386736c14e17c5c41121ee0f4ed64486.ppt
- Количество слайдов: 81

Immunofluorescence in Dermatopathology
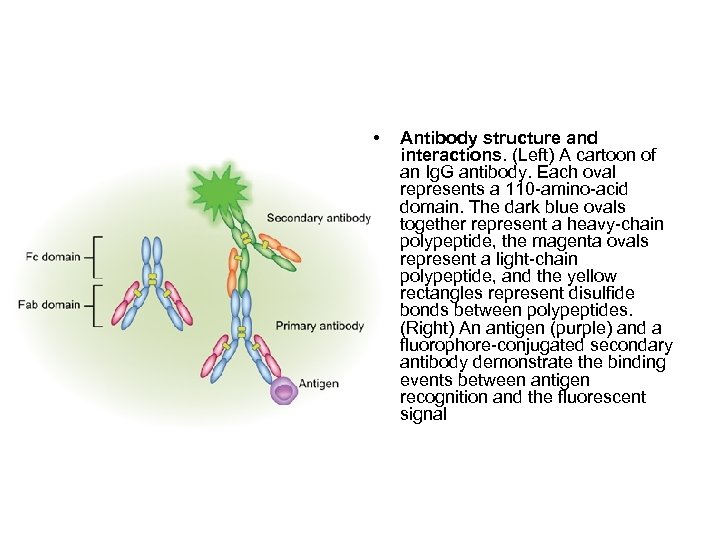
• Antibody structure and interactions. (Left) A cartoon of an Ig. G antibody. Each oval represents a 110 -amino-acid domain. The dark blue ovals together represent a heavy-chain polypeptide, the magenta ovals represent a light-chain polypeptide, and the yellow rectangles represent disulfide bonds between polypeptides. (Right) An antigen (purple) and a fluorophore-conjugated secondary antibody demonstrate the binding events between antigen recognition and the fluorescent signal
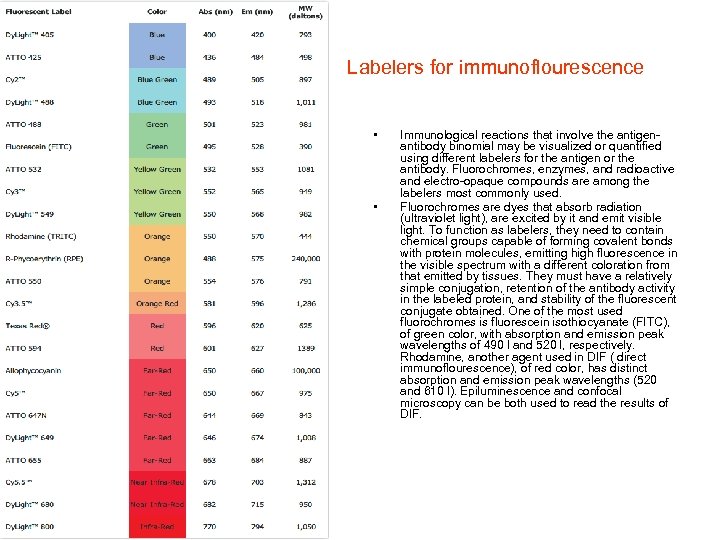
Labelers for immunoflourescence • • Immunological reactions that involve the antigenantibody binomial may be visualized or quantified using different labelers for the antigen or the antibody. Fluorochromes, enzymes, and radioactive and electro-opaque compounds are among the labelers most commonly used. Fluorochromes are dyes that absorb radiation (ultraviolet light), are excited by it and emit visible light. To function as labelers, they need to contain chemical groups capable of forming covalent bonds with protein molecules, emitting high fluorescence in the visible spectrum with a different coloration from that emitted by tissues. They must have a relatively simple conjugation, retention of the antibody activity in the labeled protein, and stability of the fluorescent conjugate obtained. One of the most used fluorochromes is fluorescein isothiocyanate (FITC), of green color, with absorption and emission peak wavelengths of 490 l and 520 l, respectively. Rhodamine, another agent used in DIF ( direct immunoflourescence), of red color, has distinct absorption and emission peak wavelengths (520 and 610 l). Epiluminescence and confocal microscopy can be both used to read the results of DIF.

• Confocal Microscope • Epiluminescence and confocal microscopy can be both used to read the results of DIF(direct immunoflourescence)

immunofluorescence studies are vital for the laboratory diagnosis of autoimmune bullous dermatosis, but they are also important in the investigation of other diseases, such as inflammatory dermatosis (lupus erythematosus, lichen planus, porphyrias, vasculitis). • • . By direct immunoflourescence (DIF), presence of immune complexes in the skin biopsy at various locations, e. g. , at the dermoepidermal junction (DEJ), upper dermal blood vessels, cytoid bodies, and intraepidermal intercellular spaces, etc. , helps us to arrive at a definite diagnosis. "Lupus band test" (LBT) is most common pattern observed on DIF examination of skin biopsies of patients suffering from connective tissue diseases. In addition, DIF microscopy of the skin has also disclosed antibodies bound to epidermal cell nuclei in several connective tissue disorders also known as in vivo ANA (antinuclear antibody) phenomenon or epidermal nuclear staining (ENS) which presents as keratinocyte nuclear fluorescence. Circulating ANAs are commonly found in patients with connective tissue disease.

Site of biopsy • • The best site and evolution time of skin lesions to perform biopsy for direct immunofluorescence examination (DIF) depend on the disease under investigation. Generally, the biopsy should have an appropriate extension (4 mm punch) and depth that involves both the epidermis and dermis in sufficient proportion. In addition, the sample will be better for analysis when fewer traumas are involved in the procedure. Fluorochromes, enzymes, and radioactive and electro-opaque compounds are among the labelers most commonly used. The following sites are recommended for biopsy: In autoimmune vesico-bullous dermatosis, the best site is the perilesional region; In collagenosis, the biospy should be done in the active lesion in evolution (avoid recent lesions, with less than 60 days); In vasculitis, preference should be given to recent lesions with up to 24 hours of evolution. After the procedure, the material can be immediately frozen in liquid nitrogen or placed in a proper transport medium - Michel's medium. 7 Michel's medium is composed of ammonium sulphate, Nethyl-maleimide, and magnesium sulphate in a citrate buffer, which allows the conservation of the specimen for up to two weeks. The specimen is then sectioned in a cryostat into 4 -micron fragments. Primary anti-human antibodies conjugated to FITC fluorescein (anti-Ig. A, anti-ig. G, anti-Ig. M, and anti-C 3) are applied to each section and the reading is done on fluorescence microscopy The indirect immunoflourescence (IIF) technique employed in studies of circulating antibodies in vesico- bullous dermatosis (VBD) uses the healthy epithelium as substrate. Substrates vary based on the protocols of each laboratory: healthy human skin obtained from prepuce, breasts or eyelids ideal ( site, good antigenicity), as a substitute for monkey esophagus

Direct immunoflourescence • • • A. Epithelium: I. Intercellular flourescence: Pemphigus vulgaris, pemphigus folacious, pemphigus herpitiformis , Paraneoplastic pemphigus , Ig. A pemphigus ( two types: subcorneal pustular dermatosis, & intra-epidermal neutrophilic dermatosis) II. Flourescence of the nuclei of keratinocytes (in vivo ANF): Lupus erythematosis, mixed connective tissue syndrome, overlap syndrome, vasculitis B. Basement membrane zone: Lupus erythematosus, Vasculitis, Lichen planus, porphyrias There are different fluorescence patterns of the BMZ. The most frequent are linear, homogeneous, granulous and reticulate. I. LINEAR IGG AND/OR C 3 DEPOSITS IN THE BASEMENT MEMBRANE ZONE: Bullous pemphigoid, pemphigoid gestationis or herpes gestationis II. MULTIPLE LINEAR DEPOSITS (IGA, IGG, IGM AND/OR C 3) IN THE BASEMENT MEMBRANE ZONE: Epidermolysis bullosa acqusita, bullous systemic lupus eryhematosus • III. MULTIPLE LINEAR DEPOSITS (IGA, IGG, IGM AND/OR C 3) IN THE BASEMENT MEMBRANE ZONE: Linear Ig. A bullous dermatosis • • C. DERMAL FLUORESCENCE: Dermatitis herpitiformis, vasculitis, lichen planus, porphyrias, lupus erythematosus
