макет_постера.pptx
- Количество слайдов: 3
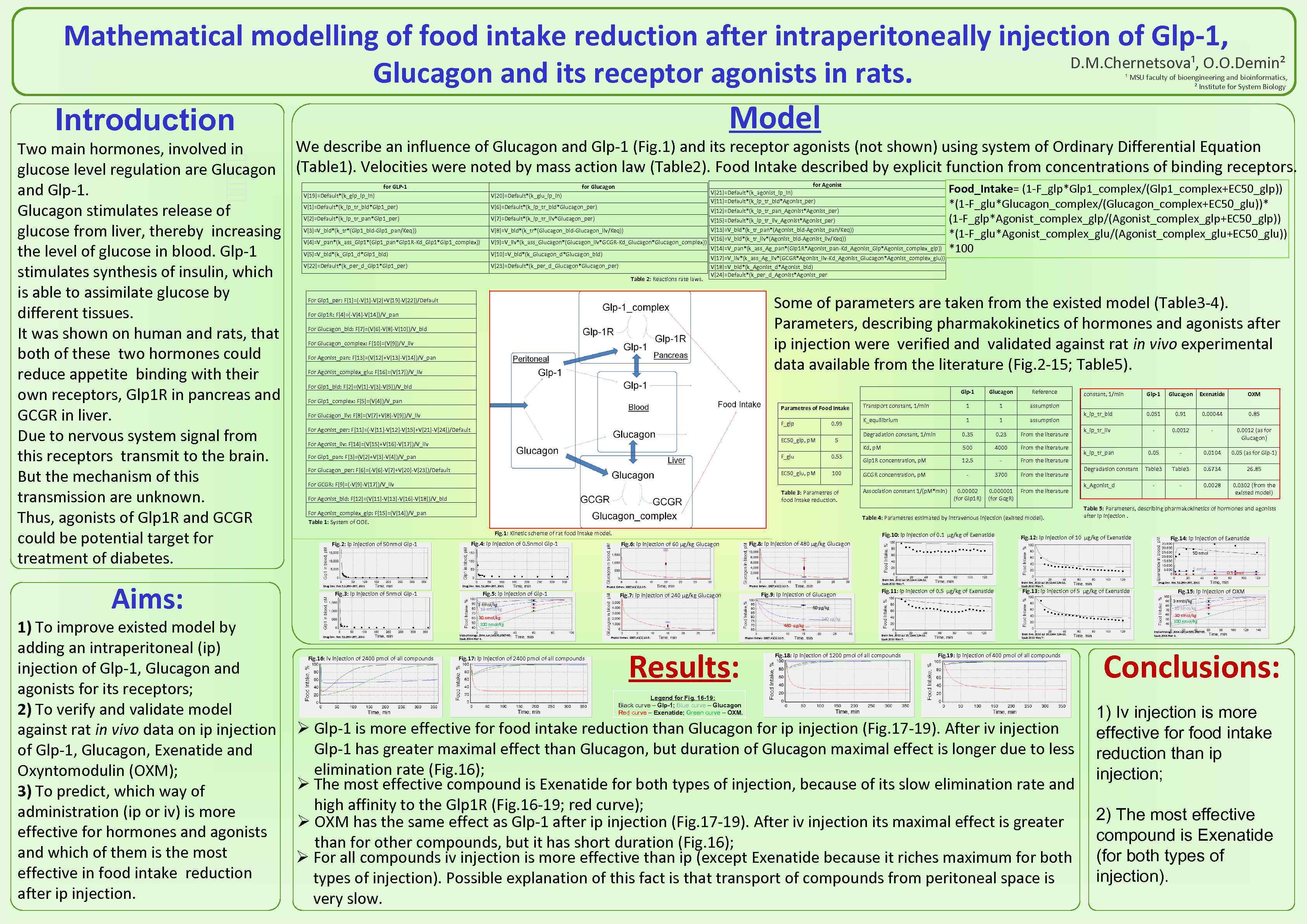 Mathematical modelling of food intake reduction after intraperitoneally injection of Glp-1, D. M. Chernetsova¹, O. O. Demin² Glucagon and its receptor agonists in rats. ¹ MSU faculty of bioengineering and bioinformatics, ² Institute for System Biology Model Introduction We describe an influence of Glucagon and Glp-1 (Fig. 1) and its receptor agonists (not shown) using system of Ordinary Differential Equation Two main hormones, involved in glucose level regulation are Glucagon (Table 1). Velocities were noted by mass action law (Table 2). Food Intake described by explicit function from concentrations of binding receptors. Food_Intake= (1 -F_glp*Glp 1_complex/(Glp 1_complex+EC 50_glp)) and Glp-1. *(1 -F_glu*Glucagon_complex/(Glucagon_complex+EC 50_glu))* Glucagon stimulates release of (1 -F_glp*Agonist_complex_glp/(Agonist_complex_glp+EC 50_glp)) glucose from liver, thereby increasing *(1 -F_glu*Agonist_complex_glu/(Agonist_complex_glu+EC 50_glu)) *100 the level of glucose in blood. Glp-1 stimulates synthesis of insulin, which is able to assimilate glucose by Some of parameters are taken from the existed model (Table 3 -4). different tissues. Parameters, describing pharmakokinetics of hormones and agonists after It was shown on human and rats, that ip injection were verified and validated against rat in vivo experimental both of these two hormones could data available from the literature (Fig. 2 -15; Table 5). reduce appetite binding with their own receptors, Glp 1 R in pancreas and GCGR in liver. Due to nervous system signal from this receptors transmit to the brain. But the mechanism of this transmission are unknown. Thus, agonists of Glp 1 R and GCGR could be potential target for treatment of diabetes. for GLP-1 for Agonist for Glucagon V[21]=Default*(k_agonist_ip_in) V[11]=Default*(k_ip_tr_bld*Agonist_per) V[19]=Default*(k_glp_ip_in) V[20]=Default*(k_glu_ip_in) V[1]=Default*(k_ip_tr_bld*Glp 1_per) V[6]=Default*(k_ip_tr_bld*Glucagon_per) V[2]=Default*(k_ip_tr_pan*Glp 1_per) V[7]=Default*(k_ip_tr_liv*Glucagon_per) V[15]=Default*(k_ip_tr_liv_Agonist*Agonist_per) V[3]=V_bld*(k_tr*(Glp 1_bld-Glp 1_pan/Keq)) V[8]=V_bld*(k_tr*(Glucagon_bld-Glucagon_liv/Keq)) V[4]=V_pan*(k_ass_Glp 1*(Glp 1_pan*Glp 1 R-Kd_Glp 1*Glp 1_complex)) V[9]=V_liv*(k_ass_Glucagon*(Glucagon_liv*GCGR-Kd_Glucagon*Glucagon_complex)) V[13]=V_bld*(k_tr_pan*(Agonist_bld-Agonist_pan/Keq)) V[16]=V_bld*(k_tr_liv*(Agonist_bld-Agonist_liv/Keq)) V[5]=V_bld*(k_Glp 1_d*Glp 1_bld) V[10]=V_bld*(k_Glucagon_d*Glucagon_bld) V[22]=Default*(k_per_d_Glp 1*Glp 1_per) V[23]=Default*(k_per_d_Glucagon*Glucagon_per) V[12]=Default*(k_ip_tr_pan_Agonist*Agonist_per) Table 2: