workprogramme Health 2012 Published the 20 th of July 2011
 Health Tema SALUTE Budget € 684 million FP 7 -HEALTH-2012 -INNOVATION-1 indicative budget of EUR 546 million with broader topics of which many are tailored for SME participation bottom-up with a minimum percentage of EU funding requested going to SMEs FP 7 -HEALTH-2012 -INNOVATION-2 as a pilot call with an indicative budget of 108 million EUR with very specific conditions OTHER ACTIVITIES indicative budget of 30 million EUR
Health Tema SALUTE Budget € 684 million FP 7 -HEALTH-2012 -INNOVATION-1 indicative budget of EUR 546 million with broader topics of which many are tailored for SME participation bottom-up with a minimum percentage of EU funding requested going to SMEs FP 7 -HEALTH-2012 -INNOVATION-2 as a pilot call with an indicative budget of 108 million EUR with very specific conditions OTHER ACTIVITIES indicative budget of 30 million EUR
 Health: objectives Ø migliorare la salute dei cittadini europei Ø rafforzare la competitività delle industrie e delle aziende europee del settore della salute. Ø partire dalle scoperte della ricerca di base per arrivare alle applicazioni cliniche Ø promuovere lo sviluppo di nuove terapie, metodi di promozione e prevenzione della salute, Ø sviluppare nuove tecnologie, strumenti diagnostici e sistemi sanitari efficenti e sostenibili anche da un punto di vista socio-economico Ø affrontare questioni sanitarie di livello mondiale come le nuove epidemie. 33
Health: objectives Ø migliorare la salute dei cittadini europei Ø rafforzare la competitività delle industrie e delle aziende europee del settore della salute. Ø partire dalle scoperte della ricerca di base per arrivare alle applicazioni cliniche Ø promuovere lo sviluppo di nuove terapie, metodi di promozione e prevenzione della salute, Ø sviluppare nuove tecnologie, strumenti diagnostici e sistemi sanitari efficenti e sostenibili anche da un punto di vista socio-economico Ø affrontare questioni sanitarie di livello mondiale come le nuove epidemie. 33
 EIP Per raggiungere gli obiettivi di EU 2020 la commissione ha avviato una partnership europea per l’innovazione su “Active and healthy ageing” (EIP AHA). Gli obiettivi sono: Healthy elderly – healthy public finances – healthy business
EIP Per raggiungere gli obiettivi di EU 2020 la commissione ha avviato una partnership europea per l’innovazione su “Active and healthy ageing” (EIP AHA). Gli obiettivi sono: Healthy elderly – healthy public finances – healthy business
 NOTE THAT: • Questo wp contribuisce a mettere in pratica le conoscenze che derivano dalla ricerca e a garantire che i risultati vadano a beneficio della società, con una ricerca maggiormente guidata dalle aziende. • Continua però a garantire lo sviluppo della ricerca di base attraverso i CP large. • Dà molta enfasi alla partecipazione delle SME (ruolo guida e % minima definita nel funding scheme) • Per progetti con risultati molto prossimi al mercato richiesta la conformità agli standard per garantire qualità, interoperabilità e libero mercato. • Dissemination actions sono fondamentali per ridurre la frammentazione delle politiche sanitarie.
NOTE THAT: • Questo wp contribuisce a mettere in pratica le conoscenze che derivano dalla ricerca e a garantire che i risultati vadano a beneficio della società, con una ricerca maggiormente guidata dalle aziende. • Continua però a garantire lo sviluppo della ricerca di base attraverso i CP large. • Dà molta enfasi alla partecipazione delle SME (ruolo guida e % minima definita nel funding scheme) • Per progetti con risultati molto prossimi al mercato richiesta la conformità agli standard per garantire qualità, interoperabilità e libero mercato. • Dissemination actions sono fondamentali per ridurre la frammentazione delle politiche sanitarie.
 NOTE THAT: Si pensa inoltre di creare il brevetto europeo e uno specifico tribunale per favorire il knowledge transfer nei MS. L’ Inoltre l’ Open access in FP 7 è d’obbligo attraverso articoli peer-reviewed che devono essere depositati in archivi istituzionali. • Tutti i topic in innovation-1 sono aperti alla partecipazione di partner provenienti da paesi terzi (i partner americani, in particolare, hanno diritto sia a partecipare sia al finanziamento).
NOTE THAT: Si pensa inoltre di creare il brevetto europeo e uno specifico tribunale per favorire il knowledge transfer nei MS. L’ Inoltre l’ Open access in FP 7 è d’obbligo attraverso articoli peer-reviewed che devono essere depositati in archivi istituzionali. • Tutti i topic in innovation-1 sono aperti alla partecipazione di partner provenienti da paesi terzi (i partner americani, in particolare, hanno diritto sia a partecipare sia al finanziamento).
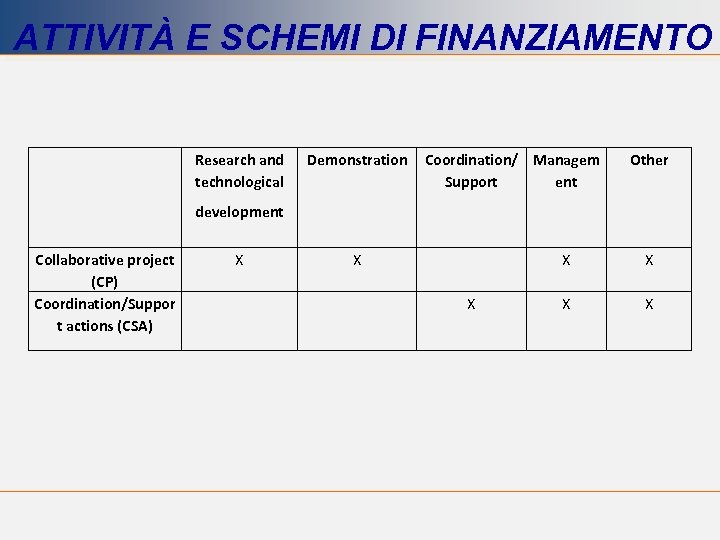 ATTIVITÀ E SCHEMI DI FINANZIAMENTO Research and technological Demonstration Coordination/ Managem Support ent Other development Collaborative project (CP) Coordination/Suppor t actions (CSA) X X X X
ATTIVITÀ E SCHEMI DI FINANZIAMENTO Research and technological Demonstration Coordination/ Managem Support ent Other development Collaborative project (CP) Coordination/Suppor t actions (CSA) X X X X
 PERCENTUALI DI FINANZIAMENTO 13
PERCENTUALI DI FINANZIAMENTO 13
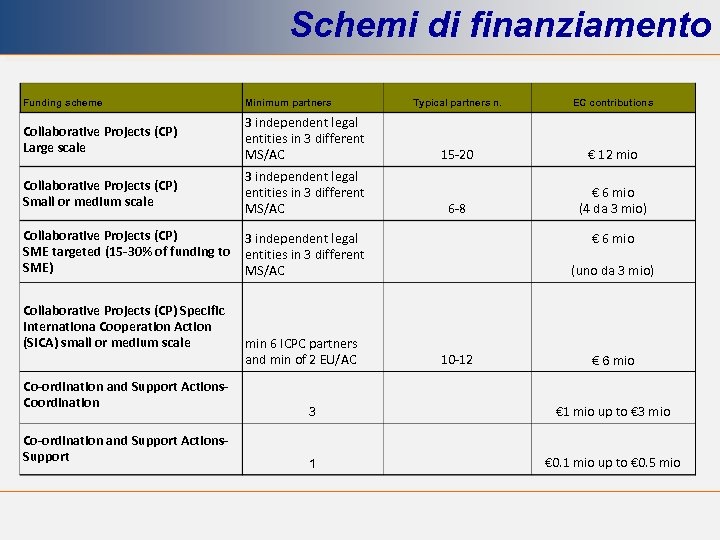 Schemi di finanziamento Funding scheme Minimum partners Collaborative Projects (CP) Large scale 3 independent legal entities in 3 different MS/AC Collaborative Projects (CP) Small or medium scale 3 independent legal entities in 3 different MS/AC Typical partners n. EC contributions 15 -20 € 12 mio 6 -8 € 6 mio (4 da 3 mio) Collaborative Projects (CP) 3 independent legal SME targeted (15 -30% of funding to entities in 3 different SME) MS/AC Collaborative Projects (CP) Specific Internationa Cooperation Action (SICA) small or medium scale Co-ordination and Support Actions. Coordination Co-ordination and Support Actions. Support min 6 ICPC partners and min of 2 EU/AC € 6 mio (uno da 3 mio) 10 -12 € 6 mio 3 € 1 mio up to € 3 mio 1 € 0. 1 mio up to € 0. 5 mio