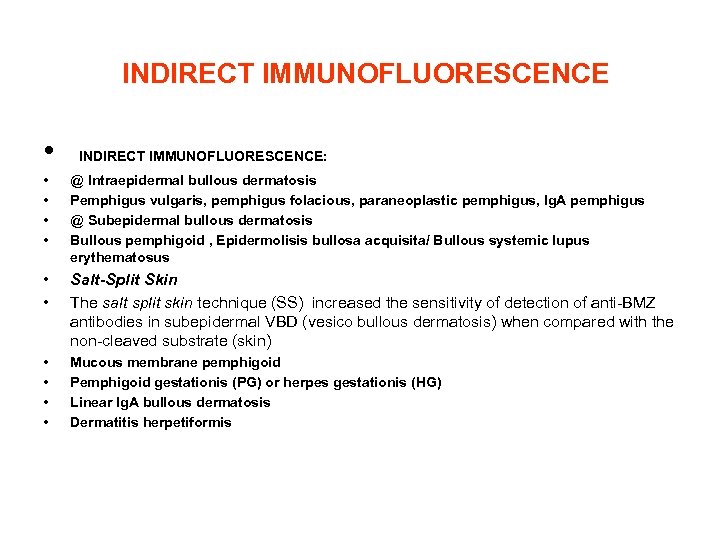
INDIRECT IMMUNOFLUORESCENCE • INDIRECT IMMUNOFLUORESCENCE: • • @ Intraepidermal bullous dermatosis Pemphigus vulgaris, pemphigus folacious, paraneoplastic pemphigus, Ig. A pemphigus @ Subepidermal bullous dermatosis Bullous pemphigoid , Epidermolisis bullosa acquisita/ Bullous systemic lupus erythematosus • • Salt-Split Skin The salt split skin technique (SS) increased the sensitivity of detection of anti-BMZ antibodies in subepidermal VBD (vesico bullous dermatosis) when compared with the non-cleaved substrate (skin) • • Mucous membrane pemphigoid Pemphigoid gestationis (PG) or herpes gestationis (HG) Linear Ig. A bullous dermatosis Dermatitis herpetiformis
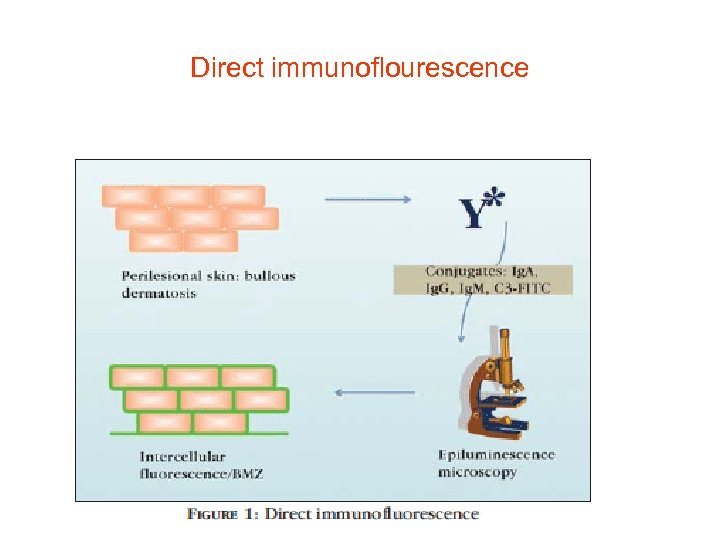
Direct immunoflourescence

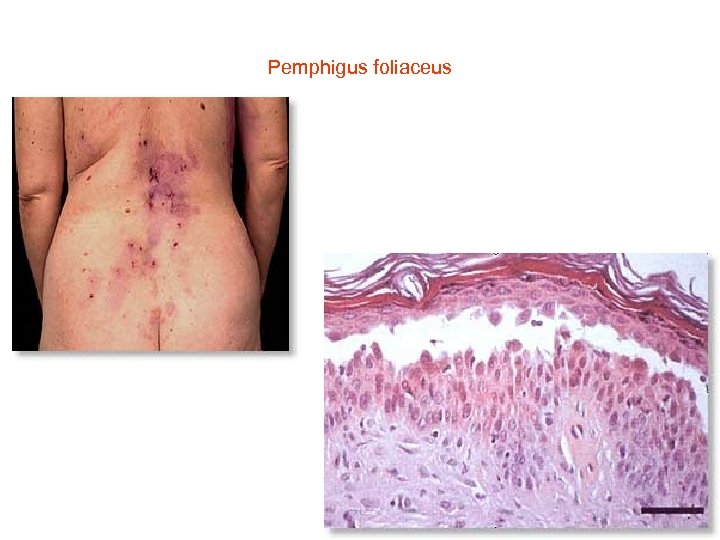
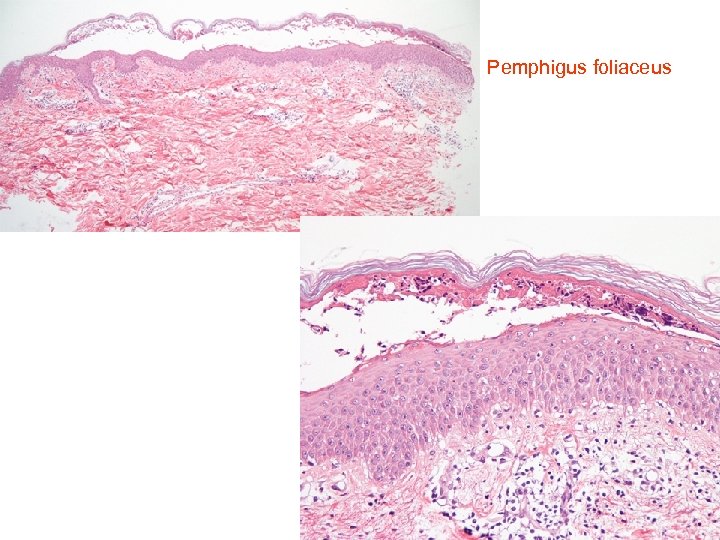
Pemphigus foliaceus • • Histology of pemphigus foliaceus includes loss of the stratum corneum, increased prominence of the granular layer, or visible superficial epidermal separation with blister formation. At higher magnification subtle acanthloysis and spongiosis can be seen within the stratum granulosum, extending into the stratum corneum. This can form separation within the superficial epidermis, or as mentioned above, lead to complete loss of the stratum corneum. The prominent granular layer is seen as hyperchromasia of the nuclei within dyskeratotic cells in this layer, similar to the grains seen in Dariers disease. In the dermis there is a predominantly superficial lymphocytic infiltrate with scattered eosinophils. Neutrophils may be more common in the Ig. A subtype. If there is clinical suspicion for pemphigus foliaceus but little to see on first inspection, remember to assess the hair follicles, as early changes may be seen here. The level of cleavage allows us to differentiate the two main forms of pemphigus: pemphigus vulgaris and pemphigus foliaceus. In pemphigus vulgaris (PV), the cleavage is suprabasal, whereas in pemphigus foliaceus (PF) it is intramalpighian. Direct immunofluorescence reveals intercellular fluorescence, of linear pattern, intraepidermal
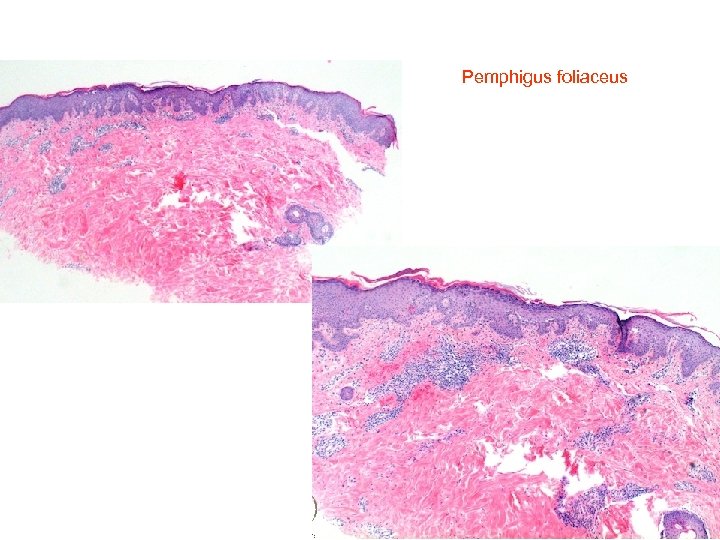
Pemphigus foliaceus
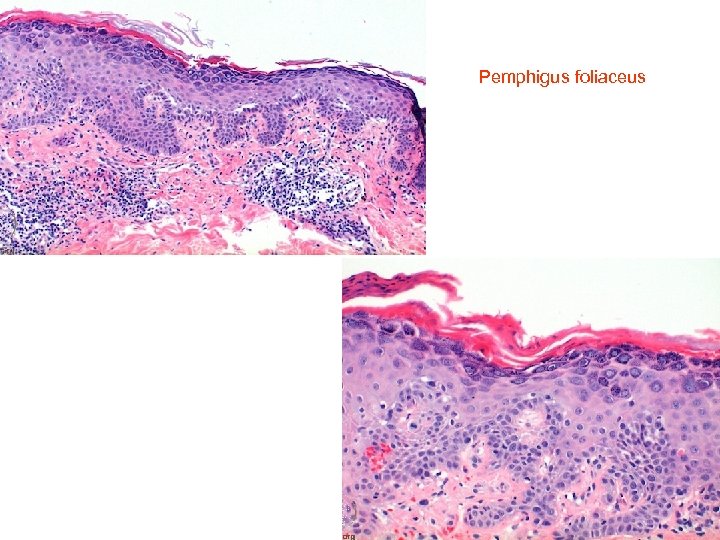
Pemphigus foliaceus
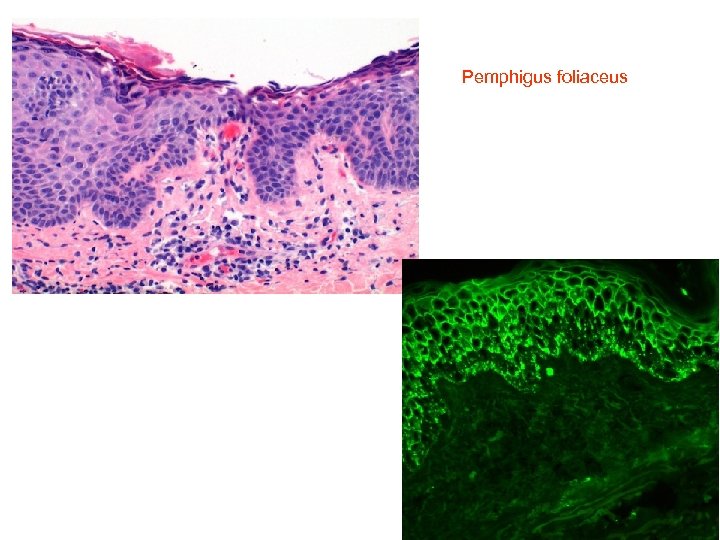
Pemphigus foliaceus
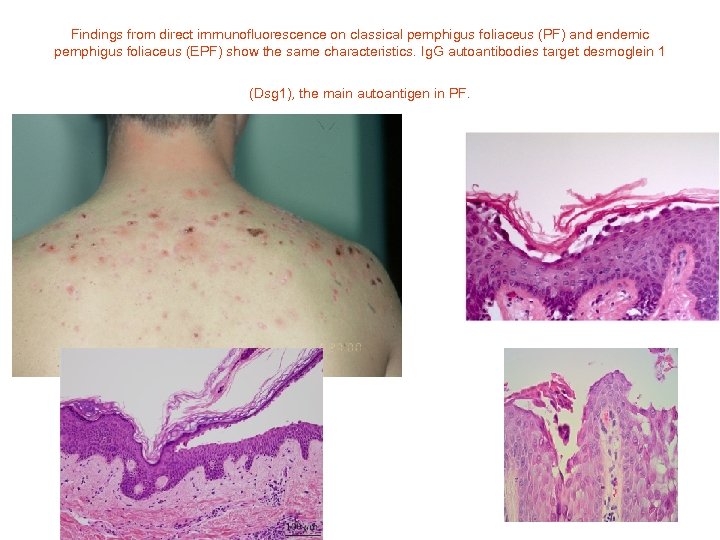
Findings from direct immunofluorescence on classical pemphigus foliaceus (PF) and endemic pemphigus foliaceus (EPF) show the same characteristics. Ig. G autoantibodies target desmoglein 1 (Dsg 1), the main autoantigen in PF.
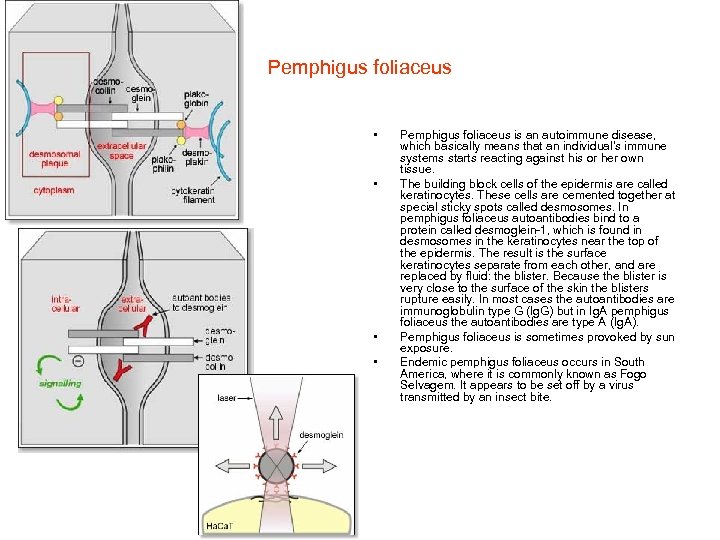
Pemphigus foliaceus • • Pemphigus foliaceus is an autoimmune disease, which basically means that an individual's immune systems starts reacting against his or her own tissue. The building block cells of the epidermis are called keratinocytes. These cells are cemented together at special sticky spots called desmosomes. In pemphigus foliaceus autoantibodies bind to a protein called desmoglein-1, which is found in desmosomes in the keratinocytes near the top of the epidermis. The result is the surface keratinocytes separate from each other, and are replaced by fluid: the blister. Because the blister is very close to the surface of the skin the blisters rupture easily. In most cases the autoantibodies are immunoglobulin type G (Ig. G) but in Ig. A pemphigus foliaceus the autoantibodies are type A (Ig. A). Pemphigus foliaceus is sometimes provoked by sun exposure. Endemic pemphigus foliaceus occurs in South America, where it is commonly known as Fogo Selvagem. It appears to be set off by a virus transmitted by an insect bite.
Pemphigus foliaceus
Pemphigus foliaceus
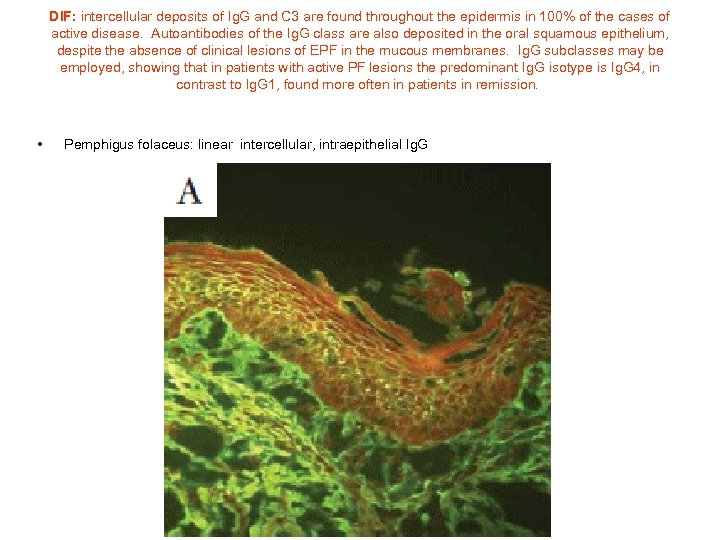
DIF: intercellular deposits of Ig. G and C 3 are found throughout the epidermis in 100% of the cases of active disease. Autoantibodies of the Ig. G class are also deposited in the oral squamous epithelium, despite the absence of clinical lesions of EPF in the mucous membranes. Ig. G subclasses may be employed, showing that in patients with active PF lesions the predominant Ig. G isotype is Ig. G 4, in contrast to Ig. G 1, found more often in patients in remission. • Pemphigus folaceus: linear intercellular, intraepithelial Ig. G
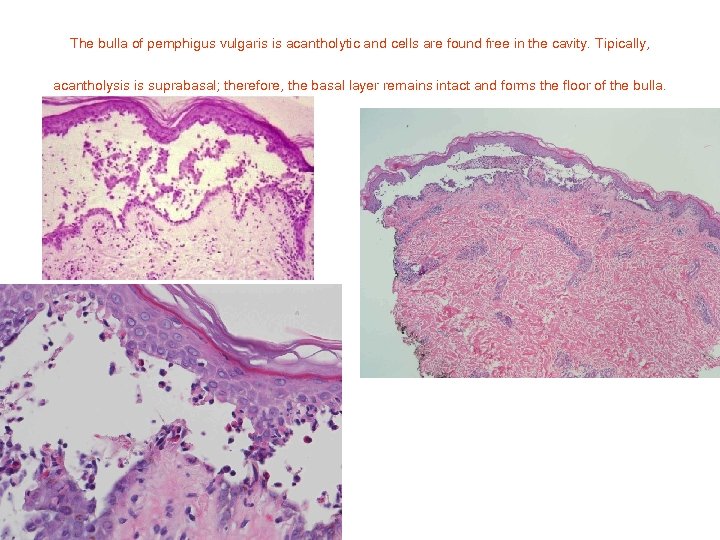
The bulla of pemphigus vulgaris is acantholytic and cells are found free in the cavity. Tipically, acantholysis is suprabasal; therefore, the basal layer remains intact and forms the floor of the bulla.
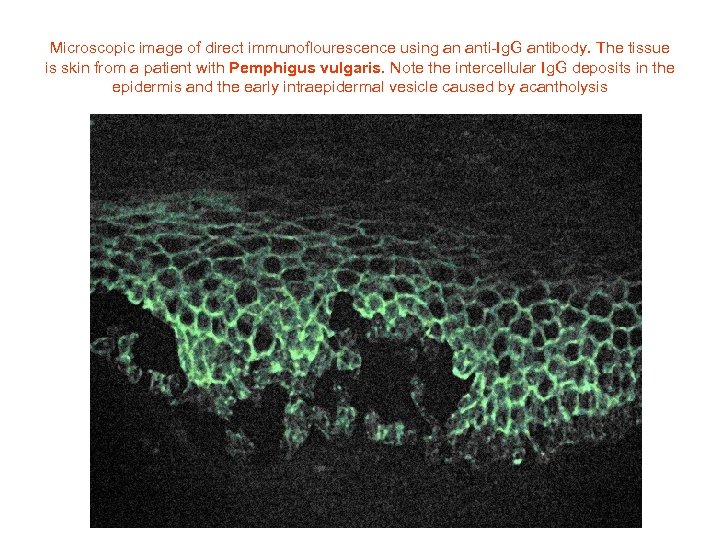
Microscopic image of direct immunoflourescence using an anti-Ig. G antibody. The tissue is skin from a patient with Pemphigus vulgaris. Note the intercellular Ig. G deposits in the epidermis and the early intraepidermal vesicle caused by acantholysis
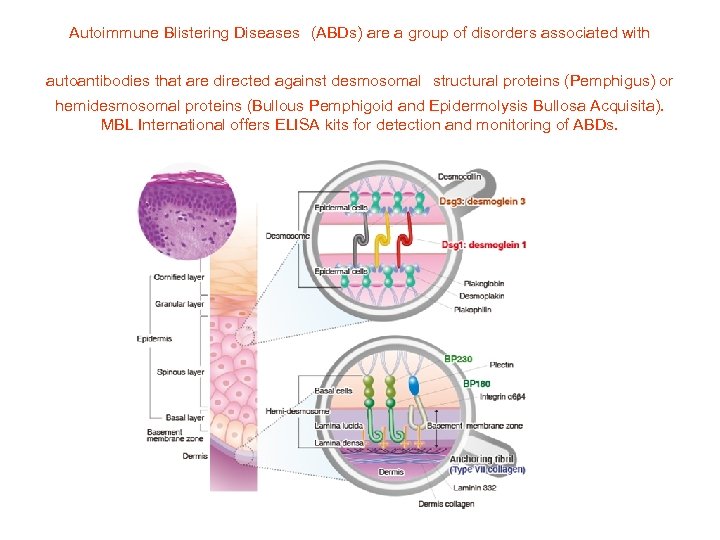
Autoimmune Blistering Diseases (ABDs) are a group of disorders associated with autoantibodies that are directed against desmosomal structural proteins (Pemphigus) or hemidesmosomal proteins (Bullous Pemphigoid and Epidermolysis Bullosa Acquisita). MBL International offers ELISA kits for detection and monitoring of ABDs.
