L 29 - Nuclear Physics 2015 Updated.pptx
- Количество слайдов: 34
 Lecture 29: Nuclear Physics 1 OUTLINE 1. Rutherford’s experiment 2. The atomic mass 3. The strong nuclear force 4. Radioactivity 5. The Geiger – Muller Counter
Lecture 29: Nuclear Physics 1 OUTLINE 1. Rutherford’s experiment 2. The atomic mass 3. The strong nuclear force 4. Radioactivity 5. The Geiger – Muller Counter
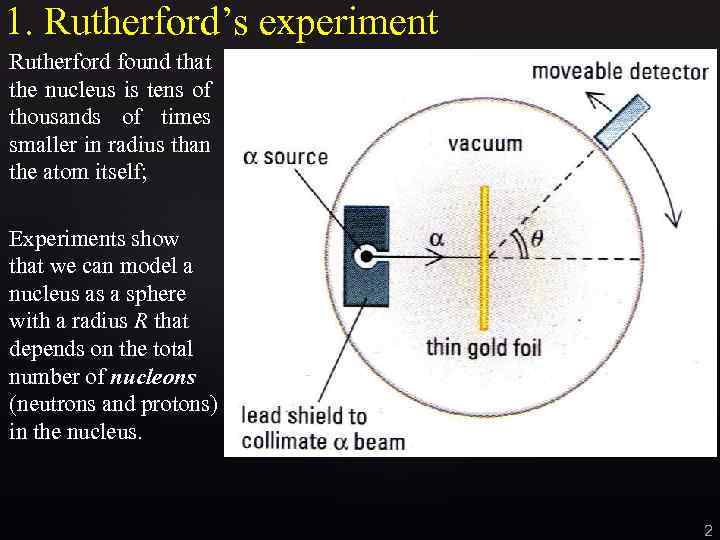 1. Rutherford’s experiment Rutherford found that the nucleus is tens of thousands of times smaller in radius than the atom itself; Experiments show that we can model a nucleus as a sphere with a radius R that depends on the total number of nucleons (neutrons and protons) in the nucleus. 2
1. Rutherford’s experiment Rutherford found that the nucleus is tens of thousands of times smaller in radius than the atom itself; Experiments show that we can model a nucleus as a sphere with a radius R that depends on the total number of nucleons (neutrons and protons) in the nucleus. 2
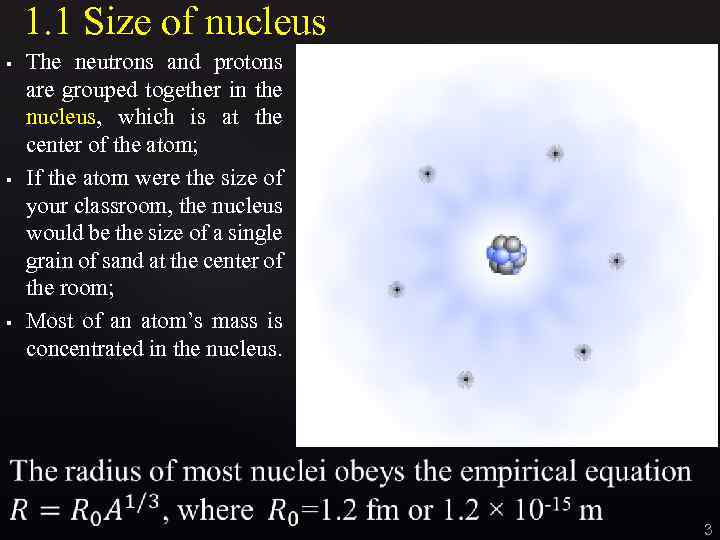 1. 1 Size of nucleus § § § The neutrons and protons are grouped together in the nucleus, which is at the center of the atom; If the atom were the size of your classroom, the nucleus would be the size of a single grain of sand at the center of the room; Most of an atom’s mass is concentrated in the nucleus. 3
1. 1 Size of nucleus § § § The neutrons and protons are grouped together in the nucleus, which is at the center of the atom; If the atom were the size of your classroom, the nucleus would be the size of a single grain of sand at the center of the room; Most of an atom’s mass is concentrated in the nucleus. 3
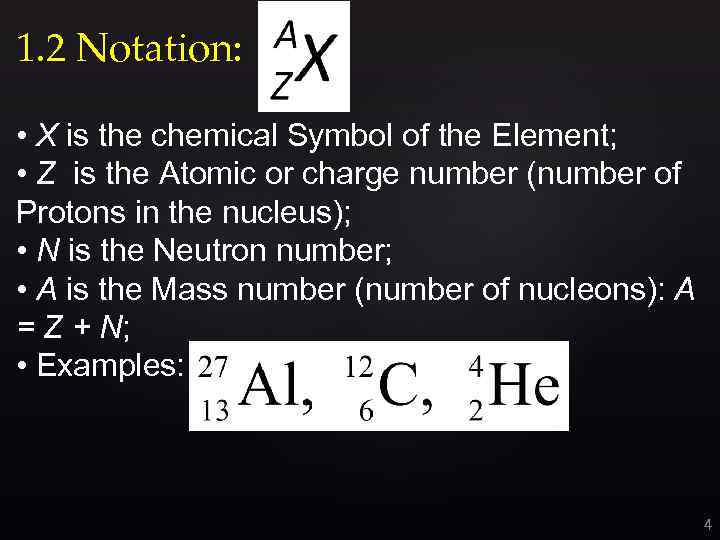 1. 2 Notation: • X is the chemical Symbol of the Element; • Z is the Atomic or charge number (number of Protons in the nucleus); • N is the Neutron number; • A is the Mass number (number of nucleons): A = Z + N; • Examples: 4