Reactions rate laws. V[14]=V_pan*(k_ass_Ag_pan*(Glp 1 R*Agonist_pan-Kd_Agonist_Glp*Agonist_complex_glp)) V[17]=V_liv*(k_ass_Ag_liv*(GCGR*Agonist_liv-Kd_Agonist_Glucagon*Agonist_complex_glu)) V[18]=V_bld*(k_Agonist_d*Agonist_bld) V[24]=Default*(k_per_d_Agonist*Agonist_per For Glp 1_per: F[1]=(-V[1]-V[2]+V[19]-V[22])/Default For Glp 1 R: F[4]=(-V[4]-V[14])/V_pan For Glucagon_bld: F[7]=(V[6]-V[8]-V[10])/V_bld For Glucagon_complex: F[10]=(V[9])/V_liv For Agonist_pan: F[13]=(V[12]+V[13]-V[14])/V_pan For Agonist_complex_glu: F[16]=(V[17])/V_liv For Glp 1_bld: F[2]=(V[1]-V[3]-V[5])/V_bld Glp-1 For Glp 1_complex: F[5]=(V[4])/V_pan Parametres of Food Intake For Glucagon_liv: F[8]=(V[7]+V[8]-V[9])/V_liv F_glp For Agonist_per: F[11]=(-V[11]-V[12]-V[15]+V[21]-V[24])/Default 0. 99 EC 50_glp, p. M For Agonist_liv: F[14]=(V[15]+V[16]-V[17])/V_liv 5 For Glp 1_pan: F[3]=(V[2]+V[3]-V[4])/V_pan F_glu 0. 53 For Glucagon_per: F[6]=(-V[6]-V[7]+V[20]-V[23])/Default EC 50_glu, p. M 100 For GCGR: F[9]=(-V[9]-V[17])/V_liv Table 3: Parametres of food intake reduction. For Agonist_bld: F[12]=(V[11]-V[13]-V[16]-V[18])/V_bld For Agonist_complex_glp: F[15]=(V[14])/V_pan Table 1: System of ODE. 1 assumption 1 1 assumption Degradation constant, 1/min 0. 35 0. 23 From the literature Kd, p. M 500 4000 From the literature Glp 1 R concentration, p. M 12. 5 - Fig. 4: ip injection of 0. 5 nmol Glp-1 GCGR concentration, p. M - Association constant 1/(p. M*min) 3700 From the literature 0. 00002 (for Glp 1 R) 0. 000001 (for Gcg. R) From the literature Fig. 10: ip injection of 0. 1 μg/kg of Exenatide Fig. 6: ip injection of 60 μg/kg Glucagon constant, 1/min Glp-1 Glucagon Exenatide OXM k_ip_tr_bld 0. 051 0. 91 0. 00044 0. 85 k_ip_tr_liv - 0. 0012 (as for Glucagon) 0. 05 - 0. 0104 0. 05 (as for Glp-1) Table 3 0. 6734 26. 85 - - 0. 0028 0. 0302 (from the existed model) From the literature Table 4: Parametres estimated by intravenous injection (existed model). Fig. 1: Kinetic scheme of rat food intake model. Fig. 2: ip injection of 50 nmol Glp-1 K_equilibrium Reference 1 Transport constant, 1/min Glucagon Fig. 8: ip injection of 480 μg/kg Glucagon k_ip_tr_pan Degradation constant k_Agonist_d Table 5: Parameters, describing pharmakokinetics of hormones and agonists after ip injection. Fig. 12: ip injection of 10 μg/kg of Exenatide Fig. 14: ip injection of Exenatide 50 nmol 5 nmol Aims: 1) To improve existed model by adding an intraperitoneal (ip) injection of Glp-1, Glucagon and agonists for its receptors; 2) To verify and validate model against rat in vivo data on ip injection of Glp-1, Glucagon, Exenatide and Oxyntomodulin (OXM); 3) To predict, which way of administration (ip or iv) is more effective for hormones and agonists and which of them is the most effective in food intake reduction after ip injection. Drug Dev. Res. 53: 260– 267, 2001 Fig. 3: ip injection of 5 nmol Glp-1 Drug Dev. Res. 53: 260– 267, 2001 Fig. 5: ip injection of Glp-1 Physiol Behav. 