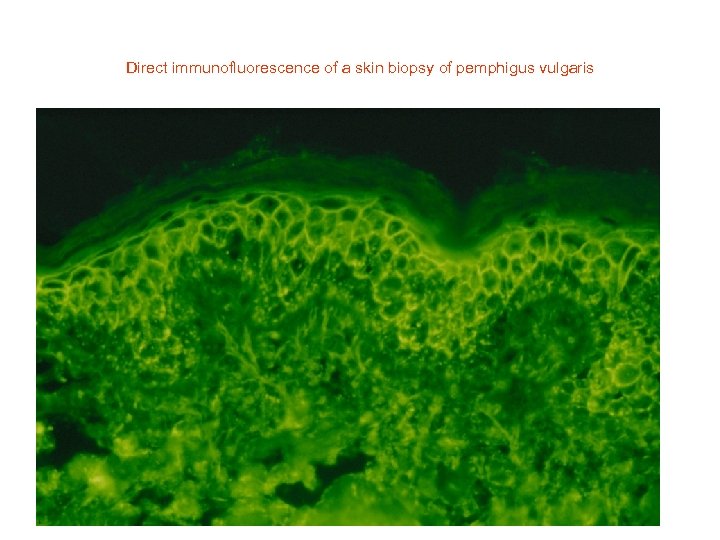
Direct immunofluorescence of a skin biopsy of pemphigus vulgaris
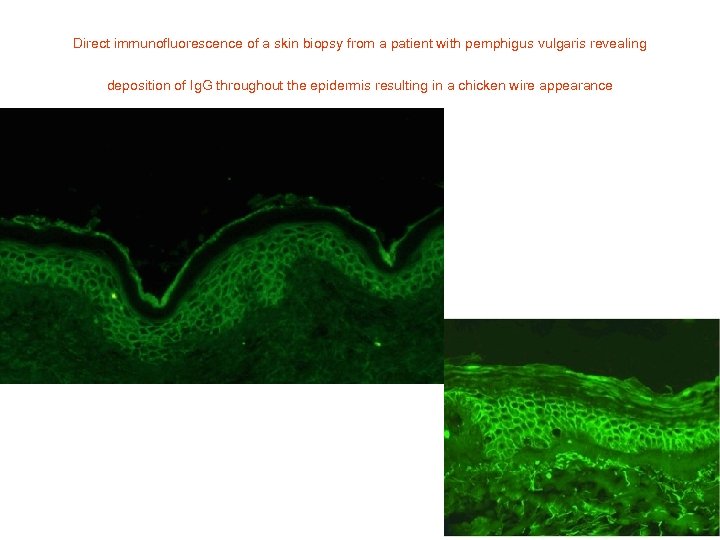
Direct immunofluorescence of a skin biopsy from a patient with pemphigus vulgaris revealing deposition of Ig. G throughout the epidermis resulting in a chicken wire appearance
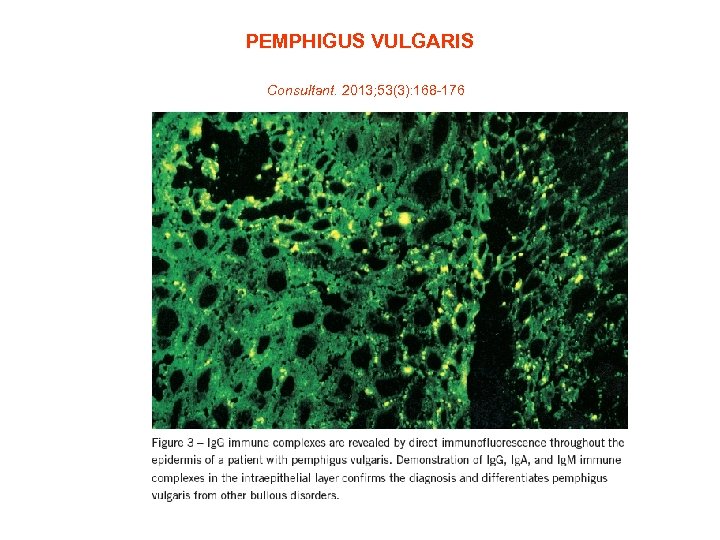
PEMPHIGUS VULGARIS Consultant. 2013; 53(3): 168 -176
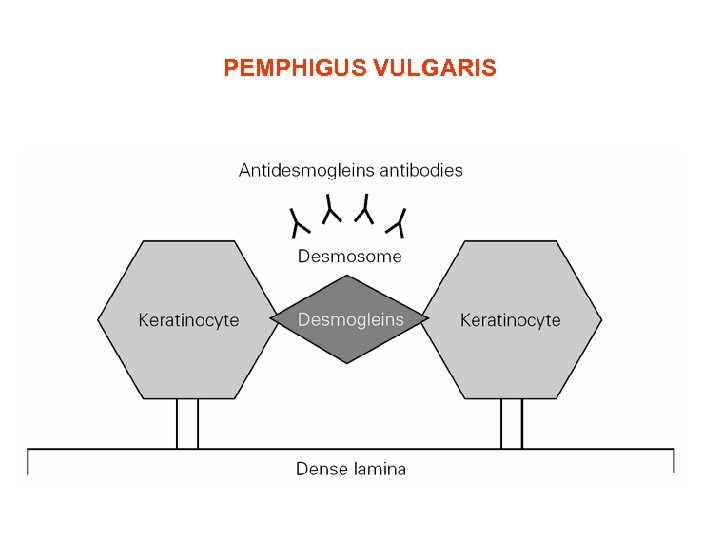
PEMPHIGUS VULGARIS
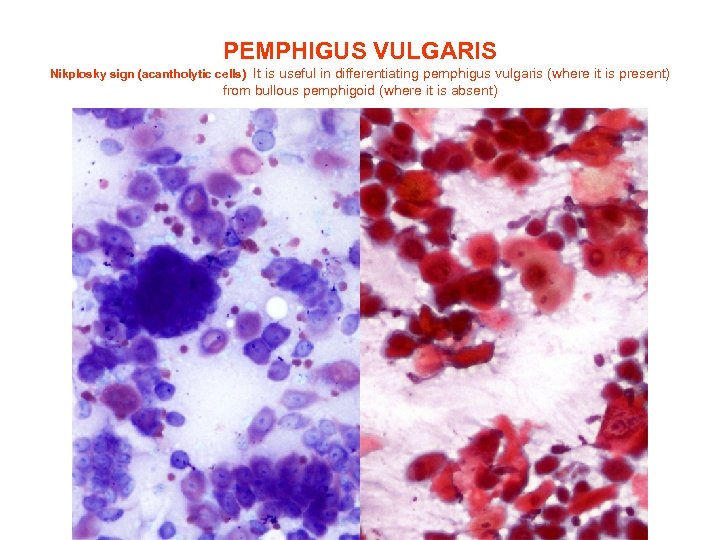
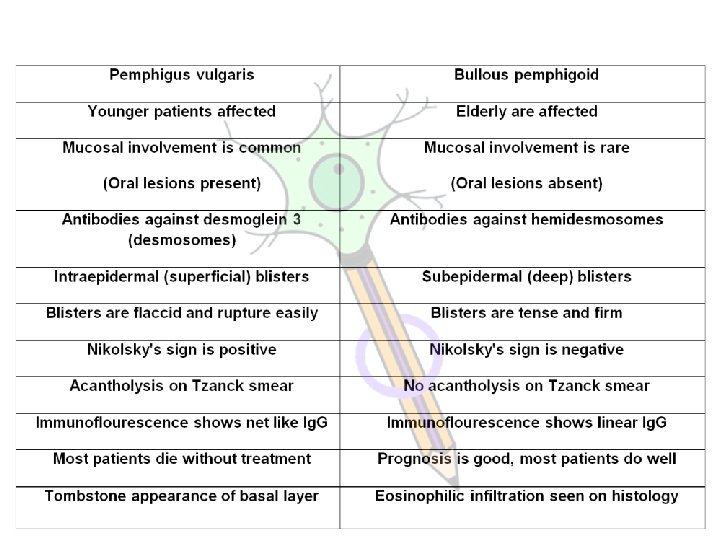
PEMPHIGUS VULGARIS Nikplosky sign (acantholytic cells) It is useful in differentiating pemphigus vulgaris (where it is present) from bullous pemphigoid (where it is absent)
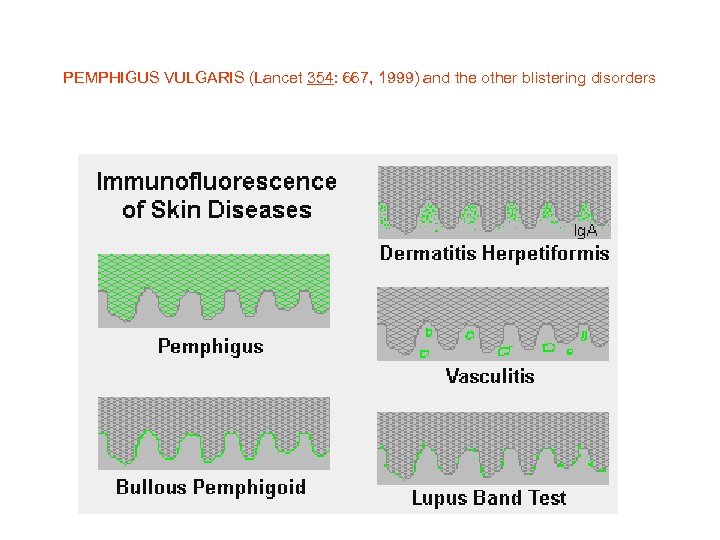
PEMPHIGUS VULGARIS (Lancet 354: 667, 1999) and the other blistering disorders
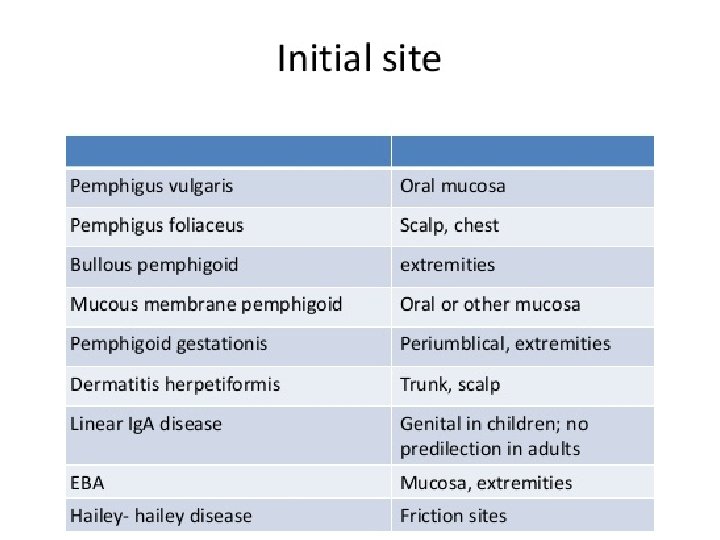
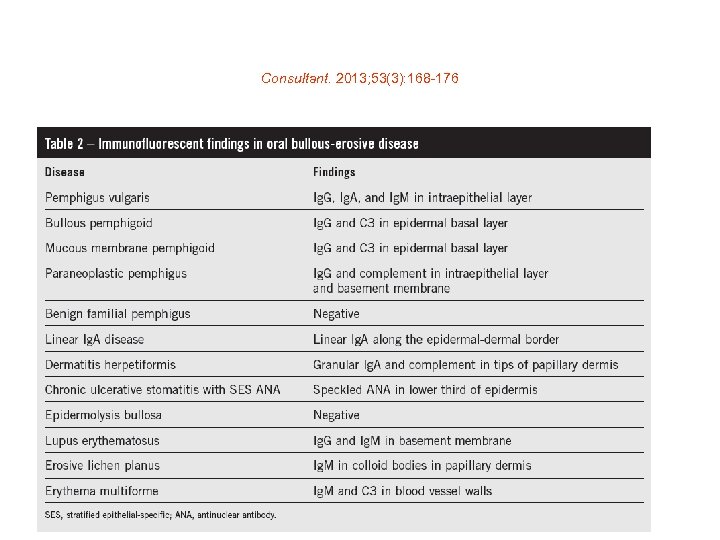
Consultant. 2013; 53(3): 168 -176
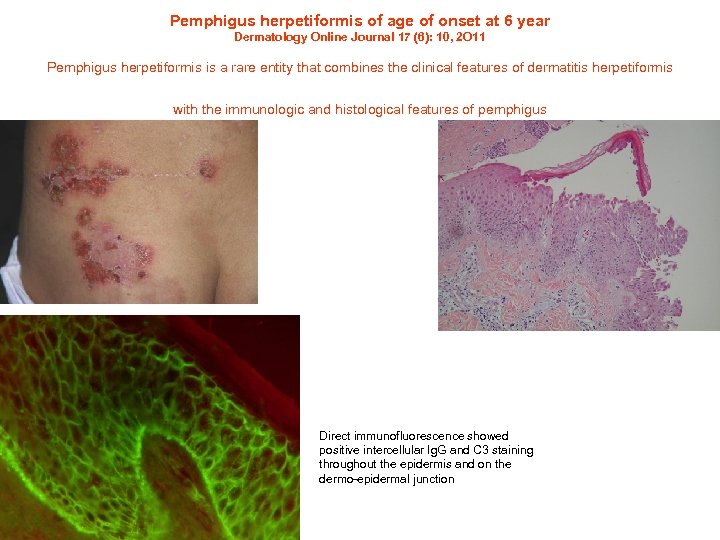
Pemphigus herpetiformis of age of onset at 6 year Dermatology Online Journal 17 (6): 10, 2 O 11 Pemphigus herpetiformis is a rare entity that combines the clinical features of dermatitis herpetiformis with the immunologic and histological features of pemphigus Direct immunofluorescence showed positive intercellular Ig. G and C 3 staining throughout the epidermis and on the dermo-epidermal junction
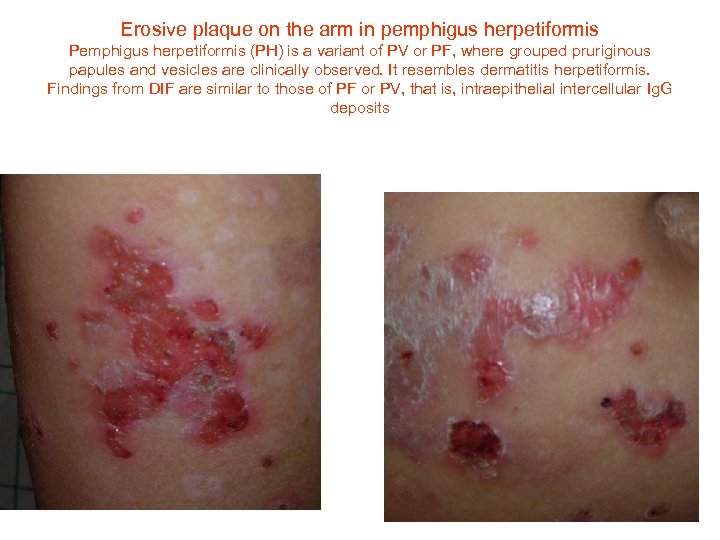
Erosive plaque on the arm in pemphigus herpetiformis Pemphigus herpetiformis (PH) is a variant of PV or PF, where grouped pruriginous papules and vesicles are clinically observed. It resembles dermatitis herpetiformis. Findings from DIF are similar to those of PF or PV, that is, intraepithelial intercellular Ig. G deposits
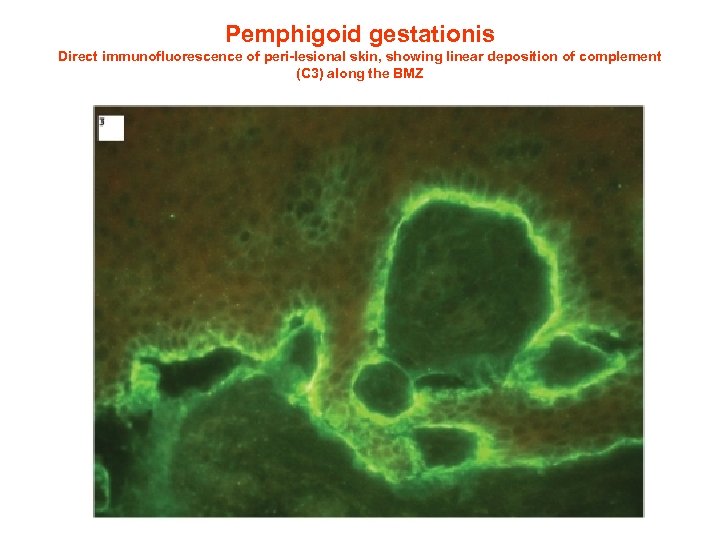
Pemphigoid gestationis Direct immunofluorescence of peri-lesional skin, showing linear deposition of complement (C 3) along the BMZ
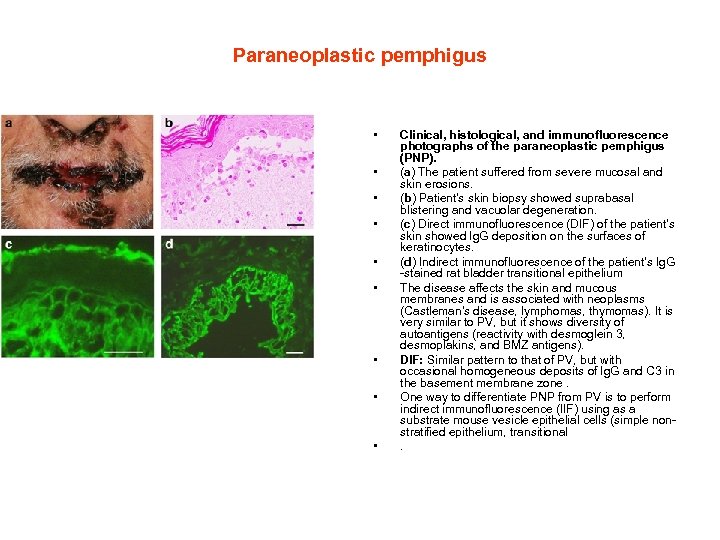
Paraneoplastic pemphigus • • • Clinical, histological, and immunofluorescence photographs of the paraneoplastic pemphigus (PNP). (a) The patient suffered from severe mucosal and skin erosions. (b) Patient's skin biopsy showed suprabasal blistering and vacuolar degeneration. (c) Direct immunofluorescence (DIF) of the patient's skin showed Ig. G deposition on the surfaces of keratinocytes. (d) Indirect immunofluorescence of the patient's Ig. G -stained rat bladder transitional epithelium The disease affects the skin and mucous membranes and is associated with neoplasms (Castleman's disease, lymphomas, thymomas). It is very similar to PV, but it shows diversity of autoantigens (reactivity with desmoglein 3, desmoplakins, and BMZ antigens). DIF: Similar pattern to that of PV, but with occasional homogeneous deposits of Ig. G and C 3 in the basement membrane zone. One way to differentiate PNP from PV is to perform indirect immunofluorescence (IIF) using as a substrate mouse vesicle epithelial cells (simple nonstratified epithelium, transitional .
