Molecular dynamics marenkov.pptx
- Количество слайдов: 27
 Molecular dynamics Marenkov E. D.
Molecular dynamics Marenkov E. D.
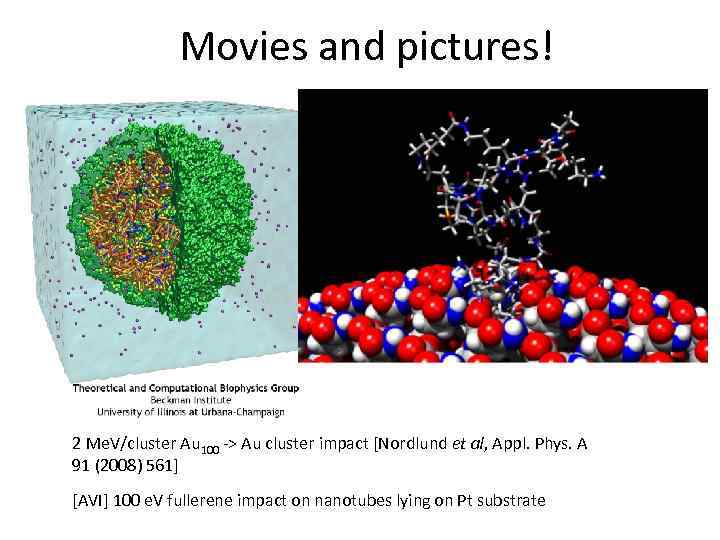 Movies and pictures! 2 Me. V/cluster Au 100 -> Au cluster impact [Nordlund et al, Appl. Phys. A 91 (2008) 561] [AVI] 100 e. V fullerene impact on nanotubes lying on Pt substrate
Movies and pictures! 2 Me. V/cluster Au 100 -> Au cluster impact [Nordlund et al, Appl. Phys. A 91 (2008) 561] [AVI] 100 e. V fullerene impact on nanotubes lying on Pt substrate
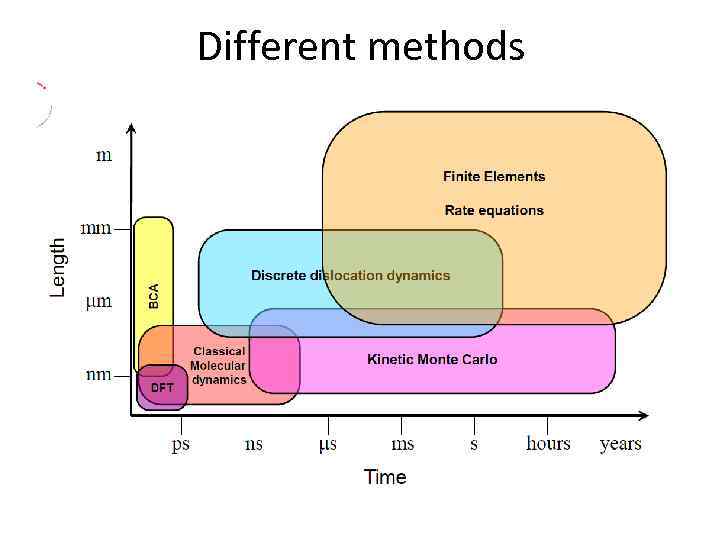 Different methods
Different methods
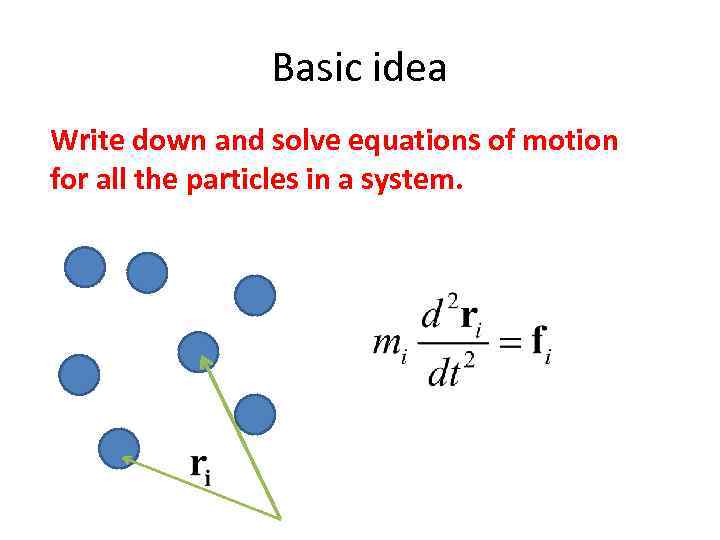 Basic idea Write down and solve equations of motion for all the particles in a system.