Schemi di finanziamento Funding scheme Minimum partners Collaborative Projects (CP) Large scale 3 independent legal entities in 3 different MS/AC Collaborative Projects (CP) Small or medium scale 3 independent legal entities in 3 different MS/AC Typical partners n. EC contributions 15 -20 € 12 mio 6 -8 € 6 mio (4 da 3 mio) Collaborative Projects (CP) 3 independent legal SME targeted (15 -30% of funding to entities in 3 different SME) MS/AC Collaborative Projects (CP) Specific Internationa Cooperation Action (SICA) small or medium scale Co-ordination and Support Actions. Coordination Co-ordination and Support Actions. Support min 6 ICPC partners and min of 2 EU/AC € 6 mio (uno da 3 mio) 10 -12 € 6 mio 3 € 1 mio up to € 3 mio 1 € 0. 1 mio up to € 0. 5 mio
 STRUTTURA DEL WORK PROGRAMME • Programme (Health) - Activity (e. g. 1. BIOTECHNOLOGY, GENERIC TOOLS AND MEDICAL TECHNOLOGIES FOR HUMAN HEALTH) - Area (e. g. 1. 4 INNOVATIVE THERAPEUTIC APPROACHES AND INTERVENTIONS) - Topic (HEALTH. 2012. 1. 4 -4: Targeted nucleic acid delivery as an innovative therapeutic or prophylactic approach 36. FP 7 HEALTH-2012 -INNOVATION-1) Per ogni topic il Work Programme indica: – Content – Funding Scheme (CP, CSA) – Expected Impact – Other Information
STRUTTURA DEL WORK PROGRAMME • Programme (Health) - Activity (e. g. 1. BIOTECHNOLOGY, GENERIC TOOLS AND MEDICAL TECHNOLOGIES FOR HUMAN HEALTH) - Area (e. g. 1. 4 INNOVATIVE THERAPEUTIC APPROACHES AND INTERVENTIONS) - Topic (HEALTH. 2012. 1. 4 -4: Targeted nucleic acid delivery as an innovative therapeutic or prophylactic approach 36. FP 7 HEALTH-2012 -INNOVATION-1) Per ogni topic il Work Programme indica: – Content – Funding Scheme (CP, CSA) – Expected Impact – Other Information
 CALL FOR PROPOSAL - TOPIC HEALTH. 2012. 1. 4 -4: Targeted nucleic acid delivery as an innovative therapeutic or prophylactic approach. FP 7 -HEALTH-2012 -INNOVATION-1. The aim of this research is to exploit technology for nucleic acid delivery through testing in clinical trials carried out within the lifetime of the project. Recent innovative developments……. . Funding scheme: Collaborative project (small or medium-scale focused research project) One or more proposals can be selected.
CALL FOR PROPOSAL - TOPIC HEALTH. 2012. 1. 4 -4: Targeted nucleic acid delivery as an innovative therapeutic or prophylactic approach. FP 7 -HEALTH-2012 -INNOVATION-1. The aim of this research is to exploit technology for nucleic acid delivery through testing in clinical trials carried out within the lifetime of the project. Recent innovative developments……. . Funding scheme: Collaborative project (small or medium-scale focused research project) One or more proposals can be selected.
 CALL FOR PROPOSAL - TOPIC Expected impact: Building on recent results the projects should link promising emerging technologies with clinical application in the area of nucleic acid delivery for prophylactic or therapeutic purposes. This would enhance European expertise and competitiveness in an important emerging market. Research will also support the European biotechnology industry, especially the SME sector. Additional eligibility criteria: The requested EU contribution per project shall not exceed EUR 6 000 Projects will only be selected for funding on the condition that the estimated EU contribution going to industry including SME(s) is 30% or more of the total estimated EU contribution for the project as a whole. This will be assessed at the end of the negotiation, before signature of the grant agreement. Proposals not fulfilling this criterion will not be funded.
CALL FOR PROPOSAL - TOPIC Expected impact: Building on recent results the projects should link promising emerging technologies with clinical application in the area of nucleic acid delivery for prophylactic or therapeutic purposes. This would enhance European expertise and competitiveness in an important emerging market. Research will also support the European biotechnology industry, especially the SME sector. Additional eligibility criteria: The requested EU contribution per project shall not exceed EUR 6 000 Projects will only be selected for funding on the condition that the estimated EU contribution going to industry including SME(s) is 30% or more of the total estimated EU contribution for the project as a whole. This will be assessed at the end of the negotiation, before signature of the grant agreement. Proposals not fulfilling this criterion will not be funded.
 Chi può fare domanda: Stati (1) • Enti, sia pubblici che privati, aventi sede in UE • Altri Paesi con specifici accordi con il Programma • Organizzazioni internazionali (Chi non può fare domanda: le persone fisiche)
Chi può fare domanda: Stati (1) • Enti, sia pubblici che privati, aventi sede in UE • Altri Paesi con specifici accordi con il Programma • Organizzazioni internazionali (Chi non può fare domanda: le persone fisiche)
 Chi può fare domanda: Stati (2) The EU MEMBER STATES (MS) are: Austria, Belgium, Bulgaria, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Netherlands, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden, United Kingdom. The ASSOCIATED COUNTRIES (AC) are: Albania, Bosnia and Herzegovina, Croatia, FYR Macedonia, Iceland, Israel, Liechtenstein, Montenegro, Norway, Serbia, Switzerland Turkey and Faroe Islands.
Chi può fare domanda: Stati (2) The EU MEMBER STATES (MS) are: Austria, Belgium, Bulgaria, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Netherlands, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden, United Kingdom. The ASSOCIATED COUNTRIES (AC) are: Albania, Bosnia and Herzegovina, Croatia, FYR Macedonia, Iceland, Israel, Liechtenstein, Montenegro, Norway, Serbia, Switzerland Turkey and Faroe Islands.