1. 2 Notation: • X is the chemical Symbol of the Element; • Z is the Atomic or charge number (number of Protons in the nucleus); • N is the Neutron number; • A is the Mass number (number of nucleons): A = Z + N; • Examples: 4
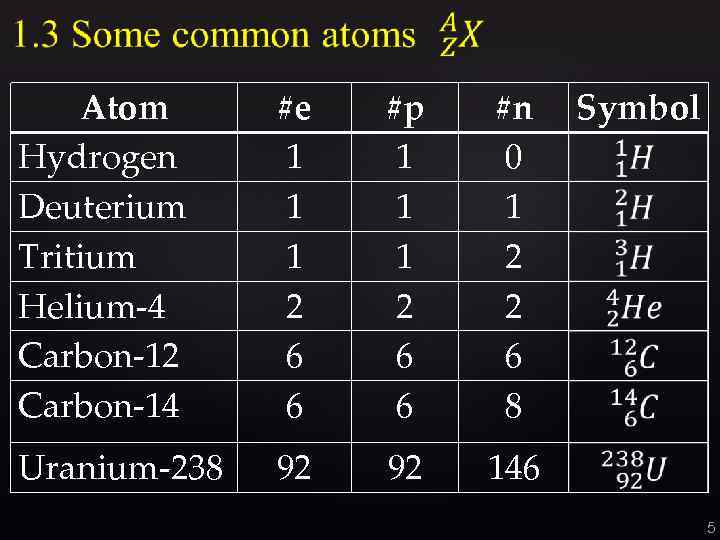 Atom Hydrogen Deuterium Tritium Helium-4 Carbon-12 Carbon-14 #e 1 1 1 2 6 6 #p 1 1 1 2 6 6 #n 0 1 2 2 6 8 Uranium-238 92 92 Symbol 146 5
Atom Hydrogen Deuterium Tritium Helium-4 Carbon-12 Carbon-14 #e 1 1 1 2 6 6 #p 1 1 1 2 6 6 #n 0 1 2 2 6 8 Uranium-238 92 92 Symbol 146 5
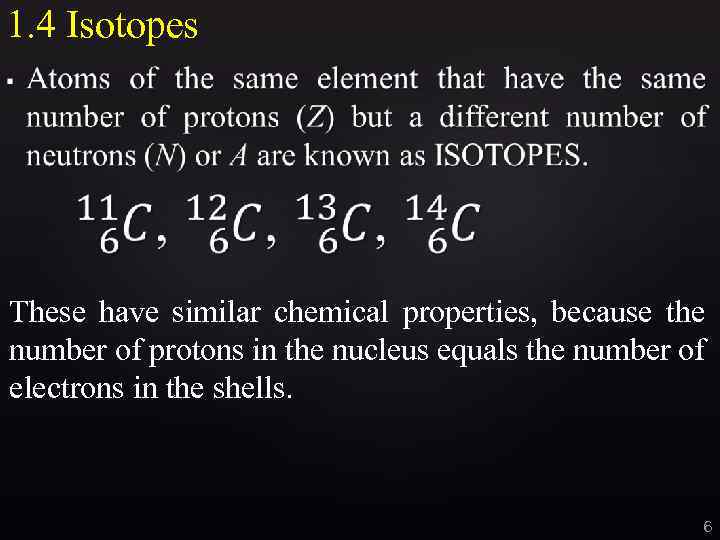 1. 4 Isotopes These have similar chemical properties, because the number of protons in the nucleus equals the number of electrons in the shells. 6
1. 4 Isotopes These have similar chemical properties, because the number of protons in the nucleus equals the number of electrons in the shells. 6
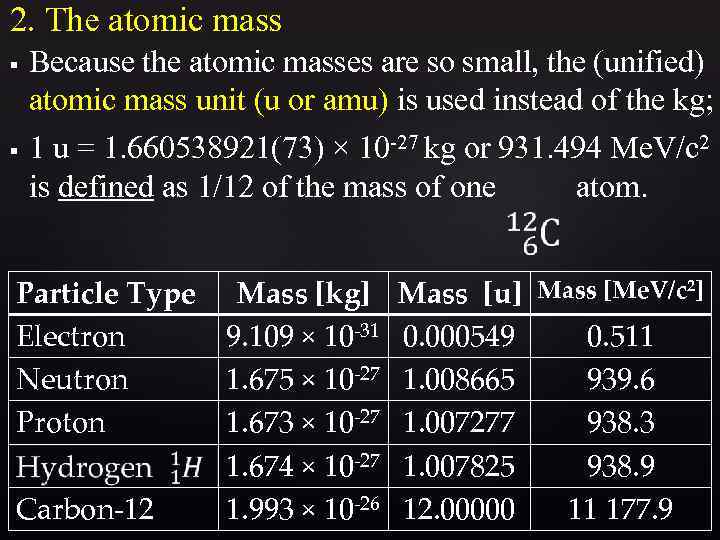 2. The atomic mass § § Because the atomic masses are so small, the (unified) atomic mass unit (u or amu) is used instead of the kg; 1 u = 1. 660538921(73) × 10 -27 kg or 931. 494 Me. V/c 2 is defined as 1/12 of the mass of one atom. Particle Type Mass [kg] Electron 9. 109 × 10 -31 Neutron 1. 675 × 10 -27 Proton 1. 673 × 10 -27 1. 674 × 10 -27 Carbon-12 1. 993 × 10 -26 Mass [u] Mass [Me. V/c 2] 0. 000549 0. 511 1. 008665 939. 6 1. 007277 938. 3 1. 007825 938. 9 12. 00000 11 177. 9
2. The atomic mass § § Because the atomic masses are so small, the (unified) atomic mass unit (u or amu) is used instead of the kg; 1 u = 1. 660538921(73) × 10 -27 kg or 931. 494 Me. V/c 2 is defined as 1/12 of the mass of one atom. Particle Type Mass [kg] Electron 9. 109 × 10 -31 Neutron 1. 675 × 10 -27 Proton 1. 673 × 10 -27 1. 674 × 10 -27 Carbon-12 1. 993 × 10 -26 Mass [u] Mass [Me. V/c 2] 0. 000549 0. 511 1. 008665 939. 6 1. 007277 938. 3 1. 007825 938. 9 12. 00000 11 177. 9
 3. The Strong Nuclear Force What are strong forces? The Helium nucleus contains two protons. They are both positively charged and will repel each other. So why don’t they go flying out the nucleus? There must be another force that holds them together … 8