Basic idea Write down and solve equations of motion for all the particles in a system.
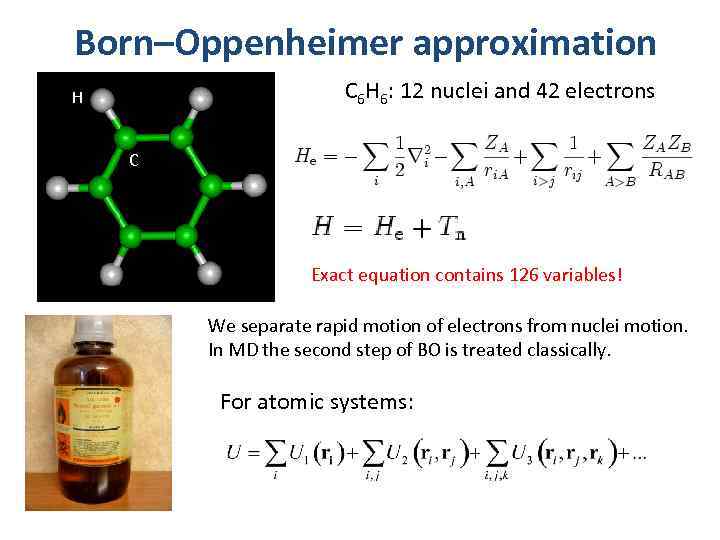 Born–Oppenheimer approximation C 6 H 6: 12 nuclei and 42 electrons H C Exact equation contains 126 variables! We separate rapid motion of electrons from nuclei motion. In MD the second step of BO is treated classically. For atomic systems:
Born–Oppenheimer approximation C 6 H 6: 12 nuclei and 42 electrons H C Exact equation contains 126 variables! We separate rapid motion of electrons from nuclei motion. In MD the second step of BO is treated classically. For atomic systems:
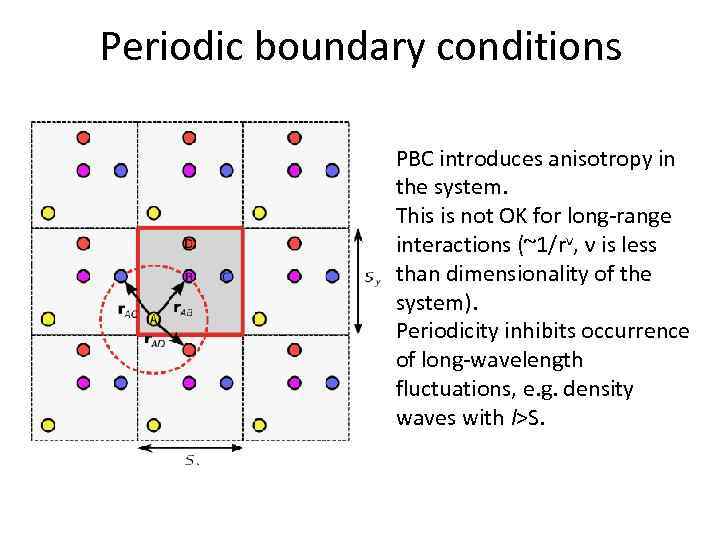 Periodic boundary conditions PBC introduces anisotropy in the system. This is not OK for long-range interactions (~1/rν, ν is less than dimensionality of the system). Periodicity inhibits occurrence of long-wavelength fluctuations, e. g. density waves with l>S.
Periodic boundary conditions PBC introduces anisotropy in the system. This is not OK for long-range interactions (~1/rν, ν is less than dimensionality of the system). Periodicity inhibits occurrence of long-wavelength fluctuations, e. g. density waves with l>S.