1987; 41(1): 31 -5. Fig. 7: ip injection of 240 μg/kg Glucagon Physiol Behav. 1987; 41(1): 31 -5. Fig. 9: ip injection of Glucagon 3 nmol/kg 10 nmol/kg Fig. 16: iv injection of 2400 pmol of all compounds Endocrinology. 2004 Jun; 145(6): 2687 -95. Epub 2004 Mar 4. Fig. 17: ip injection of 2400 pmol of all compounds Fig. 11: ip injection of 0. 5 μg/kg of Exenatide Brain Res. 2010 Jul 16; 1344: 124 -33. Epub 2010 May 7. 30 nmol/kg 100 nmol/kg 480 μg/kg Results: Physiol Behav. 1987; 41(1): 31 -5. Fig. 15: ip injection of OXM 3 nmol/kg 10 nmol/kg 240 μg/kg Physiol Behav. 1987; 41(1): 31 -5. Drug Dev. Res. 53: 260– 267, 2001 Fig. 13: ip injection of 5 μg/kg of Exenatide 60 μg/kg 30 nmol/kg 100 nmol/kg Drug Dev. Res. 53: 260– 267, 2001 Brain Res. 2010 Jul 16; 1344: 124 -33. Epub 2010 May 7. 0. 5 nmol Brain Res. 2010 Jul 16; 1344: 124 -33. Epub 2010 May 7. Fig. 18: ip injection of 1200 pmol of all compounds Brain Res. 2010 Jul 16; 1344: 124 -33. Epub 2010 May 7. Fig. 19: ip injection of 400 pmol of all compounds Legend for Fig. 16 -19: Black curve – Glp-1; Blue curve – Glucagon Red curve – Exenatide; Green curve – OXM. Ø Glp-1 is more effective for food intake reduction than Glucagon for ip injection (Fig. 17 -19). After iv injection Glp-1 has greater maximal effect than Glucagon, but duration of Glucagon maximal effect is longer due to less elimination rate (Fig. 16); Ø The most effective compound is Exenatide for both types of injection, because of its slow elimination rate and high affinity to the Glp 1 R (Fig. 16 -19; red curve); Ø OXM has the same effect as Glp-1 after ip injection (Fig. 17 -19). After iv injection its maximal effect is greater than for other compounds, but it has short duration (Fig. 16); Ø For all compounds iv injection is more effective than ip (except Exenatide because it riches maximum for both types of injection). Possible explanation of this fact is that transport of compounds from peritoneal space is very slow. Endocrinology. 2004 Jun; 145(6): 2687 -95. Epub 2004 Mar 4. Conclusions: 1) Iv injection is more effective for food intake reduction than ip injection; 2) The most effective compound is Exenatide (for both types of injection).