3. The Strong Nuclear Force What are strong forces? The Helium nucleus contains two protons. They are both positively charged and will repel each other. So why don’t they go flying out the nucleus? There must be another force that holds them together … 8
 3. The Strong Nuclear Force - continued § § It has been shown experimentally that a strong nuclear force acts on all nucleons and it is nearly independent of charge. Electrons do not “feel” this force; Holds protons and neutrons together in a nucleus; Mostly attractive, and it can exceed the Coulomb repulsive force; Short-range force, as it acts over distances comparable to the nucleus size. 9
3. The Strong Nuclear Force - continued § § It has been shown experimentally that a strong nuclear force acts on all nucleons and it is nearly independent of charge. Electrons do not “feel” this force; Holds protons and neutrons together in a nucleus; Mostly attractive, and it can exceed the Coulomb repulsive force; Short-range force, as it acts over distances comparable to the nucleus size. 9
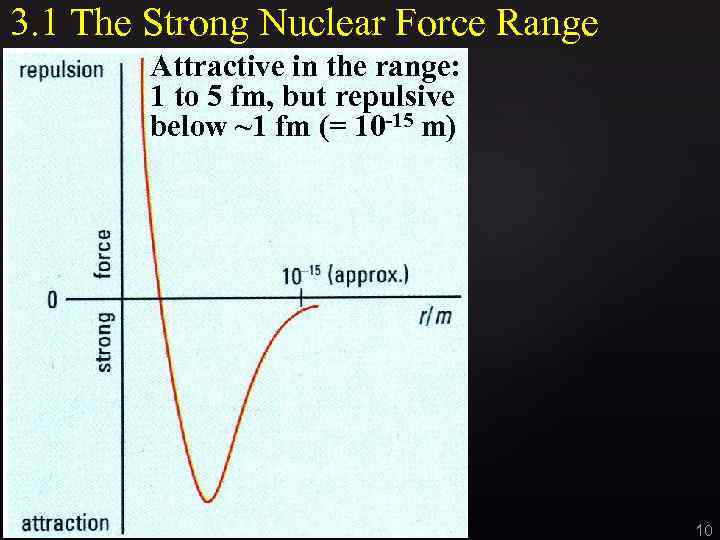 3. 1 The Strong Nuclear Force Range Attractive in the range: 1 to 5 fm, but repulsive below ~1 fm (= 10 -15 m) 10
3. 1 The Strong Nuclear Force Range Attractive in the range: 1 to 5 fm, but repulsive below ~1 fm (= 10 -15 m) 10
 3. 2 Nuclear stability § § Light nuclei (Z < ~20) are most stable if they contain equal numbers of protons and neutrons (i. e. N = Z); N > Z in heavy nuclei (Z > ~20); As the number of protons increases the electrostatic repulsion among them increases, too, and so more neutrons are required to increase the attractive nuclear force among the nucleons; Eventually the Coulomb repulsive force prevails and no stable nuclei exist for Z >83. 11
3. 2 Nuclear stability § § Light nuclei (Z < ~20) are most stable if they contain equal numbers of protons and neutrons (i. e. N = Z); N > Z in heavy nuclei (Z > ~20); As the number of protons increases the electrostatic repulsion among them increases, too, and so more neutrons are required to increase the attractive nuclear force among the nucleons; Eventually the Coulomb repulsive force prevails and no stable nuclei exist for Z >83. 11