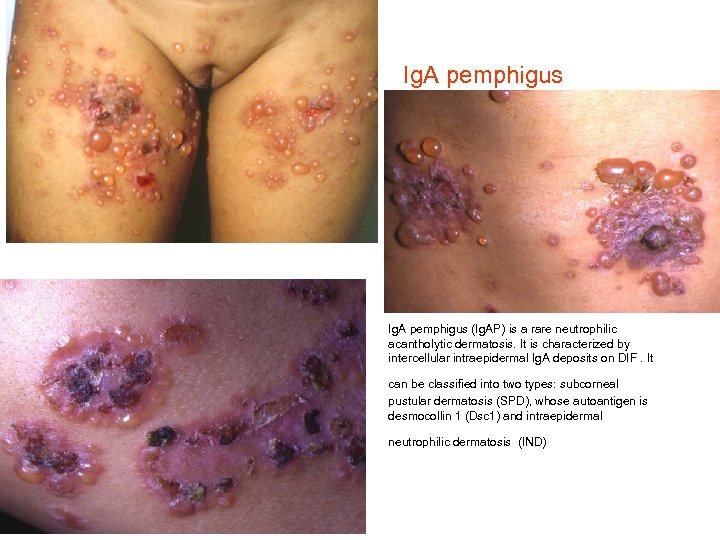
Ig. A pemphigus • Ig. A pemphigus (Ig. AP) is a rare neutrophilic acantholytic dermatosis. It is characterized by intercellular intraepidermal Ig. A deposits on DIF. It can be classified into two types: subcorneal pustular dermatosis (SPD), whose autoantigen is desmocollin 1 (Dsc 1) and intraepidermal neutrophilic dermatosis (IND)
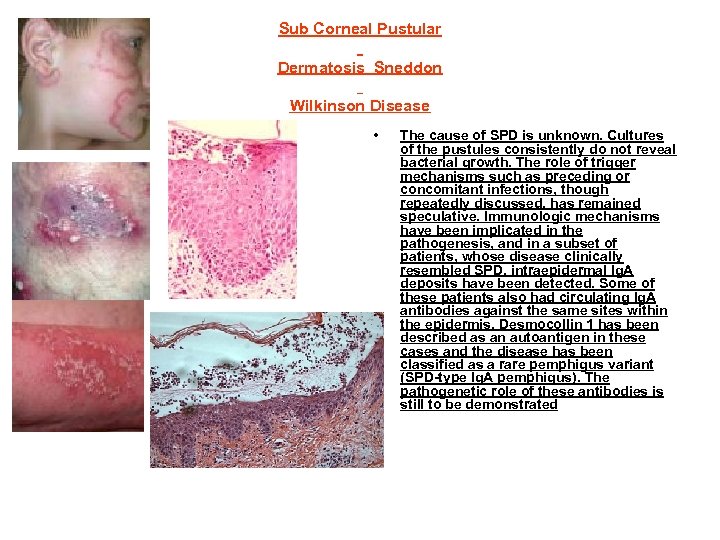
Sub Corneal Pustular Dermatosis Sneddon Wilkinson Disease • The cause of SPD is unknown. Cultures of the pustules consistently do not reveal bacterial growth. The role of trigger mechanisms such as preceding or concomitant infections, though repeatedly discussed, has remained speculative. Immunologic mechanisms have been implicated in the pathogenesis, and in a subset of patients, whose disease clinically resembled SPD, intraepidermal Ig. A deposits have been detected. Some of these patients also had circulating Ig. A antibodies against the same sites within the epidermis. Desmocollin 1 has been described as an autoantigen in these cases and the disease has been classified as a rare pemphigus variant (SPD-type Ig. A pemphigus). The pathogenetic role of these antibodies is still to be demonstrated
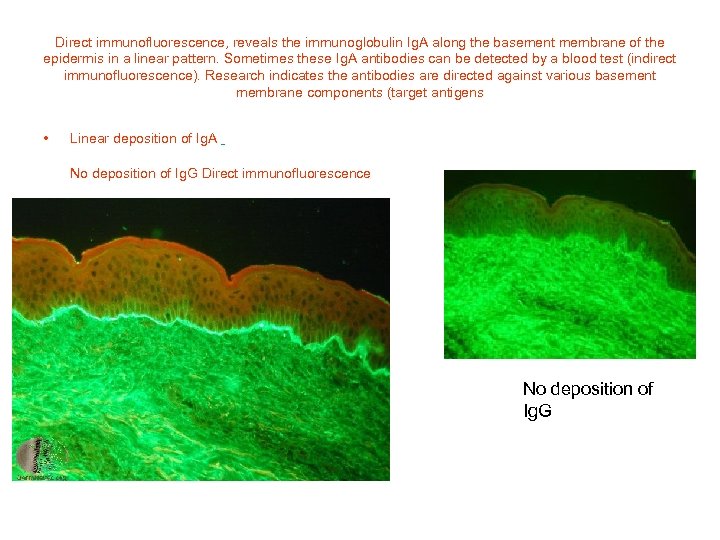
Direct immunofluorescence, reveals the immunoglobulin Ig. A along the basement membrane of the epidermis in a linear pattern. Sometimes these Ig. A antibodies can be detected by a blood test (indirect immunofluorescence). Research indicates the antibodies are directed against various basement membrane components (target antigens • Linear deposition of Ig. A No deposition of Ig. G Direct immunofluorescence No deposition of Ig. G
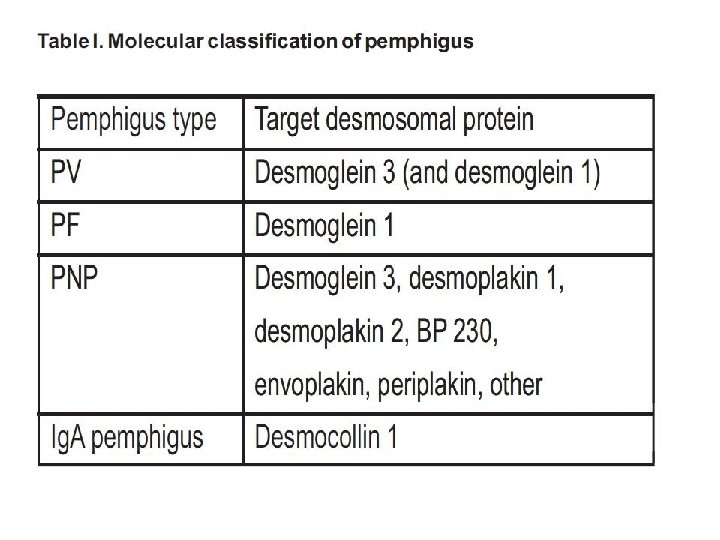
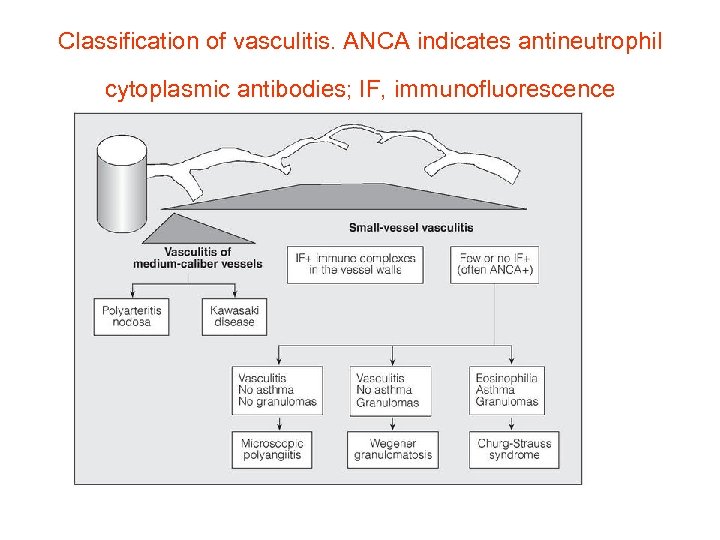
Classification of vasculitis. ANCA indicates antineutrophil cytoplasmic antibodies; IF, immunofluorescence
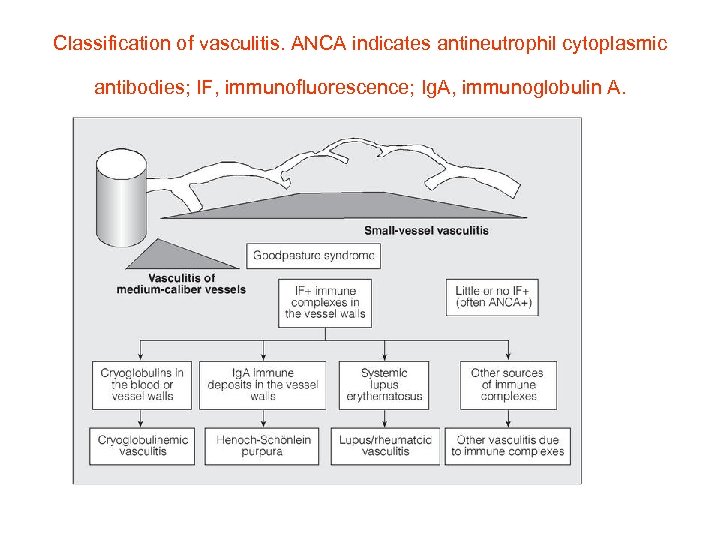
Classification of vasculitis. ANCA indicates antineutrophil cytoplasmic antibodies; IF, immunofluorescence; Ig. A, immunoglobulin A.