Mathematical modelling of food intake reduction after intraperitoneally injection of Glp-1, D. M. Chernetsova¹, O. O. Demin² Glucagon and its receptor agonists in rats. ¹ MSU faculty of bioengineering and bioinformatics, ² Institute for System Biology Model Introduction We describe an influence of Glucagon and Glp-1 (Fig. 1) and its receptor agonists (not shown) using system of Ordinary Differential Equation Two main hormones, involved in glucose level regulation are Glucagon (Table 1). Velocities were noted by mass action law (Table 2). Food Intake described by explicit function from concentrations of binding receptors. Food_Intake= (1 -F_glp*Glp 1_complex/(Glp 1_complex+EC 50_glp)) and Glp-1. *(1 -F_glu*Glucagon_complex/(Glucagon_complex+EC 50_glu))* Glucagon stimulates release of (1 -F_glp*Agonist_complex_glp/(Agonist_complex_glp+EC 50_glp)) glucose from liver, thereby increasing *(1 -F_glu*Agonist_complex_glu/(Agonist_complex_glu+EC 50_glu)) *100 the level of glucose in blood. Glp-1 stimulates synthesis of insulin, which is able to assimilate glucose by Some of parameters are taken from the existed model (Table 3 -4). different tissues. Parameters, describing pharmakokinetics of hormones and agonists after It was shown on human and rats, that ip injection were verified and validated against rat in vivo experimental both of these two hormones could data available from the literature (Fig. 2 -15; Table 5). reduce appetite binding with their own receptors, Glp 1 R in pancreas and GCGR in liver. Due to nervous system signal from this receptors transmit to the brain. But the mechanism of this transmission are unknown. Thus, agonists of Glp 1 R and GCGR could be potential target for treatment of diabetes. for GLP-1 for Agonist for Glucagon V[21]=Default*(k_agonist_ip_in) V[11]=Default*(k_ip_tr_bld*Agonist_per) V[19]=Default*(k_glp_ip_in) V[20]=Default*(k_glu_ip_in) V[1]=Default*(k_ip_tr_bld*Glp 1_per) V[6]=Default*(k_ip_tr_bld*Glucagon_per) V[2]=Default*(k_ip_tr_pan*Glp 1_per) V[7]=Default*(k_ip_tr_liv*Glucagon_per) V[15]=Default*(k_ip_tr_liv_Agonist*Agonist_per) V[3]=V_bld*(k_tr*(Glp 1_bld-Glp 1_pan/Keq)) V[8]=V_bld*(k_tr*(Glucagon_bld-Glucagon_liv/Keq)) V[4]=V_pan*(k_ass_Glp 1*(Glp 1_pan*Glp 1 R-Kd_Glp 1*Glp 1_complex)) V[9]=V_liv*(k_ass_Glucagon*(Glucagon_liv*GCGR-Kd_Glucagon*Glucagon_complex)) V[13]=V_bld*(k_tr_pan*(Agonist_bld-Agonist_pan/Keq)) V[16]=V_bld*(k_tr_liv*(Agonist_bld-Agonist_liv/Keq)) V[5]=V_bld*(k_Glp 1_d*Glp 1_bld) V[10]=V_bld*(k_Glucagon_d*Glucagon_bld) V[22]=Default*(k_per_d_Glp 1*Glp 1_per) V[23]=Default*(k_per_d_Glucagon*Glucagon_per) V[12]=Default*(k_ip_tr_pan_Agonist*Agonist_per) Table 2: Reactions rate laws. V[14]=V_pan*(k_ass_Ag_pan*(Glp 1 R*Agonist_pan-Kd_Agonist_Glp*Agonist_complex_glp)) V[17]=V_liv*(k_ass_Ag_liv*(GCGR*Agonist_liv-Kd_Agonist_Glucagon*Agonist_complex_glu)) V[18]=V_bld*(k_Agonist_d*Agonist_bld) V[24]=Default*(k_per_d_Agonist*Agonist_per For Glp 1_per: F[1]=(-V[1]-V[2]+V[19]-V[22])/Default For Glp 1 R: F[4]=(-V[4]-V[14])/V_pan For Glucagon_bld: F[7]=(V[6]-V[8]-V[10])/V_bld For Glucagon_complex: F[10]=(V[9])/V_liv For Agonist_pan: F[13]=(V[12]+V[13]-V[14])/V_pan For Agonist_complex_glu: F[16]=(V[17])/V_liv For Glp 1_bld: F[2]=(V[1]-V[3]-V[5])/V_bld Glp-1 For Glp 1_complex: F[5]=(V[4])/V_pan Parametres of Food Intake For Glucagon_liv: F[8]=(V[7]+V[8]-V[9])/V_liv F_glp For Agonist_per: F[11]=(-V[11]-V[12]-V[15]+V[21]-V[24])/Default 0. 