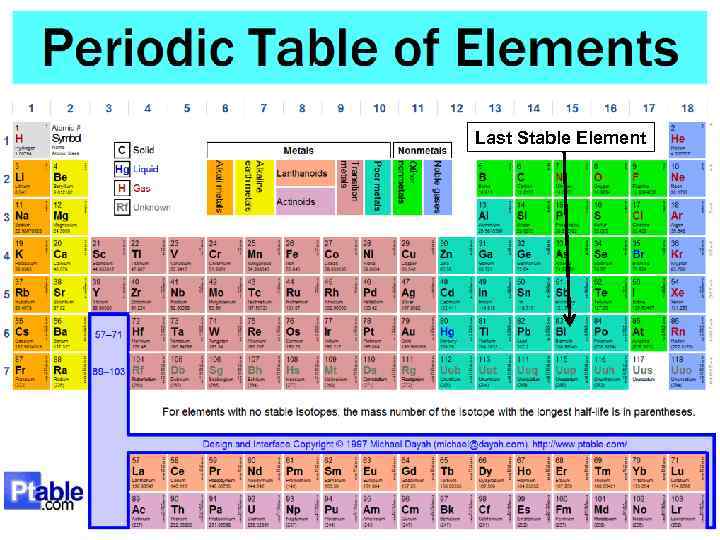 Last Stable Element 12
Last Stable Element 12
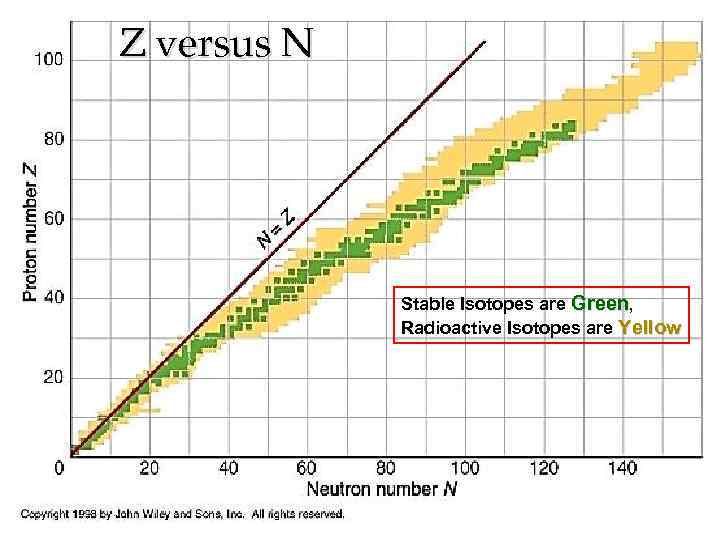 Z versus N Stable Isotopes are Green, Radioactive Isotopes are Yellow 13
Z versus N Stable Isotopes are Green, Radioactive Isotopes are Yellow 13
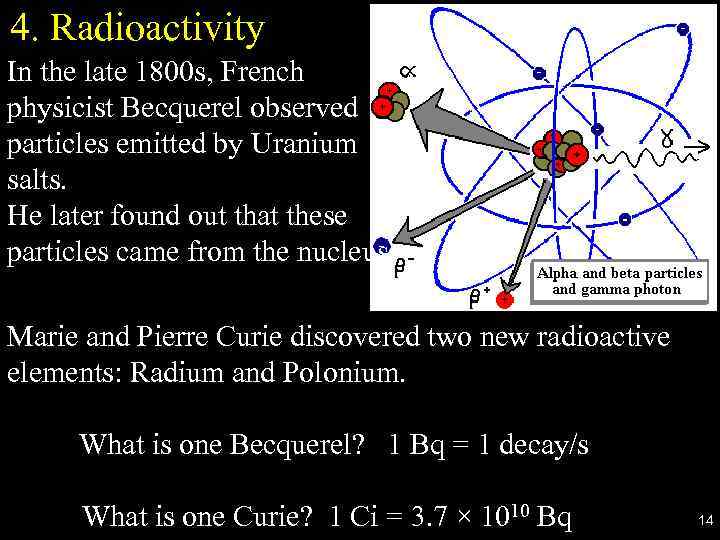 4. Radioactivity In the late 1800 s, French physicist Becquerel observed particles emitted by Uranium salts. He later found out that these particles came from the nucleus. Marie and Pierre Curie discovered two new radioactive elements: Radium and Polonium. What is one Becquerel? 1 Bq = 1 decay/s What is one Curie? 1 Ci = 3. 7 × 1010 Bq 14
4. Radioactivity In the late 1800 s, French physicist Becquerel observed particles emitted by Uranium salts. He later found out that these particles came from the nucleus. Marie and Pierre Curie discovered two new radioactive elements: Radium and Polonium. What is one Becquerel? 1 Bq = 1 decay/s What is one Curie? 1 Ci = 3. 7 × 1010 Bq 14
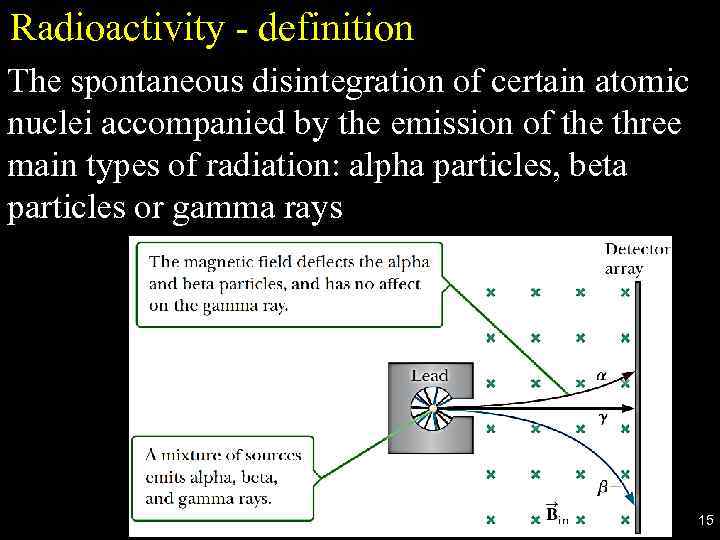 Radioactivity - definition The spontaneous disintegration of certain atomic nuclei accompanied by the emission of the three main types of radiation: alpha particles, beta particles or gamma rays 15
Radioactivity - definition The spontaneous disintegration of certain atomic nuclei accompanied by the emission of the three main types of radiation: alpha particles, beta particles or gamma rays 15
 16
16
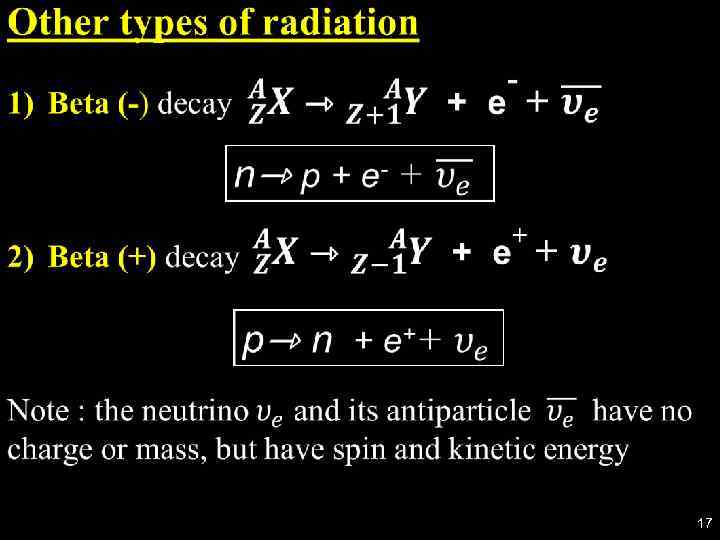 17
17
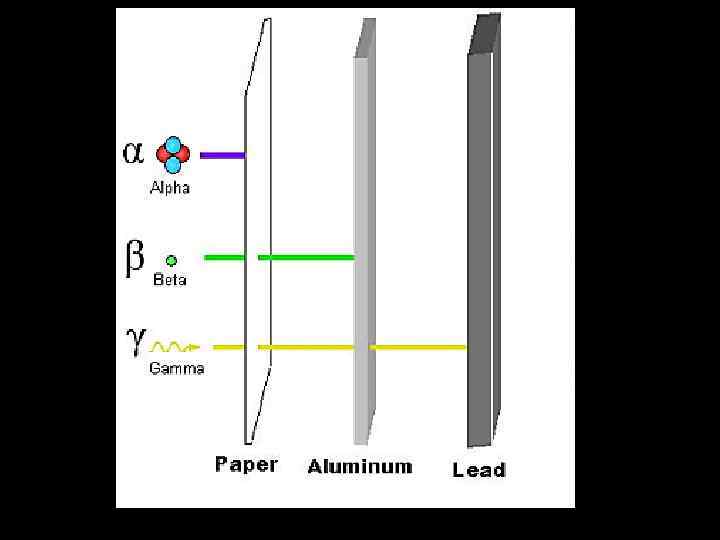
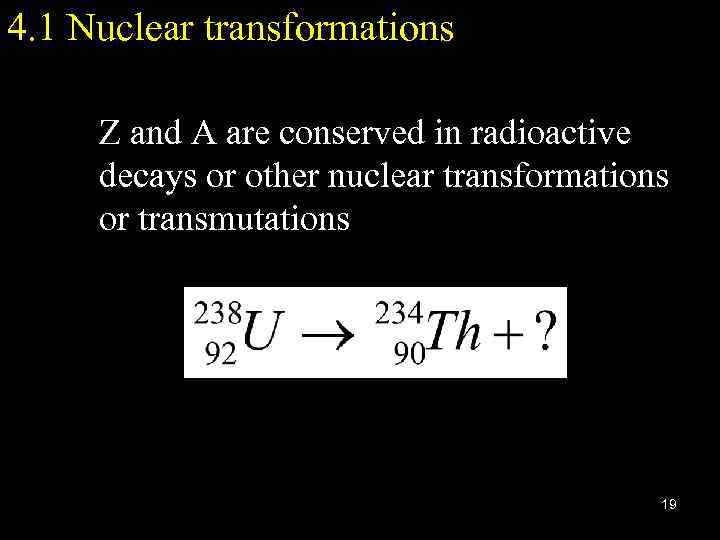 4. 1 Nuclear transformations Z and A are conserved in radioactive decays or other nuclear transformations or transmutations 19
4. 1 Nuclear transformations Z and A are conserved in radioactive decays or other nuclear transformations or transmutations 19
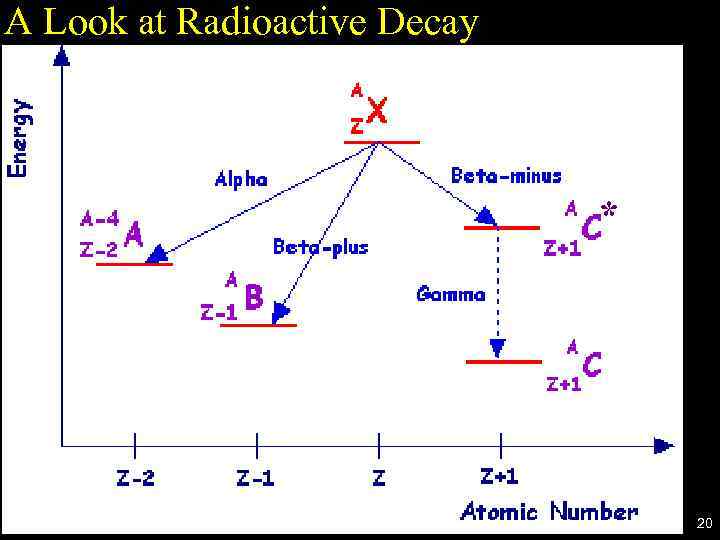 A Look at Radioactive Decay * 20