 EPSS https: //www. epss-fp 7. org/epss/
EPSS https: //www. epss-fp 7. org/epss/
 STRUTTURA DELLA PROPOSTA • PARTE A: A 1: riassunto proposta (by Coord) A 2: partecipanti (by partners and Coord) A 3: budget (by Coord) • PARTE B: contenuti tecnici e scientifici della proposta (descrizione dettagliata delle attività con obiettivi, pianificazioni e CV)
STRUTTURA DELLA PROPOSTA • PARTE A: A 1: riassunto proposta (by Coord) A 2: partecipanti (by partners and Coord) A 3: budget (by Coord) • PARTE B: contenuti tecnici e scientifici della proposta (descrizione dettagliata delle attività con obiettivi, pianificazioni e CV)
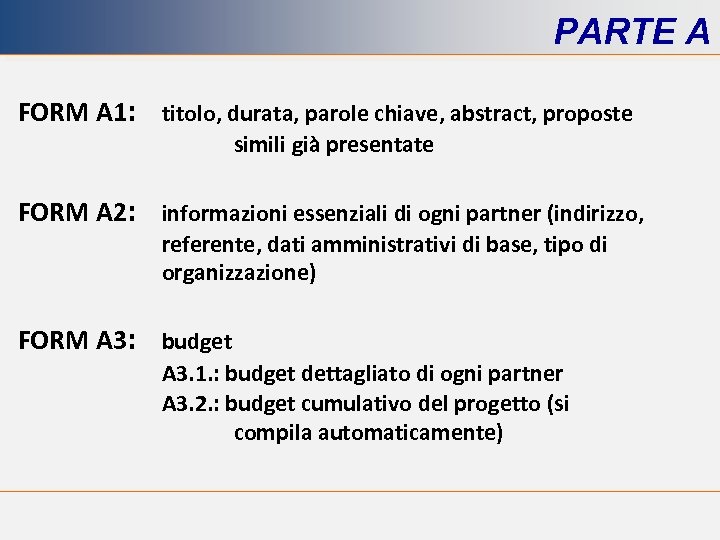 PARTE A FORM A 1: titolo, durata, parole chiave, abstract, proposte simili già presentate FORM A 2: informazioni essenziali di ogni partner (indirizzo, referente, dati amministrativi di base, tipo di organizzazione) FORM A 3: budget A 3. 1. : budget dettagliato di ogni partner A 3. 2. : budget cumulativo del progetto (si compila automaticamente)
PARTE A FORM A 1: titolo, durata, parole chiave, abstract, proposte simili già presentate FORM A 2: informazioni essenziali di ogni partner (indirizzo, referente, dati amministrativi di base, tipo di organizzazione) FORM A 3: budget A 3. 1. : budget dettagliato di ogni partner A 3. 2. : budget cumulativo del progetto (si compila automaticamente)
 PARTE B Q UAL I T A’ S CI NT I F I CA E T E CNI CA 1. CONTENUTO E OBIETTIVI 2. STATO DELL’ARTE 3. METODOLOGIA E WORK PLAN • Strategia del work plan (diagramma) • Tempistiche (Gantt chart) • Work description (work packages, deliverables, milestones, description of WPs, effort table) • Pert diagram • Risks and contingency plan
PARTE B Q UAL I T A’ S CI NT I F I CA E T E CNI CA 1. CONTENUTO E OBIETTIVI 2. STATO DELL’ARTE 3. METODOLOGIA E WORK PLAN • Strategia del work plan (diagramma) • Tempistiche (Gantt chart) • Work description (work packages, deliverables, milestones, description of WPs, effort table) • Pert diagram • Risks and contingency plan
 PARTE B I MP L E MA N T A T I O N 1. MANAGEMENT 2. INDIVIDUAL PARTICIPANTS (organismo + singoli cv) 3. CONSORTIUM • Sub-contractors • Other countries • Additional partners (excl. MS, AC, ICPC) 4. RESOURCES TO BE COMMITTED I MP A C T 1. EXPECTED IMPACTS 2. DISSEMINATION and/or EXPLOITATION OF PROJECT RESULTS, MANAGEMENT OF IPR
PARTE B I MP L E MA N T A T I O N 1. MANAGEMENT 2. INDIVIDUAL PARTICIPANTS (organismo + singoli cv) 3. CONSORTIUM • Sub-contractors • Other countries • Additional partners (excl. MS, AC, ICPC) 4. RESOURCES TO BE COMMITTED I MP A C T 1. EXPECTED IMPACTS 2. DISSEMINATION and/or EXPLOITATION OF PROJECT RESULTS, MANAGEMENT OF IPR
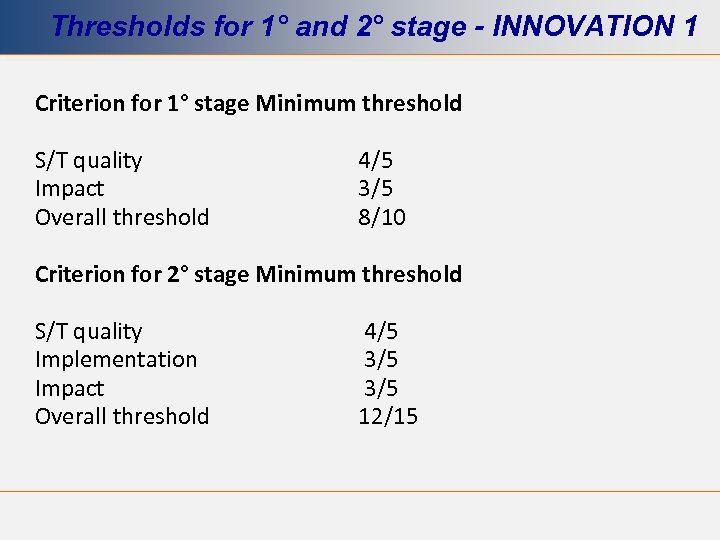 Thresholds for 1° and 2° stage - INNOVATION 1 Criterion for 1° stage Minimum threshold S/T quality Impact Overall threshold 4/5 3/5 8/10 Criterion for 2° stage Minimum threshold S/T quality Implementation Impact Overall threshold 4/5 3/5 12/15
Thresholds for 1° and 2° stage - INNOVATION 1 Criterion for 1° stage Minimum threshold S/T quality Impact Overall threshold 4/5 3/5 8/10 Criterion for 2° stage Minimum threshold S/T quality Implementation Impact Overall threshold 4/5 3/5 12/15
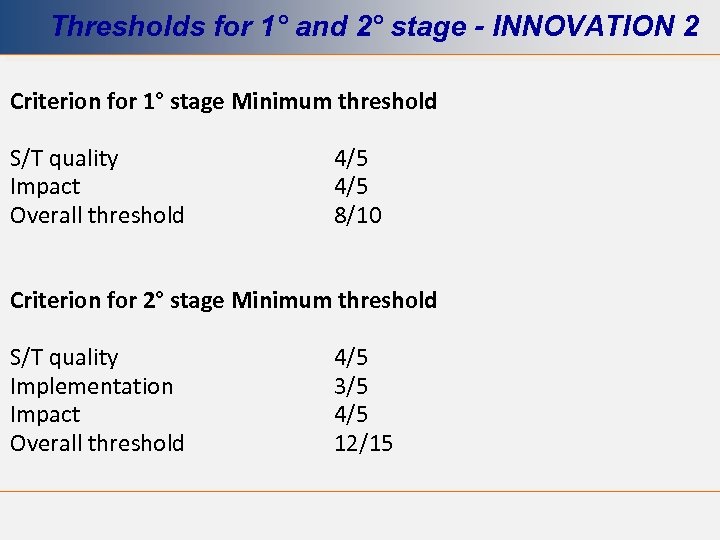 Thresholds for 1° and 2° stage - INNOVATION 2 Criterion for 1° stage Minimum threshold S/T quality Impact Overall threshold 4/5 8/10 Criterion for 2° stage Minimum threshold S/T quality Implementation Impact Overall threshold 4/5 3/5 4/5 12/15
Thresholds for 1° and 2° stage - INNOVATION 2 Criterion for 1° stage Minimum threshold S/T quality Impact Overall threshold 4/5 8/10 Criterion for 2° stage Minimum threshold S/T quality Implementation Impact Overall threshold 4/5 3/5 4/5 12/15
 CONSIGLI PRATICI • Il numero di partner può essere > di 3 purché i partner siano realmente necessari al raggiungimento degli obiettivi • Il budget è solo indicativo e definisce un tetto massimo oltre il quale non si può andare (è uno dei criteri di eleggibilità). Il contributo richiesto deve essere in linea con le reali esigenze del consorzio, ma non necessariamente al massimo. • Anche la durata del progetto deve essere definita in funzione di quanto previsto per il raggiungimento degli obiettivi (breve: da 1 a 3 anni; non meno di 3).