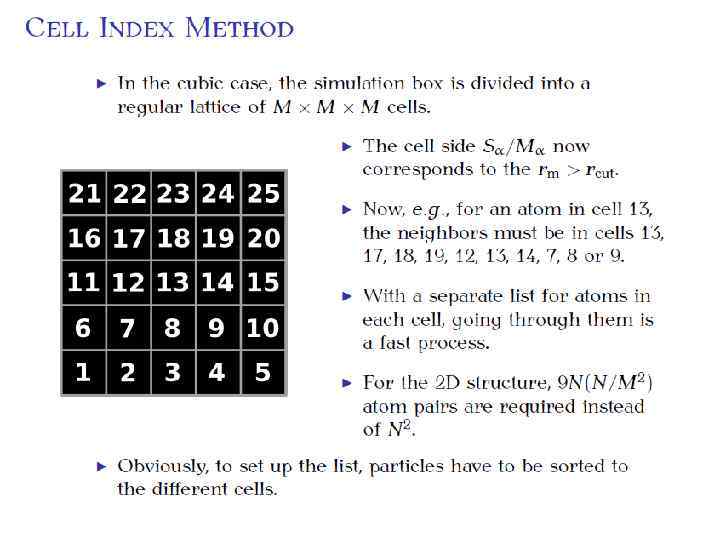
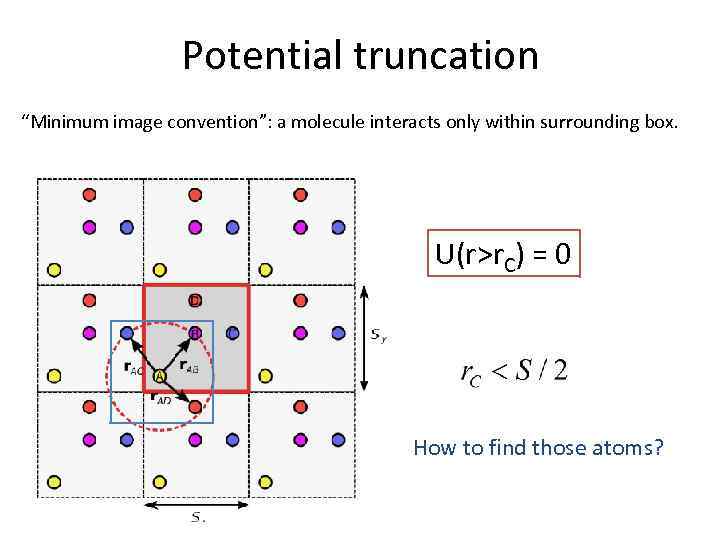 Potential truncation “Minimum image convention”: a molecule interacts only within surrounding box. U(r>r. C) = 0 How to find those atoms?
Potential truncation “Minimum image convention”: a molecule interacts only within surrounding box. U(r>r. C) = 0 How to find those atoms?

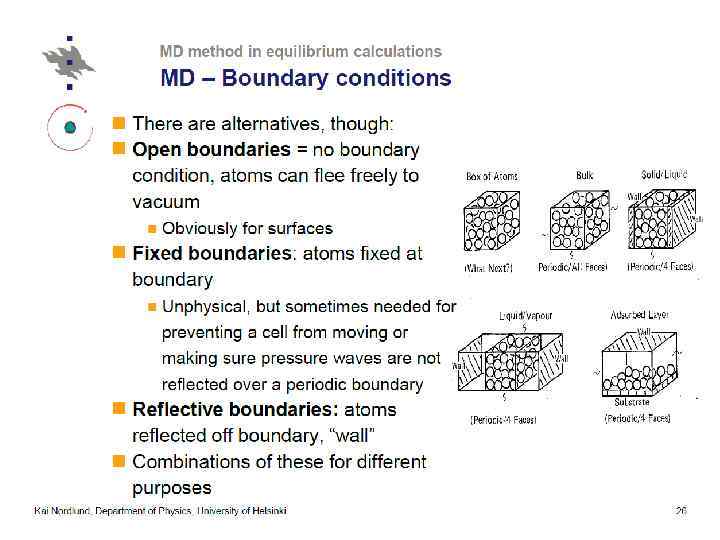
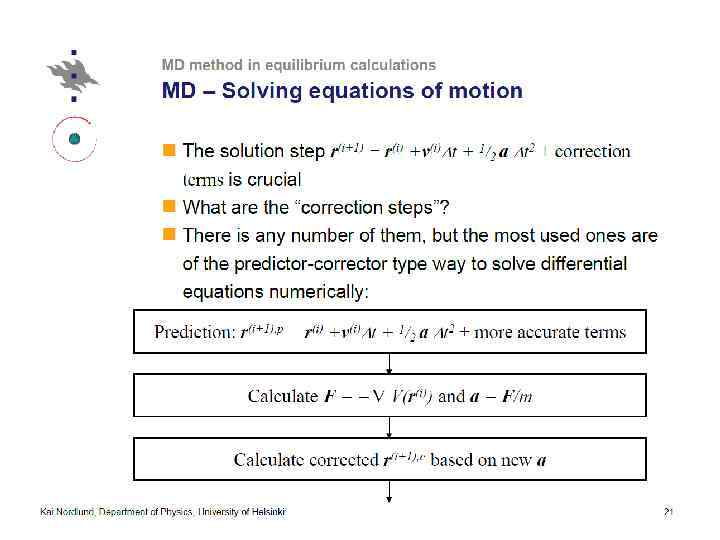
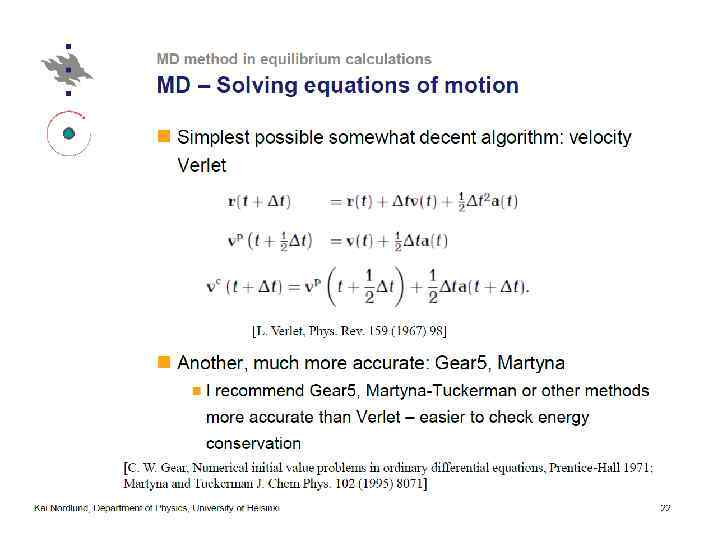
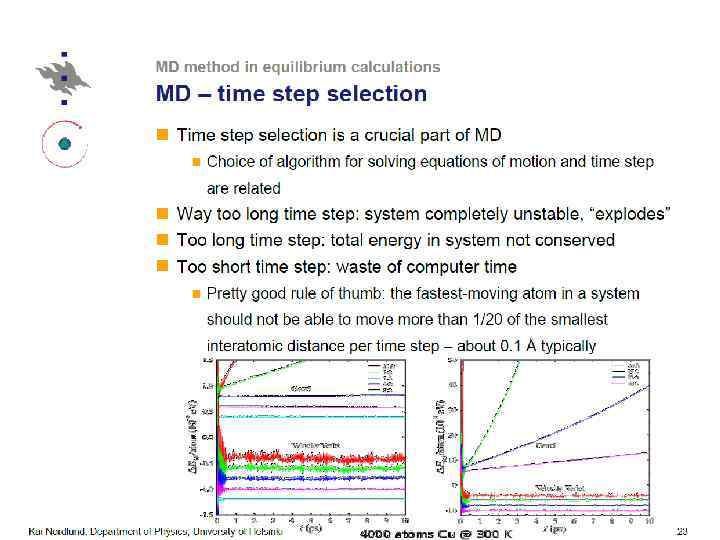