A Look at Radioactive Decay * 20
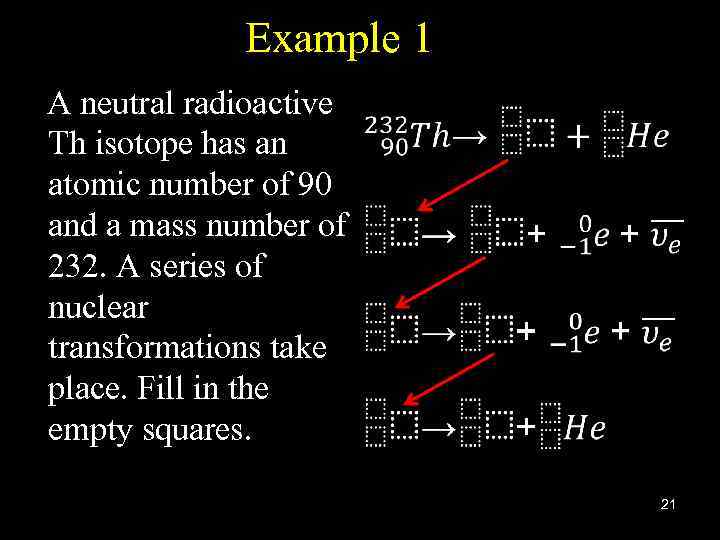 Example 1 A neutral radioactive Th isotope has an atomic number of 90 and a mass number of 232. A series of nuclear transformations take place. Fill in the empty squares. 21
Example 1 A neutral radioactive Th isotope has an atomic number of 90 and a mass number of 232. A series of nuclear transformations take place. Fill in the empty squares. 21
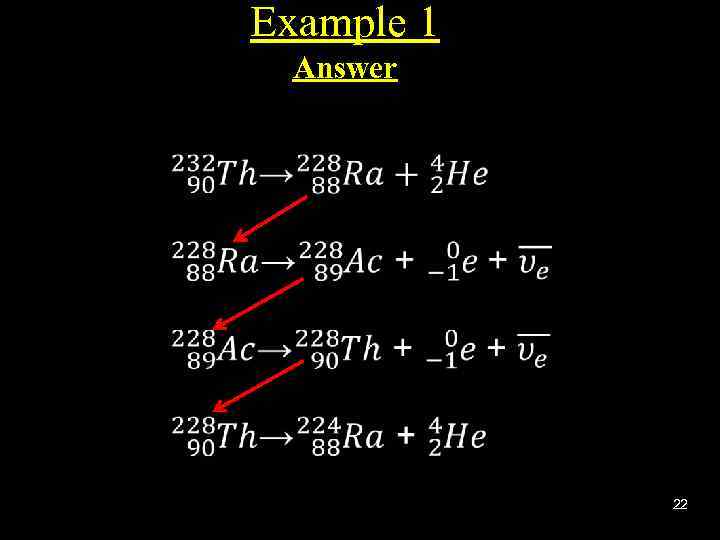 Example 1 Answer 22
Example 1 Answer 22
 4. 2 The Math of Radioactivity • Note that N is a time dependent variable. 23
4. 2 The Math of Radioactivity • Note that N is a time dependent variable. 23
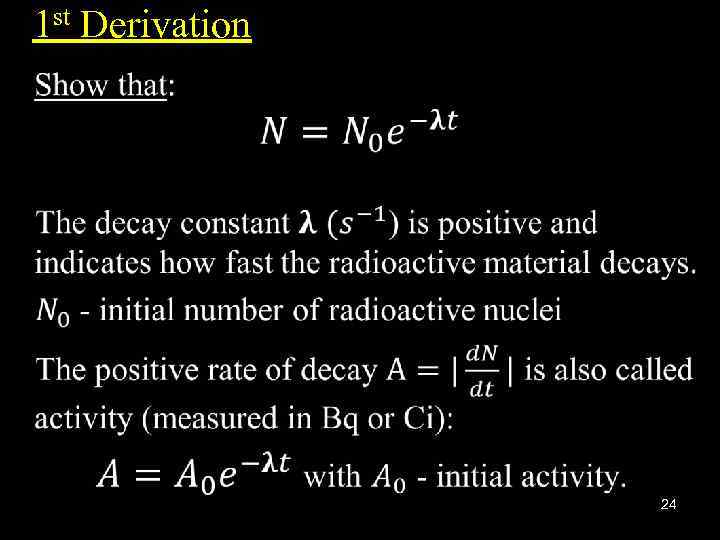 1 st Derivation • 24
1 st Derivation • 24
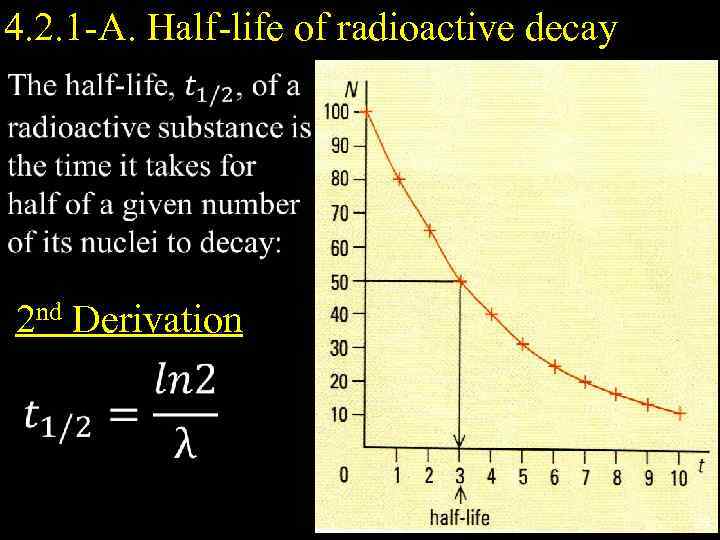 4. 2. 1 -A. Half-life of radioactive decay 2 nd Derivation 25
4. 2. 1 -A. Half-life of radioactive decay 2 nd Derivation 25
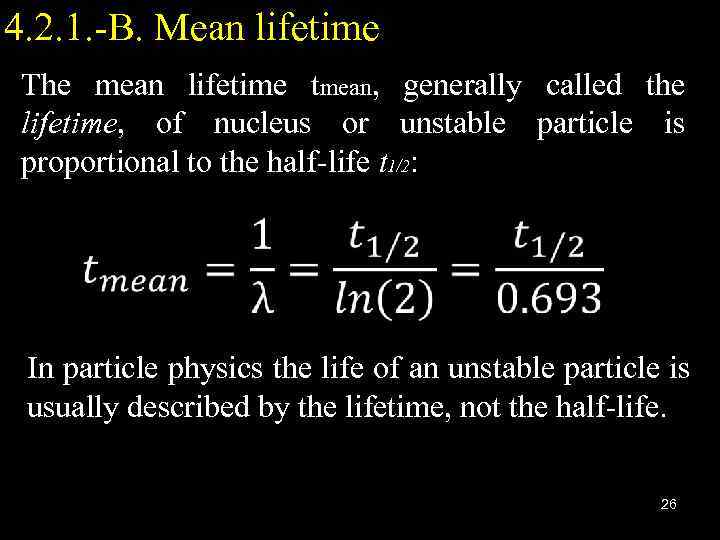 4. 2. 1. -B. Mean lifetime The mean lifetime tmean, generally called the lifetime, of nucleus or unstable particle is proportional to the half-life t 1/2: In particle physics the life of an unstable particle is usually described by the lifetime, not the half-life. 26
4. 2. 1. -B. Mean lifetime The mean lifetime tmean, generally called the lifetime, of nucleus or unstable particle is proportional to the half-life t 1/2: In particle physics the life of an unstable particle is usually described by the lifetime, not the half-life. 26
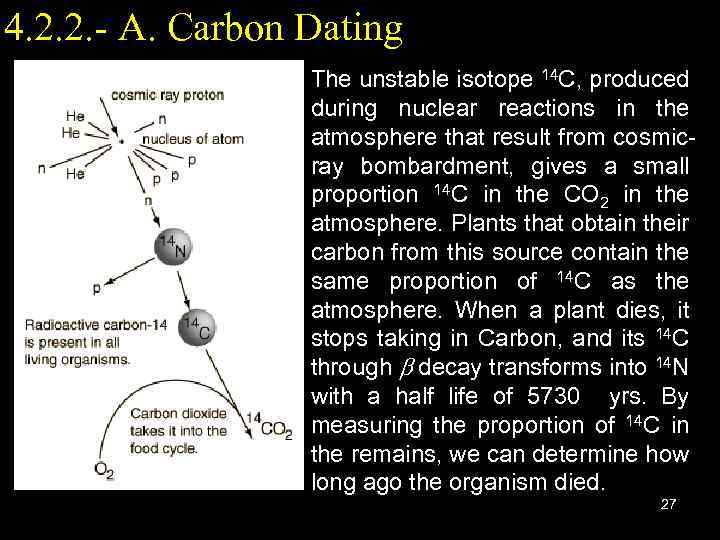 4. 2. 2. - A. Carbon Dating The unstable isotope 14 C, produced during nuclear reactions in the atmosphere that result from cosmicray bombardment, gives a small proportion 14 C in the CO 2 in the atmosphere. Plants that obtain their carbon from this source contain the same proportion of 14 C as the atmosphere. When a plant dies, it stops taking in Carbon, and its 14 C through b decay transforms into 14 N with a half life of 5730 yrs. By measuring the proportion of 14 C in the remains, we can determine how long ago the organism died. 27