CONSIGLI PRATICI • Il numero di partner può essere > di 3 purché i partner siano realmente necessari al raggiungimento degli obiettivi • Il budget è solo indicativo e definisce un tetto massimo oltre il quale non si può andare (è uno dei criteri di eleggibilità). Il contributo richiesto deve essere in linea con le reali esigenze del consorzio, ma non necessariamente al massimo. • Anche la durata del progetto deve essere definita in funzione di quanto previsto per il raggiungimento degli obiettivi (breve: da 1 a 3 anni; non meno di 3).
 CONSIGLI PRATICI • Dove possibile, la proposta dovrebbe spiegare gli aspetti statistici (come sono stati raccolti i dati, come sono stati elaborati, come è stata fatta la misura della stima dell’errore e quali I metodi di inferenza utilizzati, etc). La statistica qui risulta fondamentale e garanzia di qualità e affidabilità delle conclusioni. • Preferibile la sperimentazione clinica guidata da ricercatori accademici piuttosto che dalle industrie. Le sperimentazioni cliniche possono essere effettuate internamente da un partner o in outsourcing da una parte terza (subappaltatore) • Importante il coinvolgimento precoce dei pazienti e dei loro gruppi di sostegno nella progettazione, realizzazione e monitoraggio di una sperimentazione clinica.
CONSIGLI PRATICI • Dove possibile, la proposta dovrebbe spiegare gli aspetti statistici (come sono stati raccolti i dati, come sono stati elaborati, come è stata fatta la misura della stima dell’errore e quali I metodi di inferenza utilizzati, etc). La statistica qui risulta fondamentale e garanzia di qualità e affidabilità delle conclusioni. • Preferibile la sperimentazione clinica guidata da ricercatori accademici piuttosto che dalle industrie. Le sperimentazioni cliniche possono essere effettuate internamente da un partner o in outsourcing da una parte terza (subappaltatore) • Importante il coinvolgimento precoce dei pazienti e dei loro gruppi di sostegno nella progettazione, realizzazione e monitoraggio di una sperimentazione clinica.
 CONSIGLI PRATICI • Attenzione alla corrispondenza tra le competenze del consorzio e gli obiettivi del progetto • Rendere il documento di facile lettura (apprezzata la sintesi) • Il contenuto dei packages deve indicare chiaramente ruoli ed attività • Il progetto deve produrre un evidente miglioramento rispetto all’attuale stato dell’arte
CONSIGLI PRATICI • Attenzione alla corrispondenza tra le competenze del consorzio e gli obiettivi del progetto • Rendere il documento di facile lettura (apprezzata la sintesi) • Il contenuto dei packages deve indicare chiaramente ruoli ed attività • Il progetto deve produrre un evidente miglioramento rispetto all’attuale stato dell’arte
 Date da ricordare call identifier: FP 7 - Health-2012 -INNOVATION 1 deadline for stage 1 proposals: 04 October 2011 max page limits first stage proposal: 6 pages stage 1 evaluation: mid December 2011 indicative deadline for stage 2 proposals: February 2012 stage 2 evaluation: March 2012 Grant agreement negotiations: May 2012
Date da ricordare call identifier: FP 7 - Health-2012 -INNOVATION 1 deadline for stage 1 proposals: 04 October 2011 max page limits first stage proposal: 6 pages stage 1 evaluation: mid December 2011 indicative deadline for stage 2 proposals: February 2012 stage 2 evaluation: March 2012 Grant agreement negotiations: May 2012
 Date da ricordare call identifier: FP 7 - Health-2012 -INNOVATION 2 ( partners (MS/AC): min 3 max 5; budget 6 mio, max duration 3 years, funding for SME 50%, more importance to impacts and exploitation plan ) deadline for stage 1 proposals: 27 September 2011 max page limits first stage proposal: 3 pages stage 1 evaluation: October 2011 indicative deadline for stage 2 proposals: mid December 2011 max page limits second stage proposal: 20 pages stage 2 evaluation: January 2012 Grant agreement negotiations: February 2012
Date da ricordare call identifier: FP 7 - Health-2012 -INNOVATION 2 ( partners (MS/AC): min 3 max 5; budget 6 mio, max duration 3 years, funding for SME 50%, more importance to impacts and exploitation plan ) deadline for stage 1 proposals: 27 September 2011 max page limits first stage proposal: 3 pages stage 1 evaluation: October 2011 indicative deadline for stage 2 proposals: mid December 2011 max page limits second stage proposal: 20 pages stage 2 evaluation: January 2012 Grant agreement negotiations: February 2012
 Overview • BIOTECHNOLOGY, GENERIC TOOLS AND MEDICAL TECHNOLOGIES FOR HUMAN HEALTH • 1. 2 Detection, diagnosis and monitoring • 1. 3 Suitability, safety, efficacy of therapies • 1. 4 Innovative therapeutic approaches and interventions • 2. TRANSLATING RESEARCH FOR HUMAN HEALTH • 2. 1 Integrating biological data and processes: large-scale data gathering, systems biology • 2. 1. 1 Large-scale data gathering • 2. 1. 2 Systems biology • 2. 2 Research on the brain and related diseases, human development and ageing • 2. 2. 1 Brain and brain-related diseases • 2. 2. 2 Human development and ageing • 2. 3 Translational research in major infectious diseases: to confront major threats to public health • 2. 3. 1 Anti-microbial drug resistance 2. 3. 2 HIV/AIDS, malaria and tuberculosis • 2. 3. 3 Potentially new and re-emerging epidemics • 2. 3. 4 Neglected infectious diseases.
Overview • BIOTECHNOLOGY, GENERIC TOOLS AND MEDICAL TECHNOLOGIES FOR HUMAN HEALTH • 1. 2 Detection, diagnosis and monitoring • 1. 3 Suitability, safety, efficacy of therapies • 1. 4 Innovative therapeutic approaches and interventions • 2. TRANSLATING RESEARCH FOR HUMAN HEALTH • 2. 1 Integrating biological data and processes: large-scale data gathering, systems biology • 2. 1. 1 Large-scale data gathering • 2. 1. 2 Systems biology • 2. 2 Research on the brain and related diseases, human development and ageing • 2. 2. 1 Brain and brain-related diseases • 2. 2. 2 Human development and ageing • 2. 3 Translational research in major infectious diseases: to confront major threats to public health • 2. 3. 1 Anti-microbial drug resistance 2. 3. 2 HIV/AIDS, malaria and tuberculosis • 2. 3. 3 Potentially new and re-emerging epidemics • 2. 3. 4 Neglected infectious diseases.