 Thermostatting means controlling the temperature of particles in an MD simulation. Barostatting means controlling the pressure. Thermostats (LAMMPS) ØNose-Hoover (nvt), ØBerendsen, ØCSVR, ØLangevin, Ødirect rescaling (temp/rescale)
Thermostatting means controlling the temperature of particles in an MD simulation. Barostatting means controlling the pressure. Thermostats (LAMMPS) ØNose-Hoover (nvt), ØBerendsen, ØCSVR, ØLangevin, Ødirect rescaling (temp/rescale)
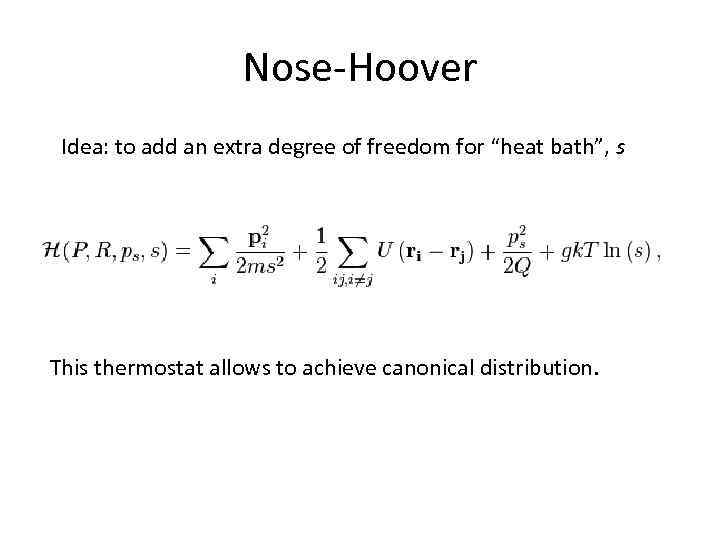 Nose-Hoover Idea: to add an extra degree of freedom for “heat bath”, s This thermostat allows to achieve canonical distribution.