4. 2. 2. - A. Carbon Dating The unstable isotope 14 C, produced during nuclear reactions in the atmosphere that result from cosmicray bombardment, gives a small proportion 14 C in the CO 2 in the atmosphere. Plants that obtain their carbon from this source contain the same proportion of 14 C as the atmosphere. When a plant dies, it stops taking in Carbon, and its 14 C through b decay transforms into 14 N with a half life of 5730 yrs. By measuring the proportion of 14 C in the remains, we can determine how long ago the organism died. 27
 4. 2. 2 Avogadro’s number • 28
4. 2. 2 Avogadro’s number • 28
 Example 2 The initial activity of 1. 00 kg of seawater containing K-40 is 12. 0 s-1. The decay constant for K-40 is 1. 60 × 10 -17 s-1. Find: a) the half-life of K-40; b) the amount (mass) of isotope initially present; c) the time taken for the activity to reduce to 10. 0 s-1. 29
Example 2 The initial activity of 1. 00 kg of seawater containing K-40 is 12. 0 s-1. The decay constant for K-40 is 1. 60 × 10 -17 s-1. Find: a) the half-life of K-40; b) the amount (mass) of isotope initially present; c) the time taken for the activity to reduce to 10. 0 s-1. 29
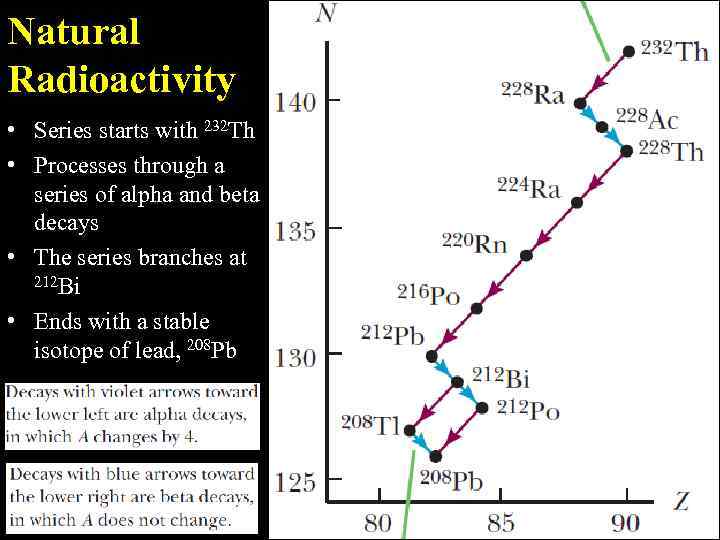 Natural Radioactivity • Series starts with 232 Th • Processes through a series of alpha and beta decays • The series branches at 212 Bi • Ends with a stable isotope of lead, 208 Pb
Natural Radioactivity • Series starts with 232 Th • Processes through a series of alpha and beta decays • The series branches at 212 Bi • Ends with a stable isotope of lead, 208 Pb
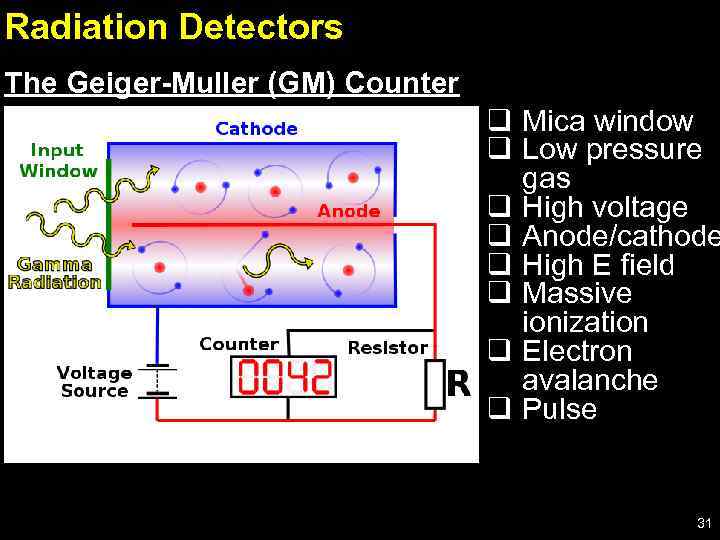 Radiation Detectors The Geiger-Muller (GM) Counter q Mica window q Low pressure gas q High voltage q Anode/cathode q High E field q Massive ionization q Electron avalanche q Pulse 31
Radiation Detectors The Geiger-Muller (GM) Counter q Mica window q Low pressure gas q High voltage q Anode/cathode q High E field q Massive ionization q Electron avalanche q Pulse 31