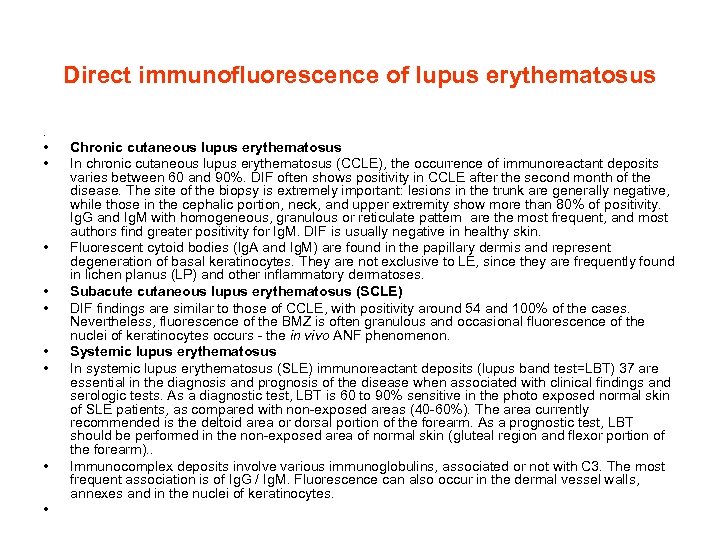
Direct immunofluorescence of lupus erythematosus . • • • Chronic cutaneous lupus erythematosus In chronic cutaneous lupus erythematosus (CCLE), the occurrence of immunoreactant deposits varies between 60 and 90%. DIF often shows positivity in CCLE after the second month of the disease. The site of the biopsy is extremely important: lesions in the trunk are generally negative, while those in the cephalic portion, neck, and upper extremity show more than 80% of positivity. Ig. G and Ig. M with homogeneous, granulous or reticulate pattern are the most frequent, and most authors find greater positivity for Ig. M. DIF is usually negative in healthy skin. Fluorescent cytoid bodies (Ig. A and Ig. M) are found in the papillary dermis and represent degeneration of basal keratinocytes. They are not exclusive to LE, since they are frequently found in lichen planus (LP) and other inflammatory dermatoses. Subacute cutaneous lupus erythematosus (SCLE) DIF findings are similar to those of CCLE, with positivity around 54 and 100% of the cases. Nevertheless, fluorescence of the BMZ is often granulous and occasional fluorescence of the nuclei of keratinocytes occurs - the in vivo ANF phenomenon. Systemic lupus erythematosus In systemic lupus erythematosus (SLE) immunoreactant deposits (lupus band test=LBT) 37 are essential in the diagnosis and prognosis of the disease when associated with clinical findings and serologic tests. As a diagnostic test, LBT is 60 to 90% sensitive in the photo exposed normal skin of SLE patients, as compared with non-exposed areas (40 -60%). The area currently recommended is the deltoid area or dorsal portion of the forearm. As a prognostic test, LBT should be performed in the non-exposed area of normal skin (gluteal region and flexor portion of the forearm). . Immunocomplex deposits involve various immunoglobulins, associated or not with C 3. The most frequent association is of Ig. G / Ig. M. Fluorescence can also occur in the dermal vessel walls, annexes and in the nuclei of keratinocytes.
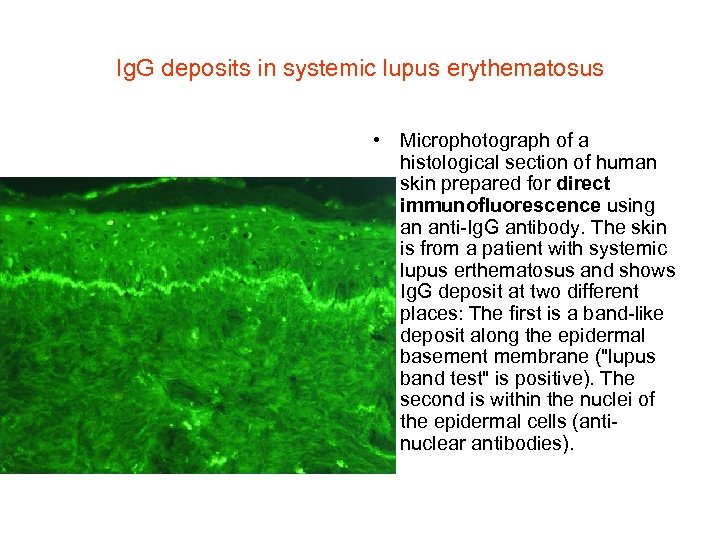
Ig. G deposits in systemic lupus erythematosus • Microphotograph of a histological section of human skin prepared for direct immunofluorescence using an anti-Ig. G antibody. The skin is from a patient with systemic lupus erthematosus and shows Ig. G deposit at two different places: The first is a band-like deposit along the epidermal basement membrane ("lupus band test" is positive). The second is within the nuclei of the epidermal cells (antinuclear antibodies).
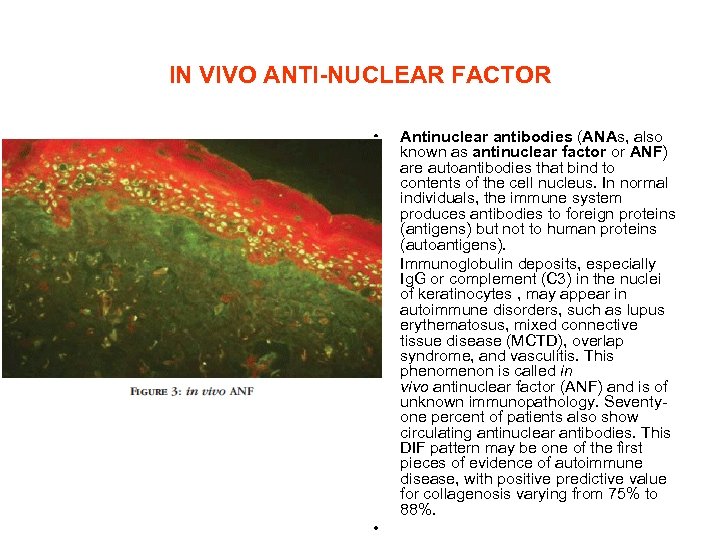
IN VIVO ANTI-NUCLEAR FACTOR • • • Antinuclear antibodies (ANAs, also known as antinuclear factor or ANF) are autoantibodies that bind to contents of the cell nucleus. In normal individuals, the immune system produces antibodies to foreign proteins (antigens) but not to human proteins (autoantigens). Immunoglobulin deposits, especially Ig. G or complement (C 3) in the nuclei of keratinocytes , may appear in autoimmune disorders, such as lupus erythematosus, mixed connective tissue disease (MCTD), overlap syndrome, and vasculitis. This phenomenon is called in vivo antinuclear factor (ANF) and is of unknown immunopathology. Seventyone percent of patients also show circulating antinuclear antibodies. This DIF pattern may be one of the first pieces of evidence of autoimmune disease, with positive predictive value for collagenosis varying from 75% to 88%.
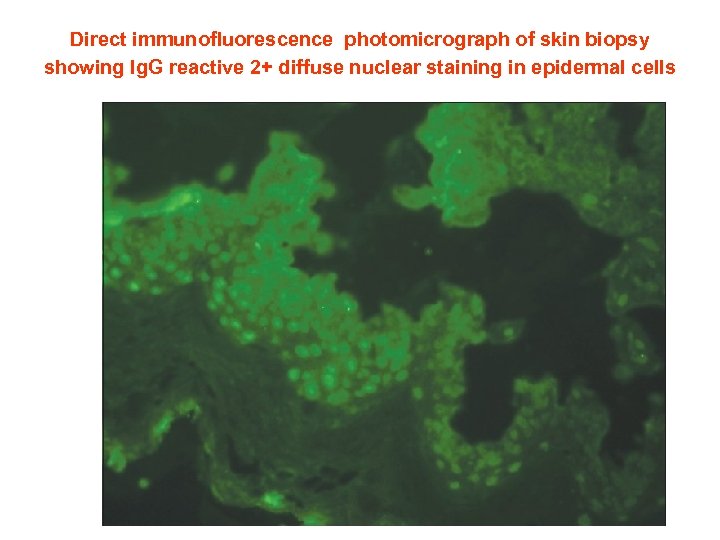
Direct immunofluorescence photomicrograph of skin biopsy showing Ig. G reactive 2+ diffuse nuclear staining in epidermal cells (ANA in vivo)

Anti-nuclear antibody • • • There are many subtypes of ANAs such as anti- RO antibodies, anti –LA antibodies, anti – Sm anti –antibodies, anti – n. RNP antibodies, anti – Scl 7 O antibodies, anti ds DNA antibodies, anti – histone antibodies, , antibodies to nuclear pore complexes, anti –centromere antibodies & anti -sp 1 OO antibodies. Each of these antibody subtypes binds to different proteins or protein complexes within the nucleus. They are found in many disorders including autoimmunity, cancer & infection, with different prevalence's of antibodies depending on the condition. This allows the use of ANAs in the diagnosis of some autoimmune disorders, including systemic lupus erythematosus, Sjogren’s syndrome, scleroderma, mixed connective tissue disease, polymyositis, , dermatomyositis, , autoimmune hepatitis & drug induced The ANA test detects the auto antibodies present in an individual's blood serum. The common tests used for detecting and quantifying ANAs are indirect immunoflourescence & enzyme linked imminosorbent assay indirect (ELISA). In immunofluorescence, the level of autoantibodies is reported as a titer. This is the highest dilution of the serum at which autoantibodies are still detectable. Positive autoantibody titers at a dilution equal to or greater than 1: 160 are usually considered as clinically significant. Positive titers of less than 1: 160 are present in up to 20% of the healthy population, especially the elderly. Although positive titers of 1: 160 or higher are strongly associated with autoimmune disorders, they are also found in 5% of healthy individuals. Autoantibody screening is useful in the diagnosis of autoimmune disorders and monitoring levels helps to predict the progression of disease A positive ANA test is seldom useful if other clinical or laboratory data supporting a diagnosis are not present Homogeneous immunoflourescence staining pattern of double stranded DNA antibodies on HEp-20 -10 cells. Interphase cells show homogeneous nuclear staining while mitotic cells show staining of the condensed chromosome regions

Anti-ds. DNA • Direct immunofluorescence of lupus erythematosus • In lupus erythematosus (LE), immunocomplexes target the nuclear components of keratinocytes and structures of the basement membrane zone. DIF aids in the diagnostic confirmation of lupus erythematosus, distinguishing it from other diseases. Ig. G, Ig. M, Ig. A, and C 3 deposits may occur, in addition to other immunoreactants in the BMZ. There are several deposit patterns in the BMZ, such as: homogeneous, fibrillar, linear, and granulous, which can be focal or continuous. Fluorescent cytoid bodies can be observed in the dermis in the dermoepidermal junction with Ig. M or Ig. A. Prevalence of immunoglobulins in the BMZ is partly determined by age, localization and morphology of the lesion, activity of the disease, and treatment • Anti-ds. DNA antibodies are a group of anti-nuclear antibodies and their target antigen is double stranded DNA. Blood tests such as enzyme linked immunosorbent assay (ELISA) and immunofluorescence are routinely performed to detect anti-ds. DNA antibodies in diagnostic laboratories. They are highly diagnostic of systemic lupus erythematosus (SLE) and are implicated in the pathogenesis of lupus nephritis • ds. DNA antibody. The variable regions (yellow) are complementary to the ds. DNA strands. These antibodies are found commonly in the sera of people with SLE
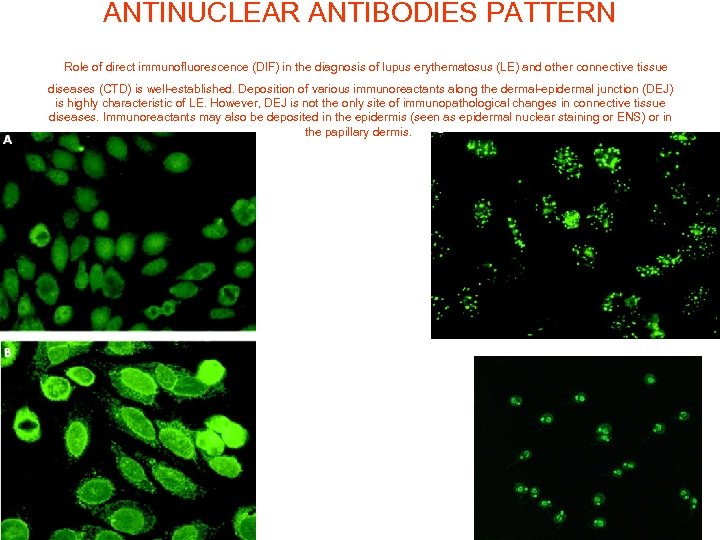
ANTINUCLEAR ANTIBODIES PATTERN Role of direct immunofluorescence (DIF) in the diagnosis of lupus erythematosus (LE) and other connective tissue diseases (CTD) is well-established. Deposition of various immunoreactants along the dermal-epidermal junction (DEJ) is highly characteristic of LE. However, DEJ is not the only site of immunopathological changes in connective tissue diseases. Immunoreactants may also be deposited in the epidermis (seen as epidermal nuclear staining or ENS) or in the papillary dermis.
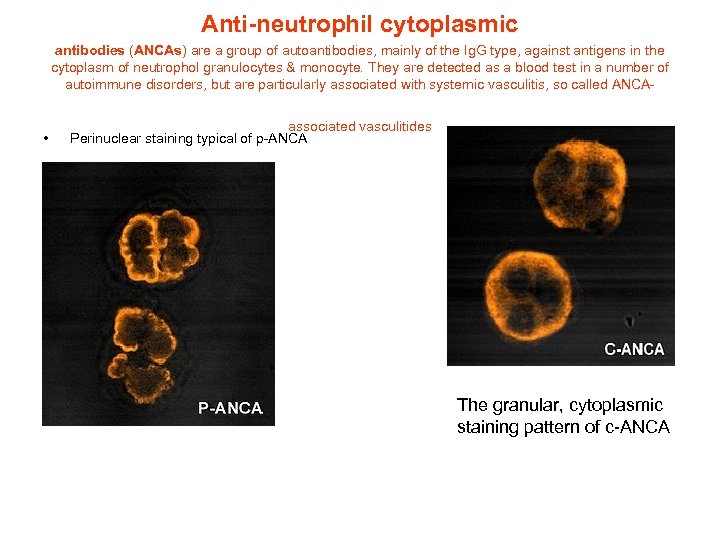
Anti-neutrophil cytoplasmic antibodies (ANCAs) are a group of autoantibodies, mainly of the Ig. G type, against antigens in the cytoplasm of neutrophol granulocytes & monocyte. They are detected as a blood test in a number of autoimmune disorders, but are particularly associated with systemic vasculitis, so called ANCA- • associated vasculitides Perinuclear staining typical of p-ANCA The granular, cytoplasmic staining pattern of c-ANCA
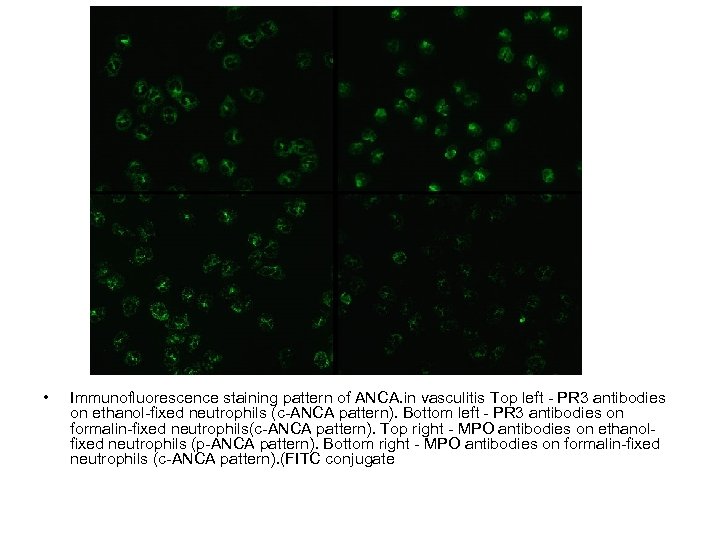
• Immunofluorescence staining pattern of ANCA. in vasculitis Top left - PR 3 antibodies on ethanol-fixed neutrophils (c-ANCA pattern). Bottom left - PR 3 antibodies on formalin-fixed neutrophils(c-ANCA pattern). Top right - MPO antibodies on ethanolfixed neutrophils (p-ANCA pattern). Bottom right - MPO antibodies on formalin-fixed neutrophils (c-ANCA pattern). (FITC conjugate
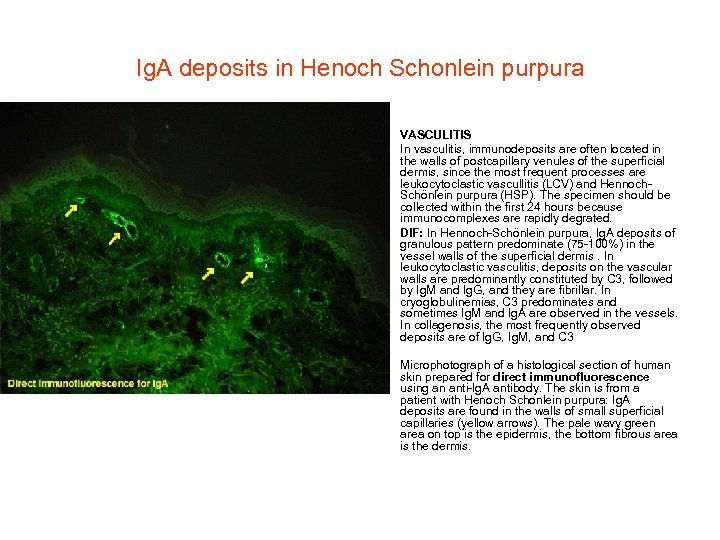
Ig. A deposits in Henoch Schonlein purpura • • VASCULITIS In vasculitis, immunodeposits are often located in the walls of postcapillary venules of the superficial dermis, since the most frequent processes are leukocytoclastic vascullitis (LCV) and Hennoch. Schönlein purpura (HSP). The specimen should be collected within the first 24 hours because immunocomplexes are rapidly degrated. DIF: In Hennoch-Schönlein purpura, Ig. A deposits of granulous pattern predominate (75 -100%) in the vessel walls of the superficial dermis. In leukocytoclastic vasculitis, deposits on the vascular walls are predominantly constituted by C 3, followed by Ig. M and Ig. G, and they are fibrillar. In cryoglobulinemias, C 3 predominates and sometimes Ig. M and Ig. A are observed in the vessels. In collagenosis, the most frequently observed deposits are of Ig. G, Ig. M, and C 3 Microphotograph of a histological section of human skin prepared for direct immunofluorescence using an anti-Ig. A antibody. The skin is from a patient with Henoch Schonlein purpura: Ig. A deposits are found in the walls of small superficial capillaries (yellow arrows). The pale wavy green area on top is the epidermis, the bottom fibrous area is the dermis.