 Overview • BIOTECHNOLOGY, GENERIC TOOLS AND MEDICAL TECHNOLOGIES FOR HUMAN HEALTH • 1. 2 Detection, diagnosis and monitoring • 1. 3 Suitability, safety, efficacy of therapies • 1. 4 Innovative therapeutic approaches and interventions • 2. TRANSLATING RESEARCH FOR HUMAN HEALTH • 2. 1 Integrating biological data and processes: large-scale data gathering, systems biology • 2. 1. 1 Large-scale data gathering • 2. 1. 2 Systems biology • 2. 2 Research on the brain and related diseases, human development and ageing • 2. 2. 1 Brain and brain-related diseases • 2. 2. 2 Human development and ageing • 2. 3 Translational research in major infectious diseases: to confront major threats to public health • 2. 3. 1 Anti-microbial drug resistance 2. 3. 2 HIV/AIDS, malaria and tuberculosis • 2. 3. 3 Potentially new and re-emerging epidemics • 2. 3. 4 Neglected infectious diseases.
Overview • BIOTECHNOLOGY, GENERIC TOOLS AND MEDICAL TECHNOLOGIES FOR HUMAN HEALTH • 1. 2 Detection, diagnosis and monitoring • 1. 3 Suitability, safety, efficacy of therapies • 1. 4 Innovative therapeutic approaches and interventions • 2. TRANSLATING RESEARCH FOR HUMAN HEALTH • 2. 1 Integrating biological data and processes: large-scale data gathering, systems biology • 2. 1. 1 Large-scale data gathering • 2. 1. 2 Systems biology • 2. 2 Research on the brain and related diseases, human development and ageing • 2. 2. 1 Brain and brain-related diseases • 2. 2. 2 Human development and ageing • 2. 3 Translational research in major infectious diseases: to confront major threats to public health • 2. 3. 1 Anti-microbial drug resistance 2. 3. 2 HIV/AIDS, malaria and tuberculosis • 2. 3. 3 Potentially new and re-emerging epidemics • 2. 3. 4 Neglected infectious diseases.
 Overview • 2. 4 Translational research in other major diseases • 2. 4. 1 Cancer • 2. 4. 2 Cardiovascular diseases • 2. 4. 3 Diabetes and obesity • 2. 4. 4 Rare diseases • 2. 4. 5 Other chronic diseases • 3. OPTIMISING THE DELIVERY OF HEALTH CARE TO EUROPEAN CITIZENS • 3. 1 Translating the results of clinical research outcome into clinical practice including better use of medicines, and appropriate use of behavioural and organisational interventions and new health therapies and technologies<) • 3. 2 Quality, efficiency and solidarity of health care systems including transitional health systems • 3. 3 Health promotion and prevention • 3. 4 International public health & health systems
Overview • 2. 4 Translational research in other major diseases • 2. 4. 1 Cancer • 2. 4. 2 Cardiovascular diseases • 2. 4. 3 Diabetes and obesity • 2. 4. 4 Rare diseases • 2. 4. 5 Other chronic diseases • 3. OPTIMISING THE DELIVERY OF HEALTH CARE TO EUROPEAN CITIZENS • 3. 1 Translating the results of clinical research outcome into clinical practice including better use of medicines, and appropriate use of behavioural and organisational interventions and new health therapies and technologies<) • 3. 2 Quality, efficiency and solidarity of health care systems including transitional health systems • 3. 3 Health promotion and prevention • 3. 4 International public health & health systems
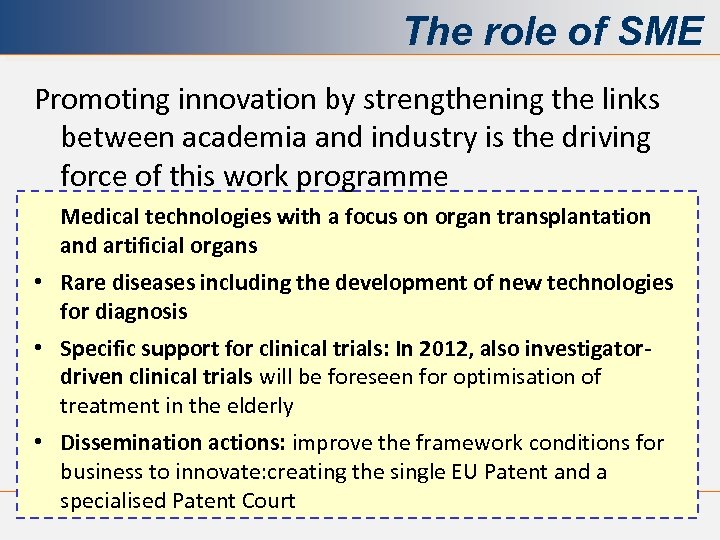 The role of SME Promoting innovation by strengthening the links between academia and industry is the driving force of this work programme Medical technologies with a focus on organ transplantation and artificial organs • Rare diseases including the development of new technologies for diagnosis • Specific support for clinical trials: In 2012, also investigatordriven clinical trials will be foreseen for optimisation of treatment in the elderly • Dissemination actions: improve the framework conditions for business to innovate: creating the single EU Patent and a specialised Patent Court
The role of SME Promoting innovation by strengthening the links between academia and industry is the driving force of this work programme Medical technologies with a focus on organ transplantation and artificial organs • Rare diseases including the development of new technologies for diagnosis • Specific support for clinical trials: In 2012, also investigatordriven clinical trials will be foreseen for optimisation of treatment in the elderly • Dissemination actions: improve the framework conditions for business to innovate: creating the single EU Patent and a specialised Patent Court
 Health Objectives: • Improving the health of European citizen and increasing competitiveness of European health related • Improving industries and services, as well as addressing the socio-economic dimension of health • Improving care and global health issues.
Health Objectives: • Improving the health of European citizen and increasing competitiveness of European health related • Improving industries and services, as well as addressing the socio-economic dimension of health • Improving care and global health issues.
 Innovation dimension of the activities 11 topics related to medical technologies for different purposes • • Detection, diagnosis & monitoring, innovative therapies, large scale data gathering, systems biology, human development and ageing, chronic diseases, health care systems and support actions.
Innovation dimension of the activities 11 topics related to medical technologies for different purposes • • Detection, diagnosis & monitoring, innovative therapies, large scale data gathering, systems biology, human development and ageing, chronic diseases, health care systems and support actions.