 GM Detector – cont’d - It detects individual radiation particles or photons; - It can be connected directly to a counter; - Radiation enters the tube through the thin mica window (good for α – particles), and ionizes the low pressure gas, e. g. air or argon; - Electricity conduction is improved at low pressure; - The free electrons are accelerated towards the anode by the high voltage producing more ionizations and free electrons; - An avalanche of electrons arrives at the anode, which is detected as a voltage pulse that can be counted electronically. 32
GM Detector – cont’d - It detects individual radiation particles or photons; - It can be connected directly to a counter; - Radiation enters the tube through the thin mica window (good for α – particles), and ionizes the low pressure gas, e. g. air or argon; - Electricity conduction is improved at low pressure; - The free electrons are accelerated towards the anode by the high voltage producing more ionizations and free electrons; - An avalanche of electrons arrives at the anode, which is detected as a voltage pulse that can be counted electronically. 32
 READING: 1)Adams and Allday (8. 11 -8. 22) 2) Serway/Vuille (29. 1, 29. 3, 29. 4, 29. 5) o o o Know that the nucleus of an atom is made of protons and neutrons, collectively called nucleons, and the meaning of the Z, N and A numbers; Be able to define the atomic mass unit (amu); Know what strong nuclear force is and which particles feel it; Be able to explain nuclear stability and the Z vs N or N vs Z trend; Be able to define Radioactivity and list the 3 types of known radiation emitted by a radioactive substance, including their general nuclear reaction equations and basic properties; Understand that radioactive decay is a spontaneous process (no external stimulation) involving the emission of particles or photons; Be able to define activity, decay constant, half-life and isotope; Know the equation relating the number of unstable nuclei to the decay rate and be able to derive the equation for the number of