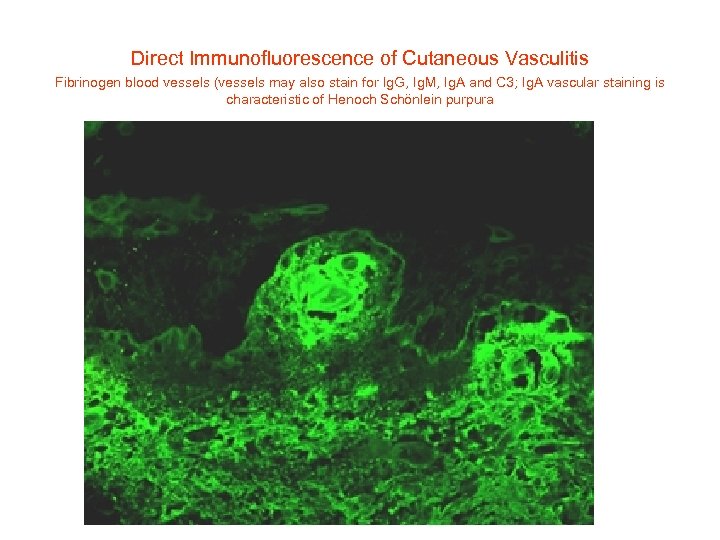
Direct Immunofluorescence of Cutaneous Vasculitis Fibrinogen blood vessels (vessels may also stain for Ig. G, Ig. M, Ig. A and C 3; Ig. A vascular staining is characteristic of Henoch Schönlein purpura
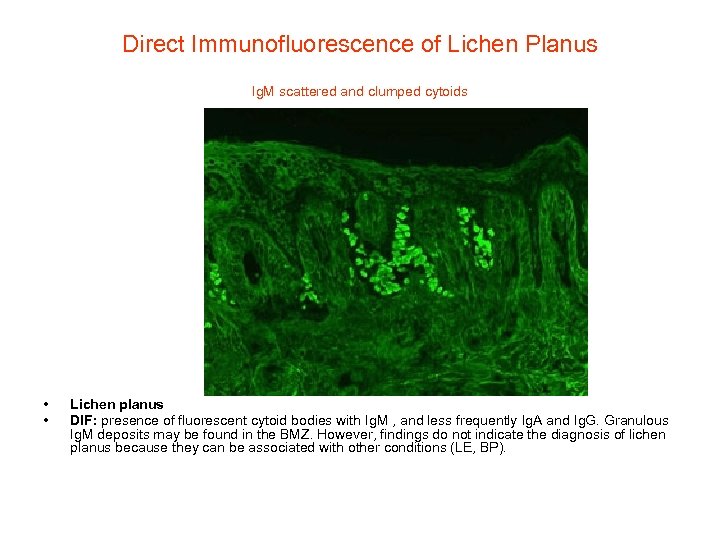
Direct Immunofluorescence of Lichen Planus Ig. M scattered and clumped cytoids • • Lichen planus DIF: presence of fluorescent cytoid bodies with Ig. M , and less frequently Ig. A and Ig. G. Granulous Ig. M deposits may be found in the BMZ. However, findings do not indicate the diagnosis of lichen planus because they can be associated with other conditions (LE, BP).
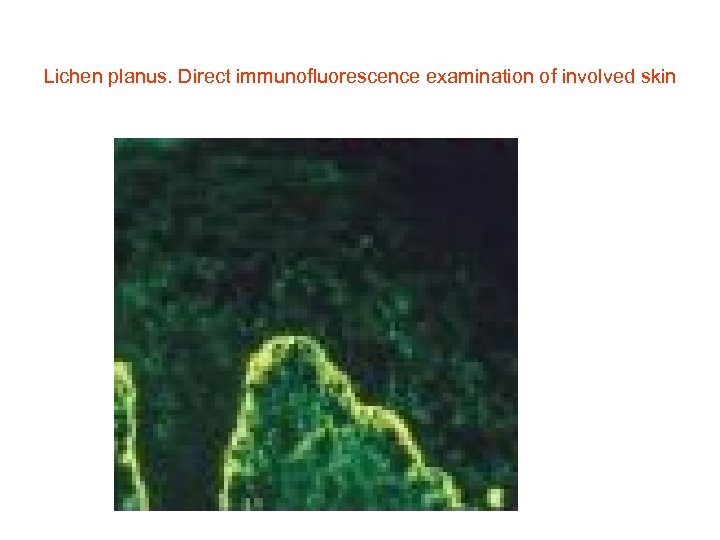
Lichen planus. Direct immunofluorescence examination of involved skin
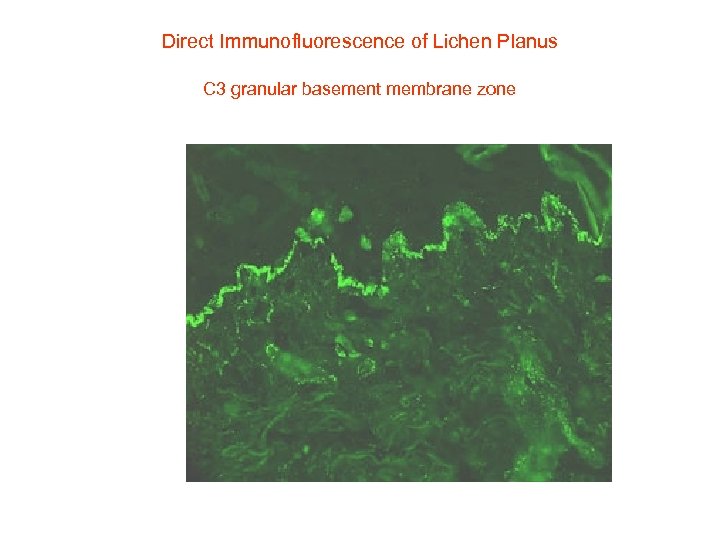
Direct Immunofluorescence of Lichen Planus C 3 granular basement membrane zone
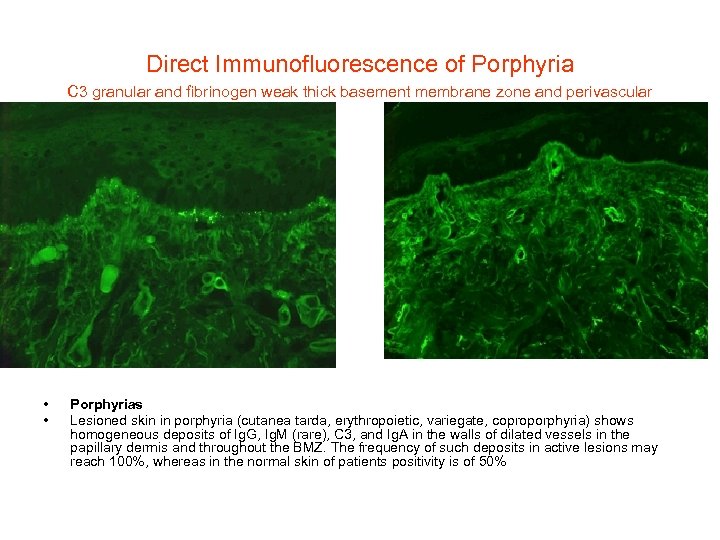
Direct Immunofluorescence of Porphyria C 3 granular and fibrinogen weak thick basement membrane zone and perivascular • • Porphyrias Lesioned skin in porphyria (cutanea tarda, erythropoietic, variegate, coproporphyria) shows homogeneous deposits of Ig. G, Ig. M (rare), C 3, and Ig. A in the walls of dilated vessels in the papillary dermis and throughout the BMZ. The frequency of such deposits in active lesions may reach 100%, whereas in the normal skin of patients positivity is of 50%
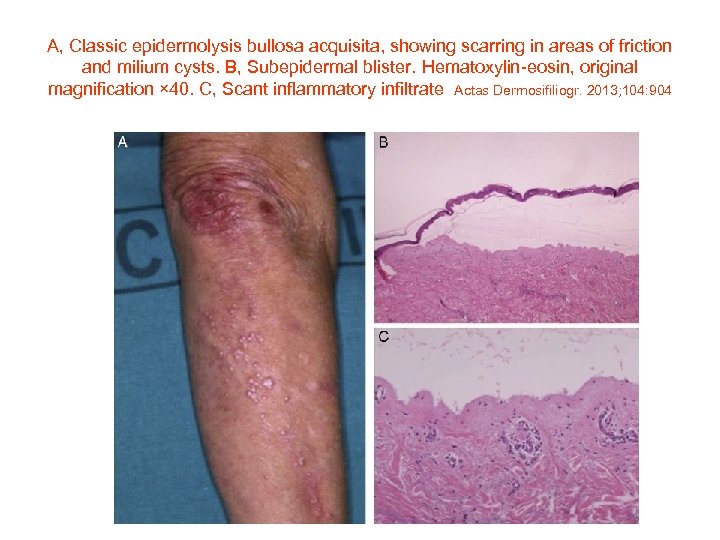
A, Classic epidermolysis bullosa acquisita, showing scarring in areas of friction and milium cysts. B, Subepidermal blister. Hematoxylin-eosin, original magnification × 40. C, Scant inflammatory infiltrate Actas Dermosifiliogr. 2013; 104: 904
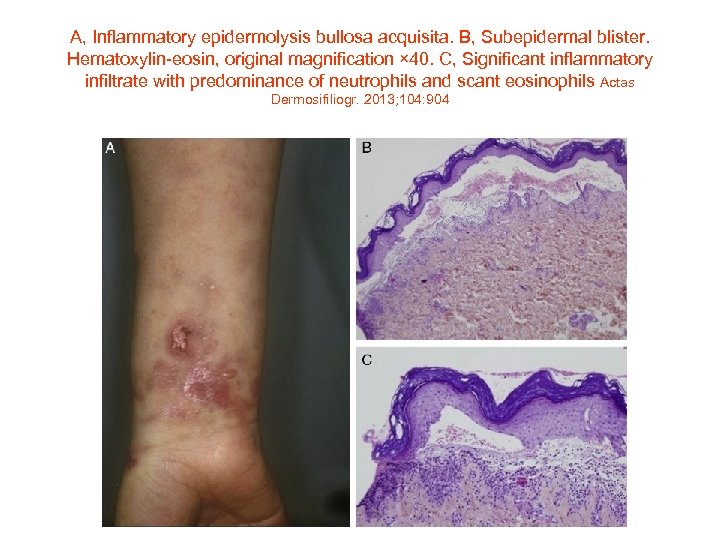
A, Inflammatory epidermolysis bullosa acquisita. B, Subepidermal blister. Hematoxylin-eosin, original magnification × 40. C, Significant inflammatory infiltrate with predominance of neutrophils and scant eosinophils Actas Dermosifiliogr. 2013; 104: 904
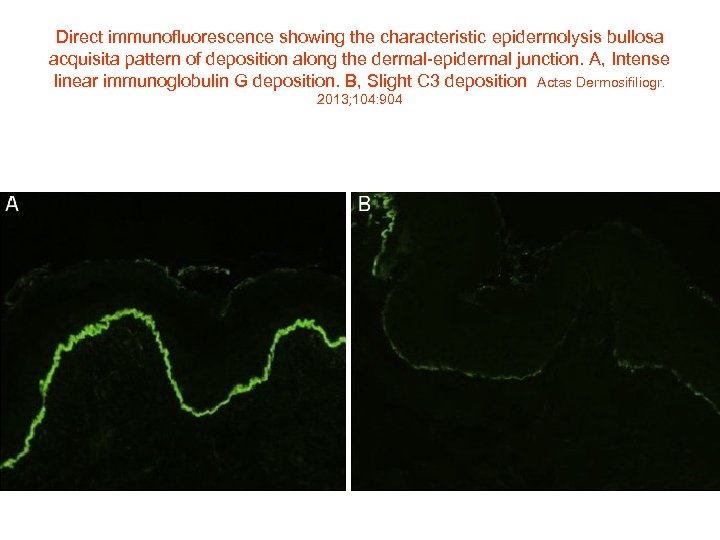
Direct immunofluorescence showing the characteristic epidermolysis bullosa acquisita pattern of deposition along the dermal-epidermal junction. A, Intense linear immunoglobulin G deposition. B, Slight C 3 deposition Actas Dermosifiliogr. 2013; 104: 904
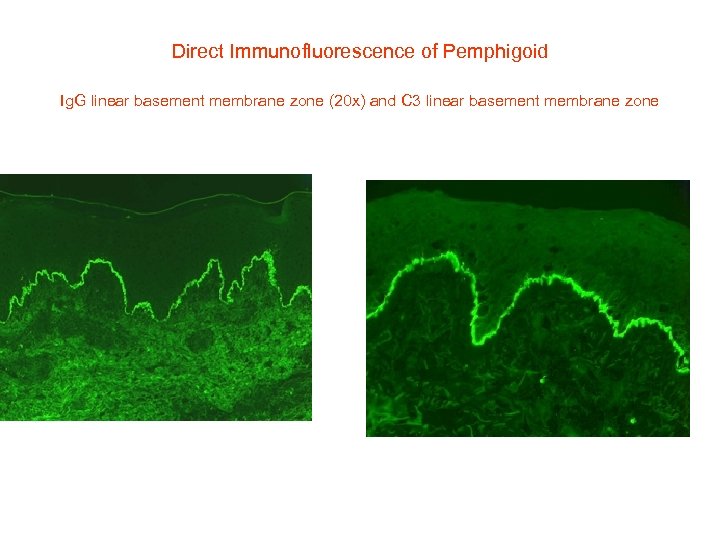
Direct Immunofluorescence of Pemphigoid Ig. G linear basement membrane zone (20 x) and C 3 linear basement membrane zone
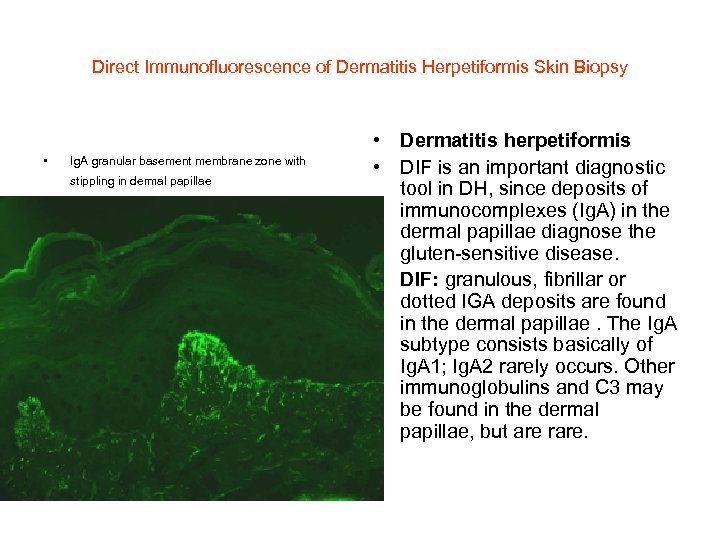
Direct Immunofluorescence of Dermatitis Herpetiformis Skin Biopsy • Ig. A granular basement membrane zone with stippling in dermal papillae • Dermatitis herpetiformis • DIF is an important diagnostic tool in DH, since deposits of immunocomplexes (Ig. A) in the dermal papillae diagnose the gluten-sensitive disease. • DIF: granulous, fibrillar or dotted IGA deposits are found in the dermal papillae. The Ig. A subtype consists basically of Ig. A 1; Ig. A 2 rarely occurs. Other immunoglobulins and C 3 may be found in the dermal papillae, but are rare.