 Innovation dimension of the activities • DETECTION, DIAGNOSIS AND MONITORING • The objectives are to develop visualisation, imaging, detection and analytical tools and technologies for biomedical research, for prediction, diagnosis, monitoring and prognosis of diseases, and for support and guidance of therapeutic interventions. The focus will be on a multidisciplinary approach integrating areas such as: molecular and cellular biology, physiology, genetics, physics, chemistry, biomedical engineering, nanotechnologies, microsystems, devices and information technologies. Funding scheme: SME-targeted Collaborative Project
Innovation dimension of the activities • DETECTION, DIAGNOSIS AND MONITORING • The objectives are to develop visualisation, imaging, detection and analytical tools and technologies for biomedical research, for prediction, diagnosis, monitoring and prognosis of diseases, and for support and guidance of therapeutic interventions. The focus will be on a multidisciplinary approach integrating areas such as: molecular and cellular biology, physiology, genetics, physics, chemistry, biomedical engineering, nanotechnologies, microsystems, devices and information technologies. Funding scheme: SME-targeted Collaborative Project
 Therapy • INNOVATIVE THERAPEUTIC APPROACHES AND INTERVENTIONS • For this call for proposals, the main focus is on transplantation, with subsidiary topics on international cooperation in stem cell research and targeted nucleic acid delivery. Topics are drafted in broad terms to encourage innovation treatment outcome for transplantation patients, better understanding of mode of action of treatments or potential treatments and be of use to the industrial, especially SME, sector
Therapy • INNOVATIVE THERAPEUTIC APPROACHES AND INTERVENTIONS • For this call for proposals, the main focus is on transplantation, with subsidiary topics on international cooperation in stem cell research and targeted nucleic acid delivery. Topics are drafted in broad terms to encourage innovation treatment outcome for transplantation patients, better understanding of mode of action of treatments or potential treatments and be of use to the industrial, especially SME, sector
 Therapy • INNOVATIVE THERAPEUTIC APPROACHES AND INTERVENTIONS • For this call for proposals, the main focus is on transplantation, with subsidiary topics on international cooperation in stem cell research and targeted nucleic acid delivery. Topics are drafted in broad terms to encourage innovation development of new tools, technologies or devices for use in transplantation -treatment outcome for transplantation patients, better understanding of mode of action of treatments or potential treatments and be of use to the industrial, especially SME, sector
Therapy • INNOVATIVE THERAPEUTIC APPROACHES AND INTERVENTIONS • For this call for proposals, the main focus is on transplantation, with subsidiary topics on international cooperation in stem cell research and targeted nucleic acid delivery. Topics are drafted in broad terms to encourage innovation development of new tools, technologies or devices for use in transplantation -treatment outcome for transplantation patients, better understanding of mode of action of treatments or potential treatments and be of use to the industrial, especially SME, sector
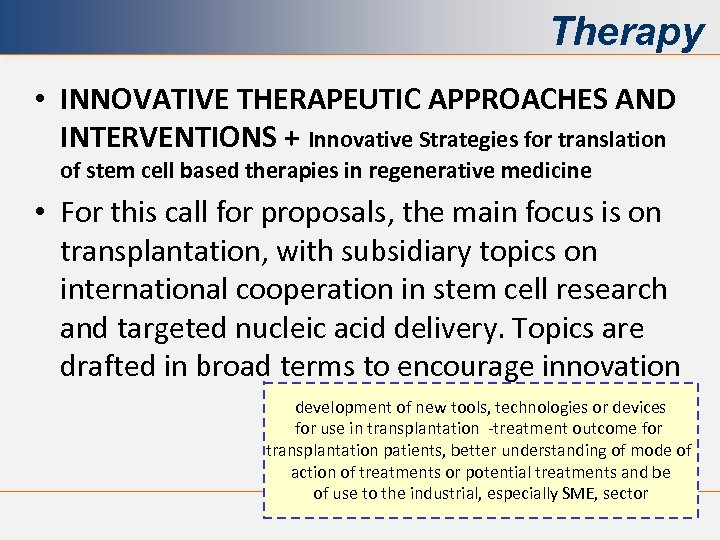 Therapy • INNOVATIVE THERAPEUTIC APPROACHES AND INTERVENTIONS + Innovative Strategies for translation of stem cell based therapies in regenerative medicine • For this call for proposals, the main focus is on transplantation, with subsidiary topics on international cooperation in stem cell research and targeted nucleic acid delivery. Topics are drafted in broad terms to encourage innovation development of new tools, technologies or devices for use in transplantation -treatment outcome for transplantation patients, better understanding of mode of action of treatments or potential treatments and be of use to the industrial, especially SME, sector
Therapy • INNOVATIVE THERAPEUTIC APPROACHES AND INTERVENTIONS + Innovative Strategies for translation of stem cell based therapies in regenerative medicine • For this call for proposals, the main focus is on transplantation, with subsidiary topics on international cooperation in stem cell research and targeted nucleic acid delivery. Topics are drafted in broad terms to encourage innovation development of new tools, technologies or devices for use in transplantation -treatment outcome for transplantation patients, better understanding of mode of action of treatments or potential treatments and be of use to the industrial, especially SME, sector
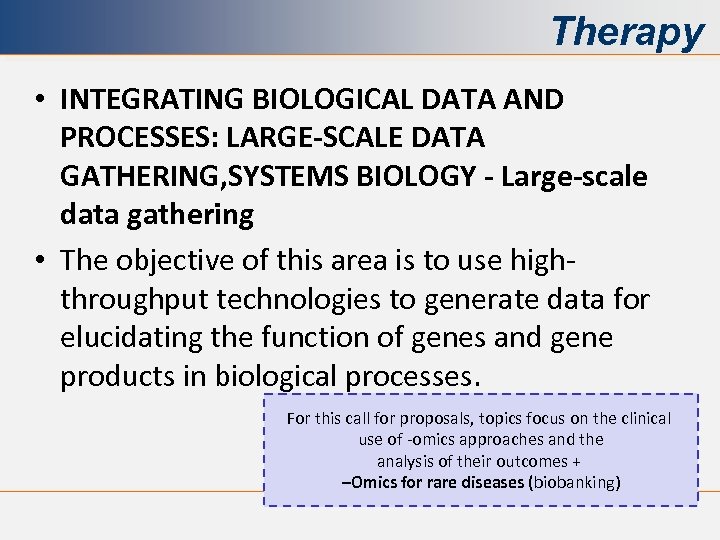 Therapy • INTEGRATING BIOLOGICAL DATA AND PROCESSES: LARGE-SCALE DATA GATHERING, SYSTEMS BIOLOGY - Large-scale data gathering • The objective of this area is to use highthroughput technologies to generate data for elucidating the function of genes and gene products in biological processes. For this call for proposals, topics focus on the clinical use of -omics approaches and the analysis of their outcomes + –Omics for rare diseases (biobanking)
Therapy • INTEGRATING BIOLOGICAL DATA AND PROCESSES: LARGE-SCALE DATA GATHERING, SYSTEMS BIOLOGY - Large-scale data gathering • The objective of this area is to use highthroughput technologies to generate data for elucidating the function of genes and gene products in biological processes. For this call for proposals, topics focus on the clinical use of -omics approaches and the analysis of their outcomes + –Omics for rare diseases (biobanking)
 Therapy • Databases, biobanks and 'clinical bioinformatics' hub for rare diseases. - Largescale data gathering • The project aims at developing an integrated platform supporting the collection and storage of -omics and clinical data, and samples collected through projects Validation of -omics-based biomarkers for diseases affecting the elderly.
Therapy • Databases, biobanks and 'clinical bioinformatics' hub for rare diseases. - Largescale data gathering • The project aims at developing an integrated platform supporting the collection and storage of -omics and clinical data, and samples collected through projects Validation of -omics-based biomarkers for diseases affecting the elderly.
 System biology • Systems medicine: SME-driven research applying systems biology approaches to address medical and clinical. Research should focus on the development, improvement and application of systems biology approaches to medical/clinical questions (i. e. Re-design of clinical trials by shortening times and costs - Re-definition of clinical phenotypes based on molecular and dynamic parameters - Development of tools for in vivo dynamic and quantitative clinically-relevant measurements at the cellular/tissue/organ level - Development of combinatorial therapies and/or chronotherapies for complex diseases) SME-targeted Collaborative Project (small-scale focused research project)
System biology • Systems medicine: SME-driven research applying systems biology approaches to address medical and clinical. Research should focus on the development, improvement and application of systems biology approaches to medical/clinical questions (i. e. Re-design of clinical trials by shortening times and costs - Re-definition of clinical phenotypes based on molecular and dynamic parameters - Development of tools for in vivo dynamic and quantitative clinically-relevant measurements at the cellular/tissue/organ level - Development of combinatorial therapies and/or chronotherapies for complex diseases) SME-targeted Collaborative Project (small-scale focused research project)
 • Innovation dimension of the activities Active and healthy ageing: enable EU citizens to lead healthy, active and independent lives until old age; contribute – to ensuring the sustainability and efficiency of social and healthcare systems; – contributing to the creation of a European and global market for innovative products – services related to healthy and active ageing.