99 EC 50_glp, p. M For Agonist_liv: F[14]=(V[15]+V[16]-V[17])/V_liv 5 For Glp 1_pan: F[3]=(V[2]+V[3]-V[4])/V_pan F_glu 0. 53 For Glucagon_per: F[6]=(-V[6]-V[7]+V[20]-V[23])/Default EC 50_glu, p. M 100 For GCGR: F[9]=(-V[9]-V[17])/V_liv Table 3: Parametres of food intake reduction. For Agonist_bld: F[12]=(V[11]-V[13]-V[16]-V[18])/V_bld For Agonist_complex_glp: F[15]=(V[14])/V_pan Table 1: System of ODE. 1 assumption 1 1 assumption Degradation constant, 1/min 0. 35 0. 23 From the literature Kd, p. M 500 4000 From the literature Glp 1 R concentration, p. M 12. 5 - Fig. 4: ip injection of 0. 5 nmol Glp-1 GCGR concentration, p. M - Association constant 1/(p. M*min) 3700 From the literature 0. 00002 (for Glp 1 R) 0. 000001 (for Gcg. R) From the literature Fig. 10: ip injection of 0. 1 μg/kg of Exenatide Fig. 6: ip injection of 60 μg/kg Glucagon constant, 1/min Glp-1 Glucagon Exenatide OXM k_ip_tr_bld 0. 051 0. 91 0. 00044 0. 85 k_ip_tr_liv - 0. 0012 (as for Glucagon) 0. 05 - 0. 0104 0. 05 (as for Glp-1) Table 3 0. 6734 26. 85 - - 0. 0028 0. 0302 (from the existed model) From the literature Table 4: Parametres estimated by intravenous injection (existed model). Fig. 1: Kinetic scheme of rat food intake model. Fig. 2: ip injection of 50 nmol Glp-1 K_equilibrium Reference 1 Transport constant, 1/min Glucagon Fig. 8: ip injection of 480 μg/kg Glucagon k_ip_tr_pan Degradation constant k_Agonist_d Table 5: Parameters, describing pharmakokinetics of hormones and agonists after ip injection. Fig. 12: ip injection of 10 μg/kg of Exenatide Fig. 14: ip injection of Exenatide 50 nmol 5 nmol Aims: 1) To improve existed model by adding an intraperitoneal (ip) injection of Glp-1, Glucagon and agonists for its receptors; 2) To verify and validate model against rat in vivo data on ip injection of Glp-1, Glucagon, Exenatide and Oxyntomodulin (OXM); 3) To predict, which way of administration (ip or iv) is more effective for hormones and agonists and which of them is the most effective in food intake reduction after ip injection. Drug Dev. Res. 53: 260– 267, 2001 Fig. 3: ip injection of 5 nmol Glp-1 Drug Dev. Res. 53: 260– 267, 2001 Fig. 5: ip injection of Glp-1 Physiol Behav. 1987; 41(1): 31 -5. Fig. 7: ip injection of 240 μg/kg Glucagon Physiol Behav. 1987; 41(1): 31 -5. Fig. 9: ip injection of Glucagon 3 nmol/kg 10 nmol/kg Fig. 16: iv injection of 2400 pmol of all compounds Endocrinology. 