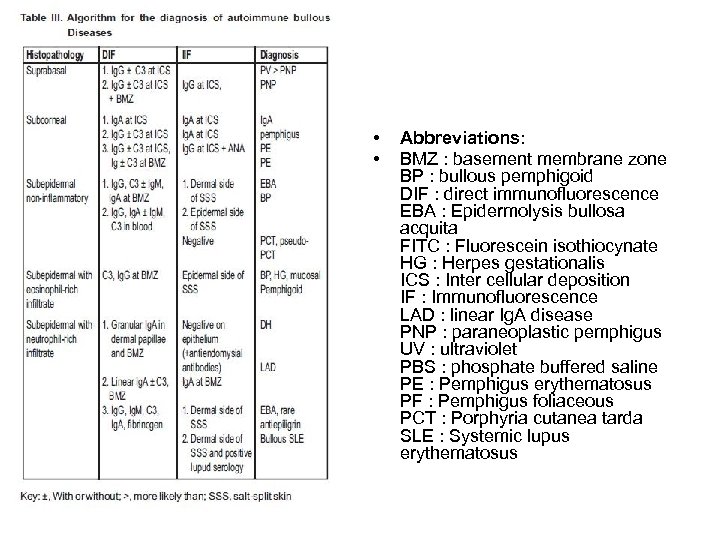
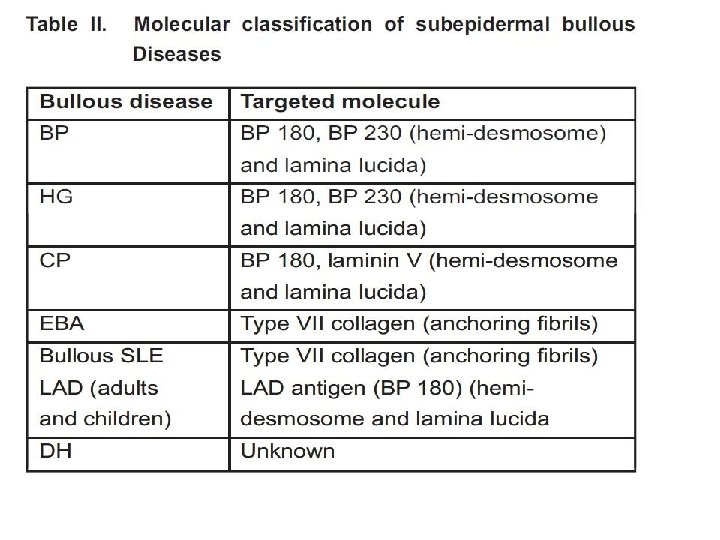
• • Abbreviations: BMZ : basement membrane zone BP : bullous pemphigoid DIF : direct immunofluorescence EBA : Epidermolysis bullosa acquita FITC : Fluorescein isothiocynate HG : Herpes gestationalis ICS : Inter cellular deposition IF : Immunofluorescence LAD : linear Ig. A disease PNP : paraneoplastic pemphigus UV : ultraviolet PBS : phosphate buffered saline PE : Pemphigus erythematosus PF : Pemphigus foliaceous PCT : Porphyria cutanea tarda SLE : Systemic lupus erythematosus

Differential diagnosis of Direct immunoflourescence • • • • Differential diagnosis of direct immunofluorescence (DIF) depends upon : 1. Primary site of immune deposition 2. Class of immune deposits 3. Number of immune deposits 4. If multiple, the identity of the most intense deposits is significant 5. Deposition in other sites besides the main sites. Inter cellular deposition (ICS) pattern ICS pattern results from binding of antibodies to desmosomal proteins around keratinocyte cell surface and is characteristic of pemphigus group. Parameters in further differential diagnosis to be studied are: a) Class of immunoglobulins deposited b) Relative intensity of fluorescence in different levels of epidermis c) Any other deposits besides ICS Ig. G in the ICS It is characteristic of all pemphigus except Ig. A pemphigus. C 3 may be present along with Ig. G deposition in the ICS and BMZ D/D: (1) Pemphigus erythematosus (PE) (2) Pemphigus foliaceous (PF) (3) Paraneoplastic pemphigus (PNP) Antibodies will be directed to BMZ also in these lesions, however those of PNP are weak, diffuse and nonspecific. Ig. A deposition in the ICS This will be seen in Ig. A pemphigus only. Clinical and histopathological D/D of Ig. A pemphigus will be PF and subcorneal pustular dermatosis which will not exhibit Ig. A deposit. BMZ deposition It is characteristic of subepidermal bullous disease. The patterns to be studied are a) Class of immunoglobulins deposited b) Number of immune deposits c) Morphology of fluorescence like continuous, discontinuous, linear, granular, homogenous. d) Deposits at other sites • • • • Exclusive BMZ deposits Ig. G and/ or C 3 deposits Ig. G and/or C 3 or multiple immunoreactants can be seen in following conditions. Ig. G, C 3/ both (1) BP (1) Mucosal pemphigoid (2) Herpes gestationalis (HG)- C 3 alone may be seen sometimes. (3) Epidermolysis bullosa acquita (EBA) (4) Bullous SLE When C 3 is more intense than Ig. G, it favors pemphigoid group. Pattern in BP and HG is linear, wavy, tubular or granular. Multiple deposits in BMZ favors EBA and bullous SLE. The deposits are homogenous, thick and broad. Ig. A depositions at the BMZ This is diagnostics of linear Ig. A disease (LAD) Deposition at the BMZ and blood vessel walls Homogenous deposition of multiple immunoreactants favors - Porphyria cutanea tarda (PCT) - Pseudo – PCT - Erythropoeitic protoporphyria. Here usually Ig. G, Ig. A ± C 3 deposit are seen. Papillary dermal deposition Granular Ig. A, C 3 in papillary dermis and BMZ is diagnostic of DH.

I have the question in the last power point a lot lately
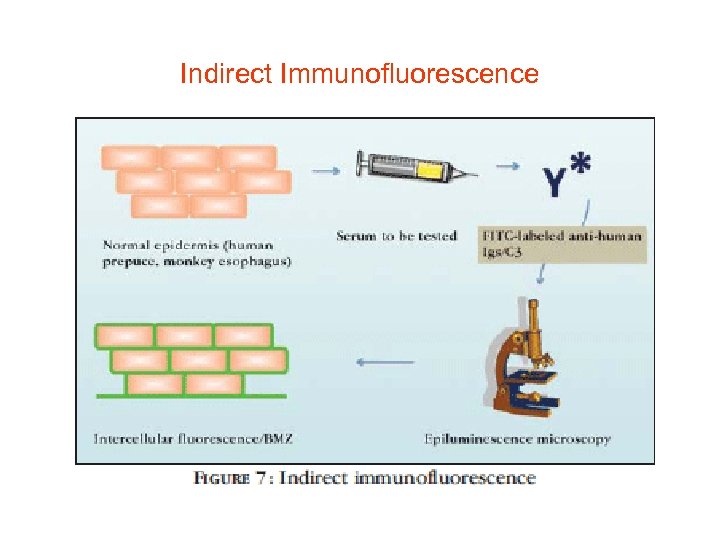
Indirect Immunofluorescence
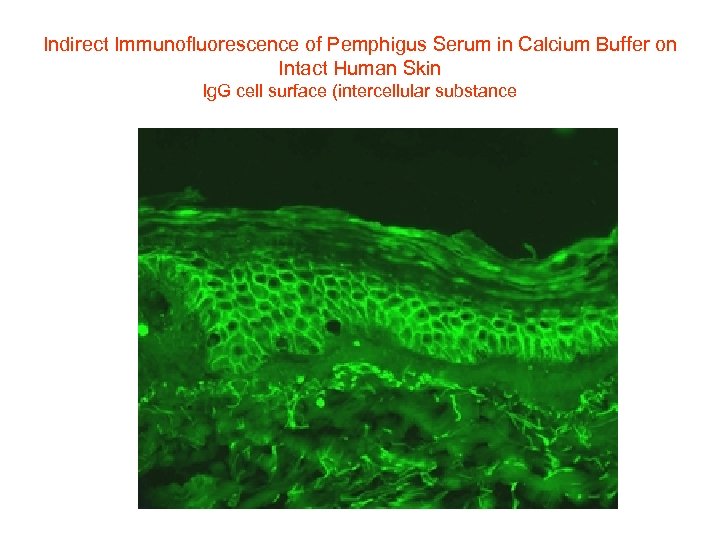
Indirect Immunofluorescence of Pemphigus Serum in Calcium Buffer on Intact Human Skin Ig. G cell surface (intercellular substance
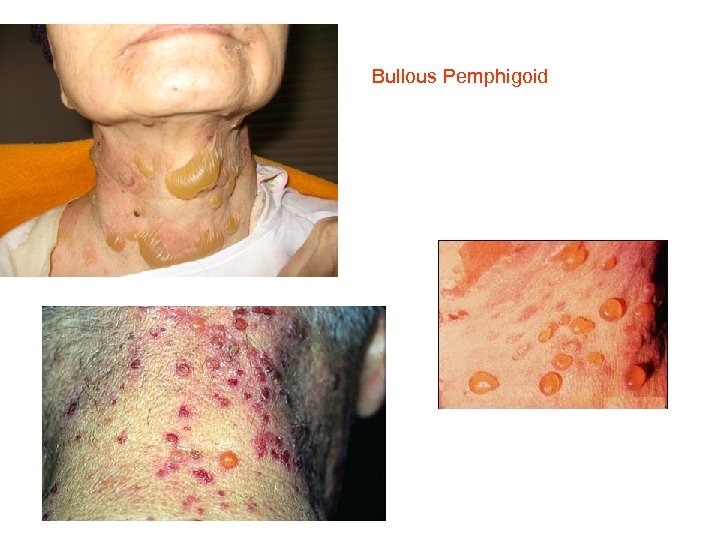
Bullous Pemphigoid
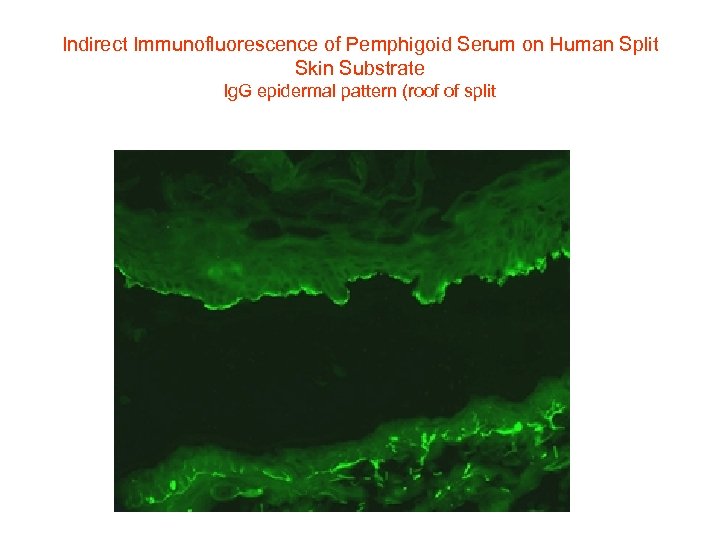
Indirect Immunofluorescence of Pemphigoid Serum on Human Split Skin Substrate Ig. G epidermal pattern (roof of split