• Innovation dimension of the activities Active and healthy ageing: enable EU citizens to lead healthy, active and independent lives until old age; contribute – to ensuring the sustainability and efficiency of social and healthcare systems; – contributing to the creation of a European and global market for innovative products – services related to healthy and active ageing.
 Aging • Active and healthy ageing: enable EU citizens to lead healthy, active and independent lives until old age; – Integrative systems biology and comparative genomics for studying human ageing and/or most common age-related diseases. – Investigator-driven clinical trials for optimisation of management of elderly patients with multiple (to encourage SME efforts towards diseases. research and innovation)Three main impacts are expected: treatments better suited to the needs of older people, lowering healthcare costs and engaging in the pre-normative setting of geriatric medicines.
Aging • Active and healthy ageing: enable EU citizens to lead healthy, active and independent lives until old age; – Integrative systems biology and comparative genomics for studying human ageing and/or most common age-related diseases. – Investigator-driven clinical trials for optimisation of management of elderly patients with multiple (to encourage SME efforts towards diseases. research and innovation)Three main impacts are expected: treatments better suited to the needs of older people, lowering healthcare costs and engaging in the pre-normative setting of geriatric medicines.
 Cross-thematic approaches • Theme Health contributes with a number of topics to the EIP "active and healthy ageing". The research part of this EIP will be established by – – – Themes Information and Communication. Technologies (ICT); Health Food Agriculture Fisheries and Biotechnology (KBBE) and Socio-economics Sciences and the Humanities (SSH).
Cross-thematic approaches • Theme Health contributes with a number of topics to the EIP "active and healthy ageing". The research part of this EIP will be established by – – – Themes Information and Communication. Technologies (ICT); Health Food Agriculture Fisheries and Biotechnology (KBBE) and Socio-economics Sciences and the Humanities (SSH).
 Statistics in health research: Appropriate study design, data processing and statistical • Analysis of results are important for the quality and efficiency of the science and reliability of conclusions, and hence also ethically. • Therefore, whenever applicable, the proposal should explain the statistical aspects.
Statistics in health research: Appropriate study design, data processing and statistical • Analysis of results are important for the quality and efficiency of the science and reliability of conclusions, and hence also ethically. • Therefore, whenever applicable, the proposal should explain the statistical aspects.
 trials for optimisation of management of elderly • Research will focus on drug therapy and other interventions for patients affected by and treated for multiple diseases • Patient advocacy groups, which can contribute to the quality, feasibility and impact of clinical trials, should be involved where appropriate. Telemedicine and other monitoring systems
trials for optimisation of management of elderly • Research will focus on drug therapy and other interventions for patients affected by and treated for multiple diseases • Patient advocacy groups, which can contribute to the quality, feasibility and impact of clinical trials, should be involved where appropriate. Telemedicine and other monitoring systems
 trials for optimisation of management of elderly • Research will focus on drug therapy and other interventions for patients affected by and treated for multiple diseases • Patient advocacy groups, which can contribute to the quality, feasibility and impact of clinical trials, should be involved where appropriate. . . treatments better suited to the needs of older people, lowering healthcare costs and engaging in the pre-normative setting of geriatric medicines. Telemedicine and other monitoring systems
trials for optimisation of management of elderly • Research will focus on drug therapy and other interventions for patients affected by and treated for multiple diseases • Patient advocacy groups, which can contribute to the quality, feasibility and impact of clinical trials, should be involved where appropriate. . . treatments better suited to the needs of older people, lowering healthcare costs and engaging in the pre-normative setting of geriatric medicines. Telemedicine and other monitoring systems
 HIV/AIDS, malaria, tuberculosis • The focus will be on promoting translational research aiming at bringing basic knowledge through to clinical application in developing new therapies, diagnostic tools and vaccines for HIV/AIDS, malaria and tuberculosis. .
HIV/AIDS, malaria, tuberculosis • The focus will be on promoting translational research aiming at bringing basic knowledge through to clinical application in developing new therapies, diagnostic tools and vaccines for HIV/AIDS, malaria and tuberculosis. .
 ERA-NET on infectious diseases • . This action shall further improve the linking, efficient integration and coordination of national/regional programmes for infectious diseases research, building on previous activities in this field. It should aim to develop new technologies and employ modern genomic approaches to advance our understanding of pathogenic organisms and interactions with their hosts as well as support the development of new tools to combat or prevent infectious diseases. .
ERA-NET on infectious diseases • . This action shall further improve the linking, efficient integration and coordination of national/regional programmes for infectious diseases research, building on previous activities in this field. It should aim to develop new technologies and employ modern genomic approaches to advance our understanding of pathogenic organisms and interactions with their hosts as well as support the development of new tools to combat or prevent infectious diseases. .
 HIV/AIDS, malaria, tuberculosis • The focus will be on promoting translational research aiming at bringing basic knowledge through to clinical application in developing new therapies, diagnostic tools and vaccines for HIV/AIDS, malaria and tuberculosis.
HIV/AIDS, malaria, tuberculosis • The focus will be on promoting translational research aiming at bringing basic knowledge through to clinical application in developing new therapies, diagnostic tools and vaccines for HIV/AIDS, malaria and tuberculosis.
 Diabetes and obesity • Taking into account state-of-the-art innovative research and technologies, the aim of this topic is to validate, in the preclinical and/or clinical setting, the performance and applicability of therapeutic devices or biological therapies aimed at improving diabetes management. This could include for instance glucose sensors, insulin delivery systems, devices that respond on low glucose levels to release glucagon or other insulin -counteracting therapies and could build on surgical, immunological, integrated physiology, cellular and bioartificial therapy approaches. .
Diabetes and obesity • Taking into account state-of-the-art innovative research and technologies, the aim of this topic is to validate, in the preclinical and/or clinical setting, the performance and applicability of therapeutic devices or biological therapies aimed at improving diabetes management. This could include for instance glucose sensors, insulin delivery systems, devices that respond on low glucose levels to release glucagon or other insulin -counteracting therapies and could build on surgical, immunological, integrated physiology, cellular and bioartificial therapy approaches. .
 Diabetes and obesity - therapy • For both diabetes and obesity, special attention will be given to juvenile diseases and factors operating in childhood. • As a healthy life-style is a pre-requisite for any containment of the steadily increasing costs of diabetes/obesity, projects should consider such aspects in their proposals whenever possible. • For this call for proposals, topics will focus on developing and testing innovation in the field of diabetes management, as well as on investigator-driven clinical trials addressing informed clinical management for type 1 diabetes, particularly in childhood and adolescence.
Diabetes and obesity - therapy • For both diabetes and obesity, special attention will be given to juvenile diseases and factors operating in childhood. • As a healthy life-style is a pre-requisite for any containment of the steadily increasing costs of diabetes/obesity, projects should consider such aspects in their proposals whenever possible. • For this call for proposals, topics will focus on developing and testing innovation in the field of diabetes management, as well as on investigator-driven clinical trials addressing informed clinical management for type 1 diabetes, particularly in childhood and adolescence.
 Rare diseases • This area should help identifying and mobilising the critical mass of expertise in order (i) to shed light on the course and/or mechanisms of rare diseases, or (ii) to test diagnostic, preventive and/or therapeutic approaches, to alleviate the negative impact of the disease on the quality of life of the patients and their families.
Rare diseases • This area should help identifying and mobilising the critical mass of expertise in order (i) to shed light on the course and/or mechanisms of rare diseases, or (ii) to test diagnostic, preventive and/or therapeutic approaches, to alleviate the negative impact of the disease on the quality of life of the patients and their families.