Nose-Hoover Idea: to add an extra degree of freedom for “heat bath”, s This thermostat allows to achieve canonical distribution.
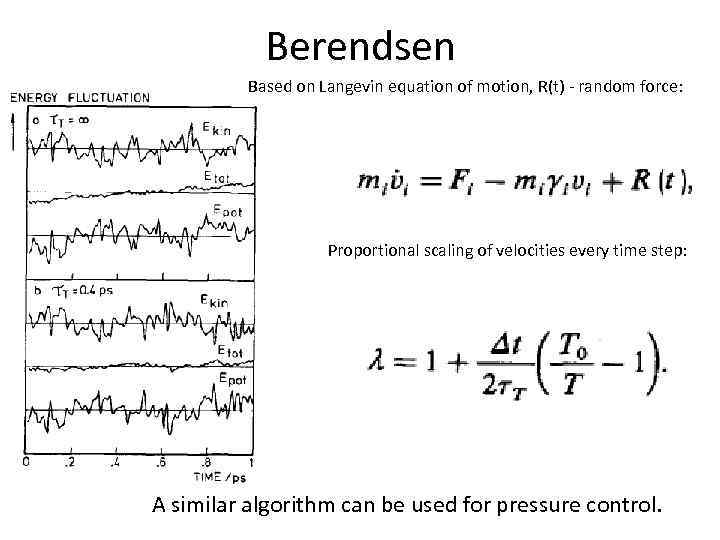 Berendsen Based on Langevin equation of motion, R(t) - random force: Proportional scaling of velocities every time step: A similar algorithm can be used for pressure control.
Berendsen Based on Langevin equation of motion, R(t) - random force: Proportional scaling of velocities every time step: A similar algorithm can be used for pressure control.
 MD potentials There is a huge amount of different potentials developed for MD. But still finding a proper potential is a key to successful simulation. Lennard-Jones type interactions LAMMPS default potentials Coarse-grain interactions atomic interactions meta interactions toy
MD potentials There is a huge amount of different potentials developed for MD. But still finding a proper potential is a key to successful simulation. Lennard-Jones type interactions LAMMPS default potentials Coarse-grain interactions atomic interactions meta interactions toy
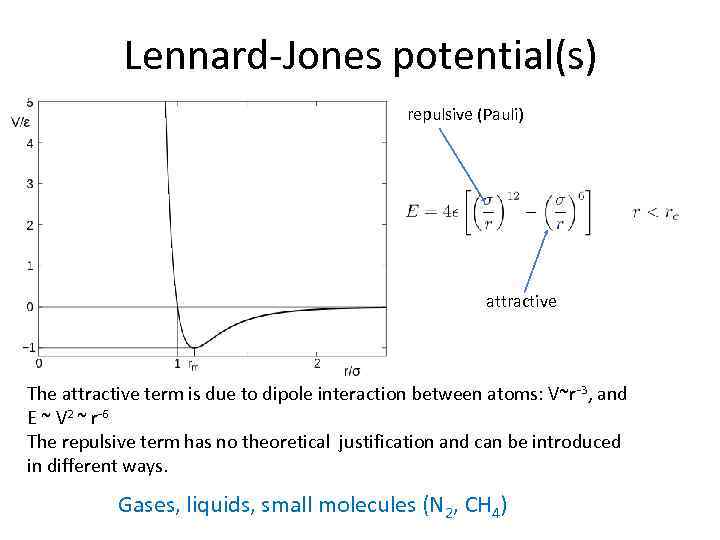 Lennard-Jones potential(s) repulsive (Pauli) attractive The attractive term is due to dipole interaction between atoms: V~r -3, and E ~ V 2 ~ r-6 The repulsive term has no theoretical justification and can be introduced in different ways. Gases, liquids, small molecules (N 2, CH 4)