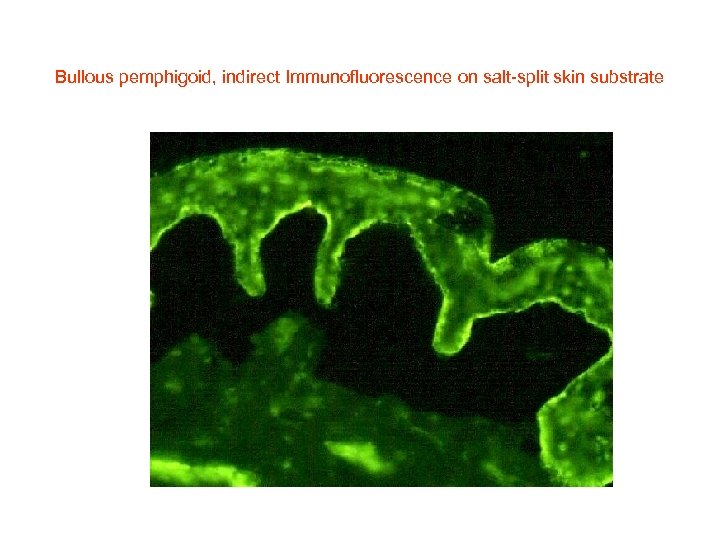
Bullous pemphigoid, indirect Immunofluorescence on salt-split skin substrate
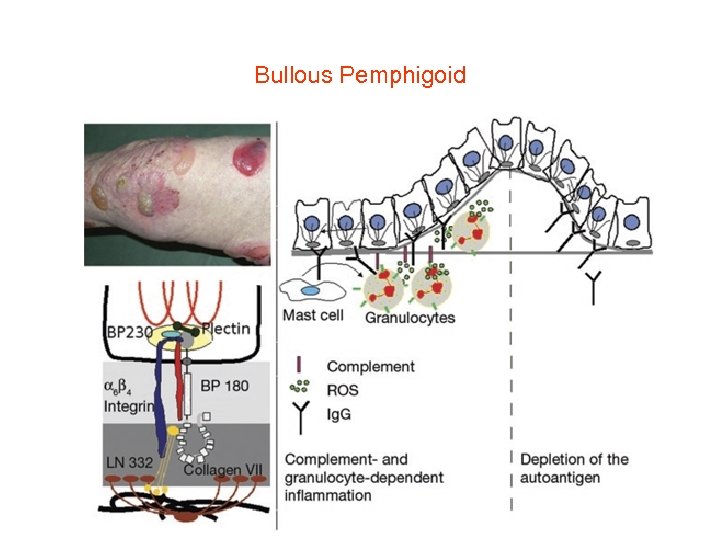
Bullous Pemphigoid
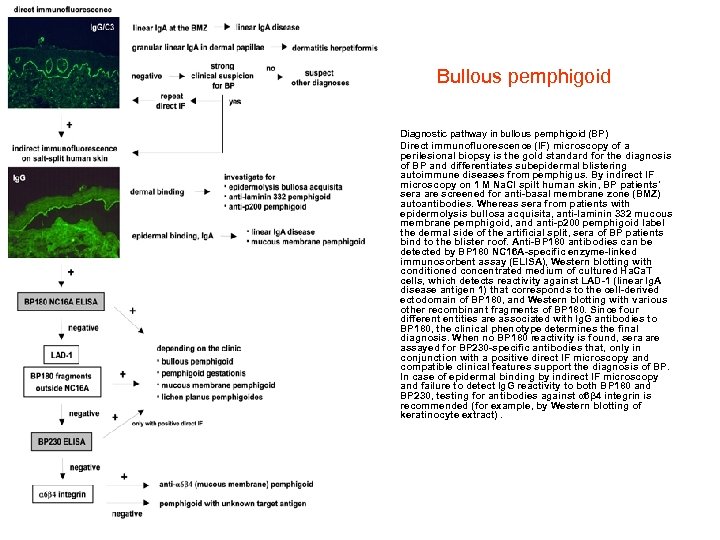
Bullous pemphigoid • • Diagnostic pathway in bullous pemphigoid (BP) Direct immunofluorescence (IF) microscopy of a perilesional biopsy is the gold standard for the diagnosis of BP and differentiates subepidermal blistering autoimmune diseases from pemphigus. By indirect IF microscopy on 1 M Na. Cl spilt human skin, BP patients' sera are screened for anti-basal membrane zone (BMZ) autoantibodies. Whereas sera from patients with epidermolysis bullosa acquisita, anti-laminin 332 mucous membrane pemphigoid, and anti-p 200 pemphigoid label the dermal side of the artificial split, sera of BP patients bind to the blister roof. Anti-BP 180 antibodies can be detected by BP 180 NC 16 A-specific enzyme-linked immunosorbent assay (ELISA), Western blotting with conditioned concentrated medium of cultured Ha. Ca. T cells, which detects reactivity against LAD-1 (linear Ig. A disease antigen 1) that corresponds to the cell-derived ectodomain of BP 180, and Western blotting with various other recombinant fragments of BP 180. Since four different entities are associated with Ig. G antibodies to BP 180, the clinical phenotype determines the final diagnosis. When no BP 180 reactivity is found, sera are assayed for BP 230 -specific antibodies that, only in conjunction with a positive direct IF microscopy and compatible clinical features support the diagnosis of BP. In case of epidermal binding by indirect IF microscopy and failure to detect Ig. G reactivity to both BP 180 and BP 230, testing for antibodies against α 6β 4 integrin is recommended (for example, by Western blotting of keratinocyte extract).
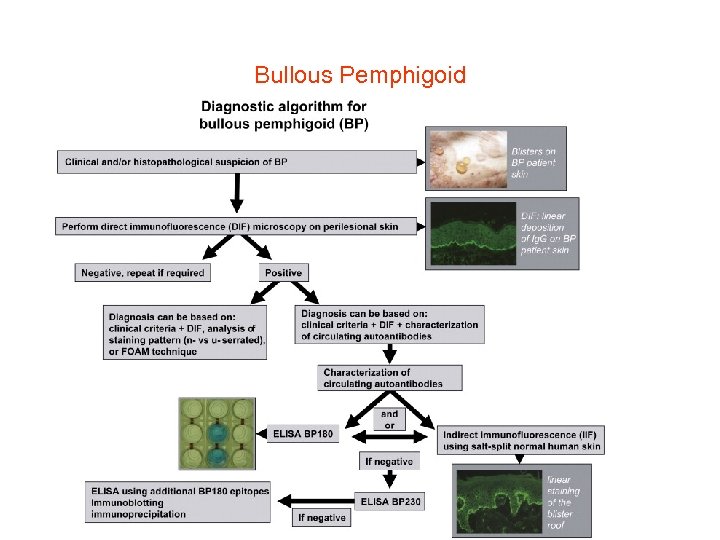
Bullous Pemphigoid
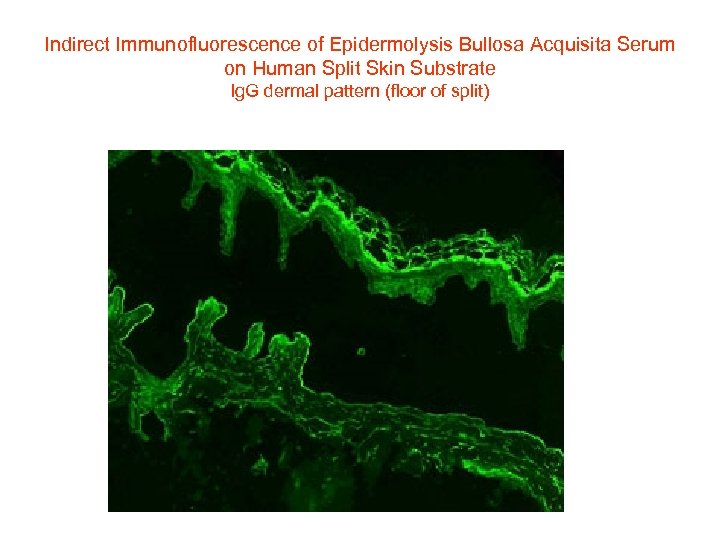
Indirect Immunofluorescence of Epidermolysis Bullosa Acquisita Serum on Human Split Skin Substrate Ig. G dermal pattern (floor of split)
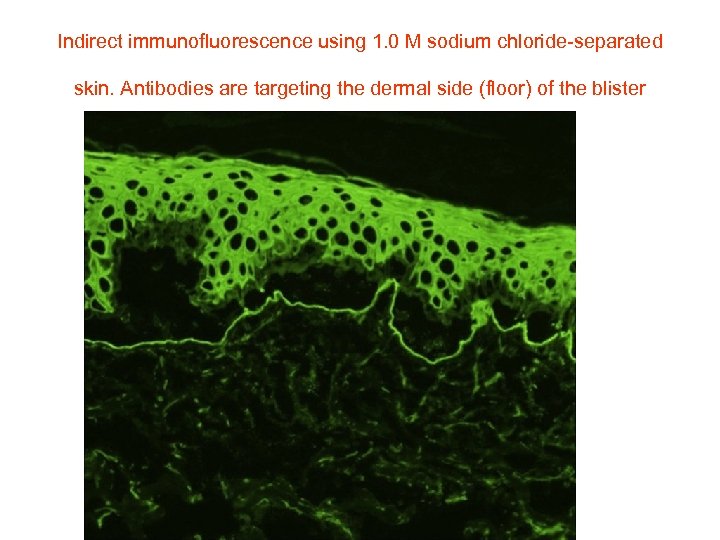
Indirect immunofluorescence using 1. 0 M sodium chloride-separated skin. Antibodies are targeting the dermal side (floor) of the blister
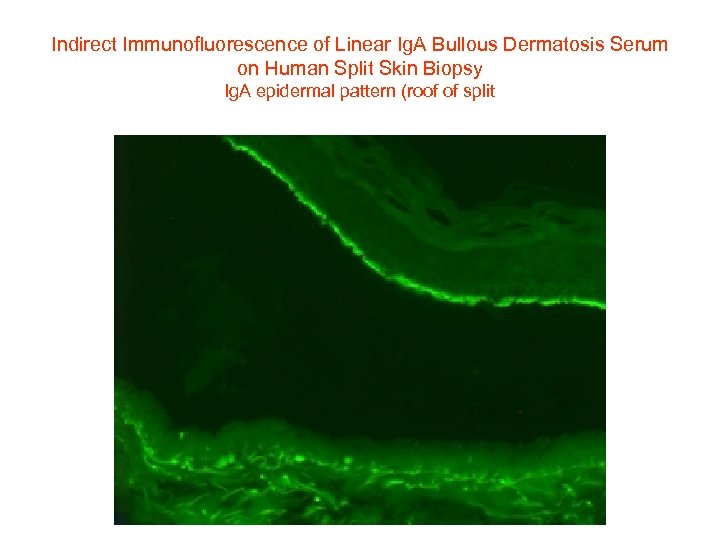
Indirect Immunofluorescence of Linear Ig. A Bullous Dermatosis Serum on Human Split Skin Biopsy Ig. A epidermal pattern (roof of split
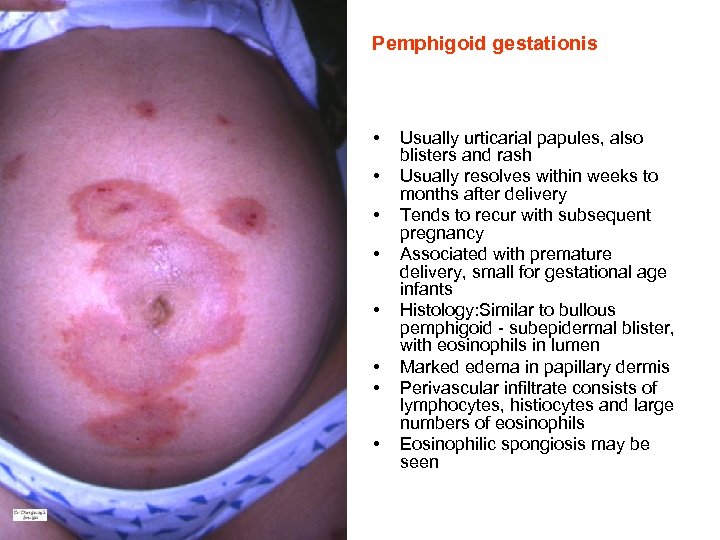
Pemphigoid gestationis • • Usually urticarial papules, also blisters and rash Usually resolves within weeks to months after delivery Tends to recur with subsequent pregnancy Associated with premature delivery, small for gestational age infants Histology: Similar to bullous pemphigoid - subepidermal blister, with eosinophils in lumen Marked edema in papillary dermis Perivascular infiltrate consists of lymphocytes, histiocytes and large numbers of eosinophils Eosinophilic spongiosis may be seen
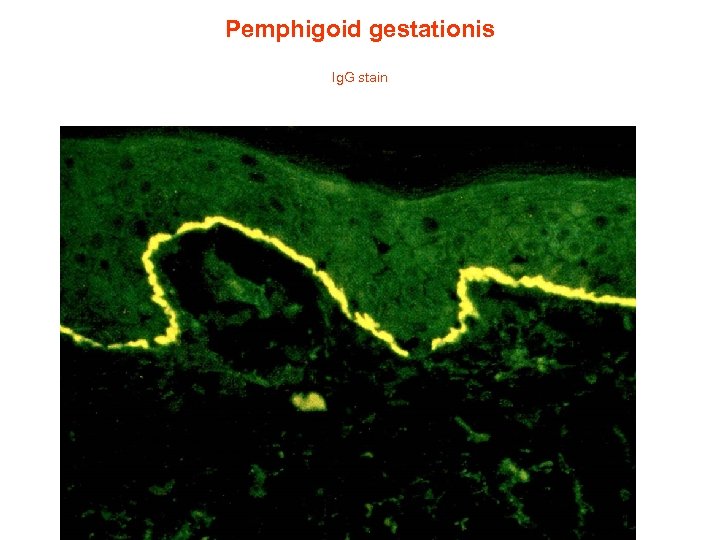
Pemphigoid gestationis Ig. G stain
Differential diagnosis of INDIRECT IMMUNOFLUORESCENCE f • • • Indirect IF This is tested in serum. - ANA for Systemic lupus erythematosus (SLE) - Ig. G anti-ICS antibodies – Pemphigus - Ig. A anti-ICS antibodies – Ig. A pemphigus - Ig. G anti BMZ - SLE, BP, HG, EBV, Bullous SLE Diseases with immune deposits along the dermoepidermal junction LE Dermatomyositis Systemic sclerosis LCV Rheumatoid arthritis BP HG EBA DH Linear Ig. A bullous dermatosis PCT Pseudoporphyria LP Rosacea Chronic active hepatitis Primary biliary cirrhosis

386736c14e17c5c41121ee0f4ed64486.ppt