 Other chronic diseases • For this call for proposals the focus will be on non -lethal diseases and chronic conditions with a high impact on the quality of life at old age such as functional and sensory impairment and chronic inflammatory diseases. It is expected that collaborative research in this area will lead to improved diagnostics of the chronic conditions, develop tools and/or intervention strategies, which may contribute to delaying the onset of chronic diseases, their efficient treatment, and improving quality of life.
Other chronic diseases • For this call for proposals the focus will be on non -lethal diseases and chronic conditions with a high impact on the quality of life at old age such as functional and sensory impairment and chronic inflammatory diseases. It is expected that collaborative research in this area will lead to improved diagnostics of the chronic conditions, develop tools and/or intervention strategies, which may contribute to delaying the onset of chronic diseases, their efficient treatment, and improving quality of life.
 Technological approaches to combating sensory impairments • Examples of possible areas to be considered: strategies aiming at prevention of damage and rejuvenation of sensory cells and systems, treatment of sensory diseases, implantable devices, cell based approaches, including stem cells, and development of artificial organs or their parts. Full attention needs to be paid to safety, bio-compatibility, interoperability and regulatory aspects as appropriate.
Technological approaches to combating sensory impairments • Examples of possible areas to be considered: strategies aiming at prevention of damage and rejuvenation of sensory cells and systems, treatment of sensory diseases, implantable devices, cell based approaches, including stem cells, and development of artificial organs or their parts. Full attention needs to be paid to safety, bio-compatibility, interoperability and regulatory aspects as appropriate.
 Quality of the health system • QUALITY, EFFICIENCY AND SOLIDARITY OF HEALTH CARE SYSTEMS INCLUDING TRANSITIONAL HEALTH SYSTEMS - There is a clear need for a more systematic mapping of variations in health care practice, for understanding their causes and assessing their consequences for individual health improvement.
Quality of the health system • QUALITY, EFFICIENCY AND SOLIDARITY OF HEALTH CARE SYSTEMS INCLUDING TRANSITIONAL HEALTH SYSTEMS - There is a clear need for a more systematic mapping of variations in health care practice, for understanding their causes and assessing their consequences for individual health improvement.
 Quality of the health system • Improving the organisation of health service delivery – • Quality of cost information for patient care. • Patient-centred care and patient involvement • Skill mix and management of human resources. – formation of new health professionals
Quality of the health system • Improving the organisation of health service delivery – • Quality of cost information for patient care. • Patient-centred care and patient involvement • Skill mix and management of human resources. – formation of new health professionals
 New methodologies for health technology assessment. • Complex interventions consisting of a wide spectrum of technologies and multidisciplinary delivery modes should be addressed, such as personalised medicines, public health interventions, organisational interventions and information and communication technologies related to health.
New methodologies for health technology assessment. • Complex interventions consisting of a wide spectrum of technologies and multidisciplinary delivery modes should be addressed, such as personalised medicines, public health interventions, organisational interventions and information and communication technologies related to health.
 Social innovation for active and healthy ageing. . • Social innovation for active and healthy ageing should aim to develop innovative approaches to promote better quality of life and improved wellbeing for the elderly. Proposals should develop new ideas (products, services and/or models) that simultaneously meet social needs and create newsocial relationships. Such research, with a holistic approach to well-being
Social innovation for active and healthy ageing. . • Social innovation for active and healthy ageing should aim to develop innovative approaches to promote better quality of life and improved wellbeing for the elderly. Proposals should develop new ideas (products, services and/or models) that simultaneously meet social needs and create newsocial relationships. Such research, with a holistic approach to well-being
 Research on health systems and services in low- and middle income Countries. • Research should combine inter- and intra-country comparisons, quantitative and qualitative approaches with experience about best practices with a view to increase and sustain universal health coverage. Research could also develop plans for improved management of the health workforce in low-resource settings such as rural areas and urban slums.
Research on health systems and services in low- and middle income Countries. • Research should combine inter- and intra-country comparisons, quantitative and qualitative approaches with experience about best practices with a view to increase and sustain universal health coverage. Research could also develop plans for improved management of the health workforce in low-resource settings such as rural areas and urban slums.
 Network to encourage knowledge transfer activity in FP-funded health research. • 1) It will create platforms for shared learning and networking forscientists, hospitals, program managers and policy makers in a continuous manner. 2) It will establish a mechanism for identifying and promoting good knowledge management and knowledge transfer practices in the EU Member States and Associated Countries, providing evidence on best practice on the transfer of knowledge, including standardisation. 3) It will give visibility to the best achievements at the European level, including impact of legislation and tax incentives on technology transfer and innovative SMEs. 4) It will create an on-line repository of best practices for further reference and actively promote them
Network to encourage knowledge transfer activity in FP-funded health research. • 1) It will create platforms for shared learning and networking forscientists, hospitals, program managers and policy makers in a continuous manner. 2) It will establish a mechanism for identifying and promoting good knowledge management and knowledge transfer practices in the EU Member States and Associated Countries, providing evidence on best practice on the transfer of knowledge, including standardisation. 3) It will give visibility to the best achievements at the European level, including impact of legislation and tax incentives on technology transfer and innovative SMEs. 4) It will create an on-line repository of best practices for further reference and actively promote them
 Preparing the future for health research and innovation-funded health research. • Different actors from academia, industry, national programmes and other relevant organisations, should come together to develop a strategy plan for the further development of the targeted health research area with high impact on competitiveness, healthcare systems and benefit for European citizens' health.
Preparing the future for health research and innovation-funded health research. • Different actors from academia, industry, national programmes and other relevant organisations, should come together to develop a strategy plan for the further development of the targeted health research area with high impact on competitiveness, healthcare systems and benefit for European citizens' health.
 SCHEDA D’INTERESSE Se ancora non l’avete fatto, vi suggeriamo di iscrivervi alla SCHEDA D’INTERESSE al seguente link www. swas. polito. it/intra/ Sezione "Servizi Europoli" - "Scheda di interesse". Per ricevere comunicazioni sui programmi di finanziamento della Commissione Europea
SCHEDA D’INTERESSE Se ancora non l’avete fatto, vi suggeriamo di iscrivervi alla SCHEDA D’INTERESSE al seguente link www. swas. polito. it/intra/ Sezione "Servizi Europoli" - "Scheda di interesse". Per ricevere comunicazioni sui programmi di finanziamento della Commissione Europea
 ALCUNI SITI WEB UTILI: Indirizzo del programma: http: //ec. europa. eu/research/participants/portal/pa ge/fp 7_calls? Ricerca partner: http: //cordis. europa. eu/fp 7/partners_en. html National Contact point: Caterina Buonocare buonocore@apre. it; Nicola Bergonzi bergonzi@apre. it;
ALCUNI SITI WEB UTILI: Indirizzo del programma: http: //ec. europa. eu/research/participants/portal/pa ge/fp 7_calls? Ricerca partner: http: //cordis. europa. eu/fp 7/partners_en. html National Contact point: Caterina Buonocare buonocore@apre. it; Nicola Bergonzi bergonzi@apre. it;
 Contatti Guido Pagana Dauin Tel. 4077 Mail: guido. pagana@polito. it Maria Onorato Presidio Scienze di Base tel. 3307 Mail: maria. onorato@polito. it presidi@polito. it Laura Fulci Responsabile Ufficio Fund Raising Europeo Tel +39 011 0906282 Mail: laura. fulci@polito. it europoli@polito. it
Contatti Guido Pagana Dauin Tel. 4077 Mail: guido. pagana@polito. it Maria Onorato Presidio Scienze di Base tel. 3307 Mail: maria. onorato@polito. it presidi@polito. it Laura Fulci Responsabile Ufficio Fund Raising Europeo Tel +39 011 0906282 Mail: laura. fulci@polito. it europoli@polito. it