remaining nuclei as a function of time and initial number of nuclei; Know the equation for activity as a function of the number of unstable nuclei and time, and be able to derive an equation for half-life as a function of the decay constant; Be able to plot activity, and the number of unstable nuclei vs. time; Know what nuclear transformations or transmutations are and be able to use the conservation of Z and A to find unknown radioactive decay elements; Understand be able to solve the recommended Lecture, PSC and CW questions. 33
READING: 1)Adams and Allday (8. 11 -8. 22) 2) Serway/Vuille (29. 1, 29. 3, 29. 4, 29. 5) o o o Know that the nucleus of an atom is made of protons and neutrons, collectively called nucleons, and the meaning of the Z, N and A numbers; Be able to define the atomic mass unit (amu); Know what strong nuclear force is and which particles feel it; Be able to explain nuclear stability and the Z vs N or N vs Z trend; Be able to define Radioactivity and list the 3 types of known radiation emitted by a radioactive substance, including their general nuclear reaction equations and basic properties; Understand that radioactive decay is a spontaneous process (no external stimulation) involving the emission of particles or photons; Be able to define activity, decay constant, half-life and isotope; Know the equation relating the number of unstable nuclei to the decay rate and be able to derive the equation for the number of remaining nuclei as a function of time and initial number of nuclei; Know the equation for activity as a function of the number of unstable nuclei and time, and be able to derive an equation for half-life as a function of the decay constant; Be able to plot activity, and the number of unstable nuclei vs. time; Know what nuclear transformations or transmutations are and be able to use the conservation of Z and A to find unknown radioactive decay elements; Understand be able to solve the recommended Lecture, PSC and CW questions. 33
 Answers to Examples 34
Answers to Examples 34