Lennard-Jones potential(s) repulsive (Pauli) attractive The attractive term is due to dipole interaction between atoms: V~r -3, and E ~ V 2 ~ r-6 The repulsive term has no theoretical justification and can be introduced in different ways. Gases, liquids, small molecules (N 2, CH 4)
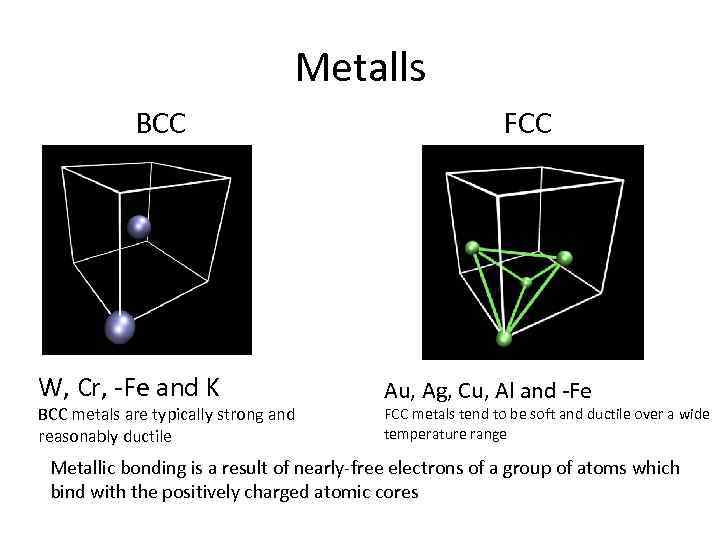 Metalls BCC W, Cr, -Fe and K BCC metals are typically strong and reasonably ductile FCC Au, Ag, Cu, Al and -Fe FCC metals tend to be soft and ductile over a wide temperature range Metallic bonding is a result of nearly-free electrons of a group of atoms which bind with the positively charged atomic cores
Metalls BCC W, Cr, -Fe and K BCC metals are typically strong and reasonably ductile FCC Au, Ag, Cu, Al and -Fe FCC metals tend to be soft and ductile over a wide temperature range Metallic bonding is a result of nearly-free electrons of a group of atoms which bind with the positively charged atomic cores
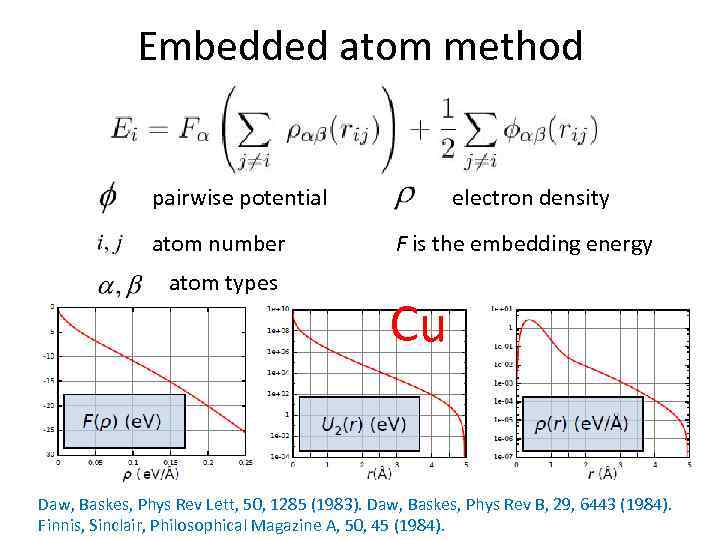 Embedded atom method pairwise potential atom number atom types electron density F is the embedding energy Cu Daw, Baskes, Phys Rev Lett, 50, 1285 (1983). Daw, Baskes, Phys Rev B, 29, 6443 (1984). Finnis, Sinclair, Philosophical Magazine A, 50, 45 (1984).
Embedded atom method pairwise potential atom number atom types electron density F is the embedding energy Cu Daw, Baskes, Phys Rev Lett, 50, 1285 (1983). Daw, Baskes, Phys Rev B, 29, 6443 (1984). Finnis, Sinclair, Philosophical Magazine A, 50, 45 (1984).