2004 Jun; 145(6): 2687 -95. Epub 2004 Mar 4. Fig. 17: ip injection of 2400 pmol of all compounds Fig. 11: ip injection of 0. 5 μg/kg of Exenatide Brain Res. 2010 Jul 16; 1344: 124 -33. Epub 2010 May 7. 30 nmol/kg 100 nmol/kg 480 μg/kg Results: Physiol Behav. 1987; 41(1): 31 -5. Fig. 15: ip injection of OXM 3 nmol/kg 10 nmol/kg 240 μg/kg Physiol Behav. 1987; 41(1): 31 -5. Drug Dev. Res. 53: 260– 267, 2001 Fig. 13: ip injection of 5 μg/kg of Exenatide 60 μg/kg 30 nmol/kg 100 nmol/kg Drug Dev. Res. 53: 260– 267, 2001 Brain Res. 2010 Jul 16; 1344: 124 -33. Epub 2010 May 7. 0. 5 nmol Brain Res. 2010 Jul 16; 1344: 124 -33. Epub 2010 May 7. Fig. 18: ip injection of 1200 pmol of all compounds Brain Res. 2010 Jul 16; 1344: 124 -33. Epub 2010 May 7. Fig. 19: ip injection of 400 pmol of all compounds Legend for Fig. 16 -19: Black curve – Glp-1; Blue curve – Glucagon Red curve – Exenatide; Green curve – OXM. Ø Glp-1 is more effective for food intake reduction than Glucagon for ip injection (Fig. 17 -19). After iv injection Glp-1 has greater maximal effect than Glucagon, but duration of Glucagon maximal effect is longer due to less elimination rate (Fig. 16); Ø The most effective compound is Exenatide for both types of injection, because of its slow elimination rate and high affinity to the Glp 1 R (Fig. 16 -19; red curve); Ø OXM has the same effect as Glp-1 after ip injection (Fig. 17 -19). After iv injection its maximal effect is greater than for other compounds, but it has short duration (Fig. 16); Ø For all compounds iv injection is more effective than ip (except Exenatide because it riches maximum for both types of injection). Possible explanation of this fact is that transport of compounds from peritoneal space is very slow. Endocrinology. 2004 Jun; 145(6): 2687 -95. Epub 2004 Mar 4. Conclusions: 1) Iv injection is more effective for food intake reduction than ip injection; 2) The most effective compound is Exenatide (for both types of injection).
 Тема работы D. M. Chernetsova¹, O. O. Demin² ¹ MSU faculty of bioengineering and bioinformatics, ² Institute for System Biology Введение Модель Цели: Результаты: Выводы:
Тема работы D. M. Chernetsova¹, O. O. Demin² ¹ MSU faculty of bioengineering and bioinformatics, ² Institute for System Biology Введение Модель Цели: Результаты: Выводы:
 Тема работы D. M. Chernetsova¹, O. O. Demin² ¹ MSU faculty of bioengineering and bioinformatics, ² Institute for System Biology Введение Цели Выводы: Результаты: Материалы и методы Ссылки/благодарности
Тема работы D. M. Chernetsova¹, O. O. Demin² ¹ MSU faculty of bioengineering and bioinformatics, ² Institute for System Biology Введение Цели Выводы: Результаты: Материалы и методы Ссылки/благодарности