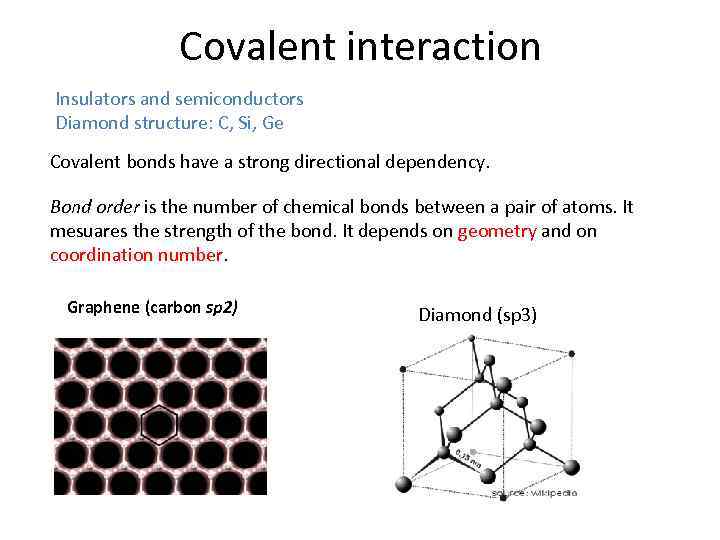 Covalent interaction Insulators and semiconductors Diamond structure: C, Si, Ge Covalent bonds have a strong directional dependency. Bond order is the number of chemical bonds between a pair of atoms. It mesuares the strength of the bond. It depends on geometry and on coordination number. Graphene (carbon sp 2) Diamond (sp 3)
Covalent interaction Insulators and semiconductors Diamond structure: C, Si, Ge Covalent bonds have a strong directional dependency. Bond order is the number of chemical bonds between a pair of atoms. It mesuares the strength of the bond. It depends on geometry and on coordination number. Graphene (carbon sp 2) Diamond (sp 3)
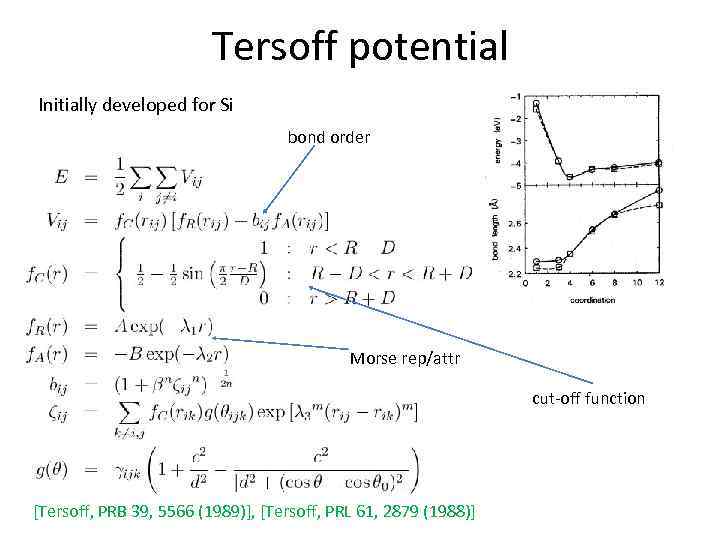 Tersoff potential Initially developed for Si bond order Morse rep/attr cut-off function [Tersoff, PRB 39, 5566 (1989)], [Tersoff, PRL 61, 2879 (1988)]
Tersoff potential Initially developed for Si bond order Morse rep/attr cut-off function [Tersoff, PRB 39, 5566 (1989)], [Tersoff, PRL 61, 2879 (1988)]
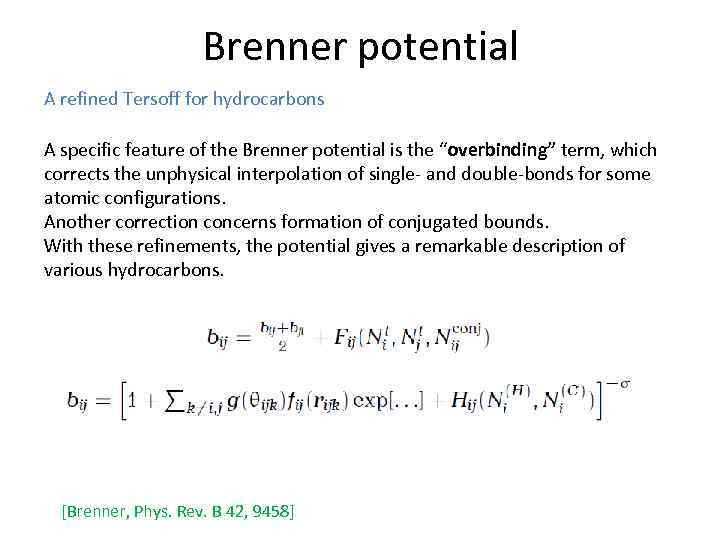 Brenner potential A refined Tersoff for hydrocarbons A specific feature of the Brenner potential is the “overbinding” term, which corrects the unphysical interpolation of single- and double-bonds for some atomic configurations. Another correction concerns formation of conjugated bounds. With these refinements, the potential gives a remarkable description of various hydrocarbons. [Brenner, Phys. Rev. B 42, 9458]
Brenner potential A refined Tersoff for hydrocarbons A specific feature of the Brenner potential is the “overbinding” term, which corrects the unphysical interpolation of single- and double-bonds for some atomic configurations. Another correction concerns formation of conjugated bounds. With these refinements, the potential gives a remarkable description of various hydrocarbons. [Brenner, Phys. Rev. B 42, 9458]
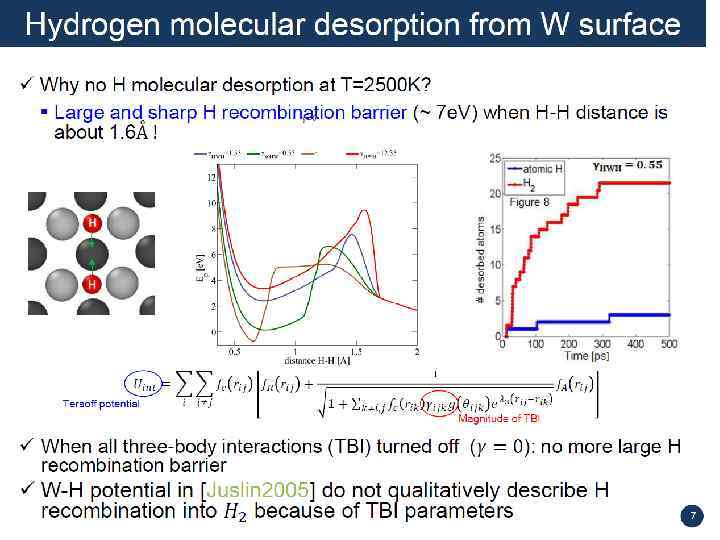
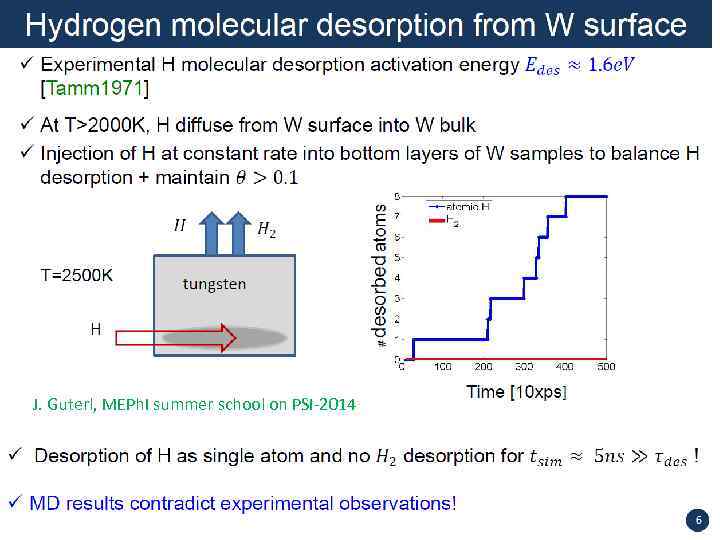 J. Guterl, MEPh. I summer school on PSI-2014
J. Guterl, MEPh. I summer school on PSI-2014
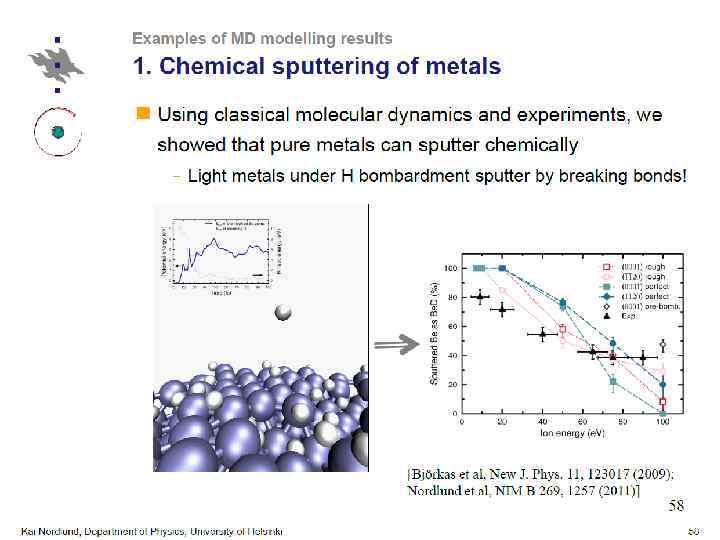
 Conclusions ØMD is a powerful method for modeling of microscopic processes, e. g. atom-surface interactions ØMain principle difficulty lies in constructing a proper potential ØMD should be used with care and only for suitable tasks. Understanding of underlying principles is a must for any simulation
Conclusions ØMD is a powerful method for modeling of microscopic processes, e. g. atom-surface interactions ØMain principle difficulty lies in constructing a proper potential ØMD should be used with care and only for suitable tasks. Understanding of underlying principles is a must for any simulation


