General Approaches to Polymer Synthesis 1. Addition Chain



39682-ch5_polycondensation_processes1_f2006_daly.ppt
- Количество слайдов: 30
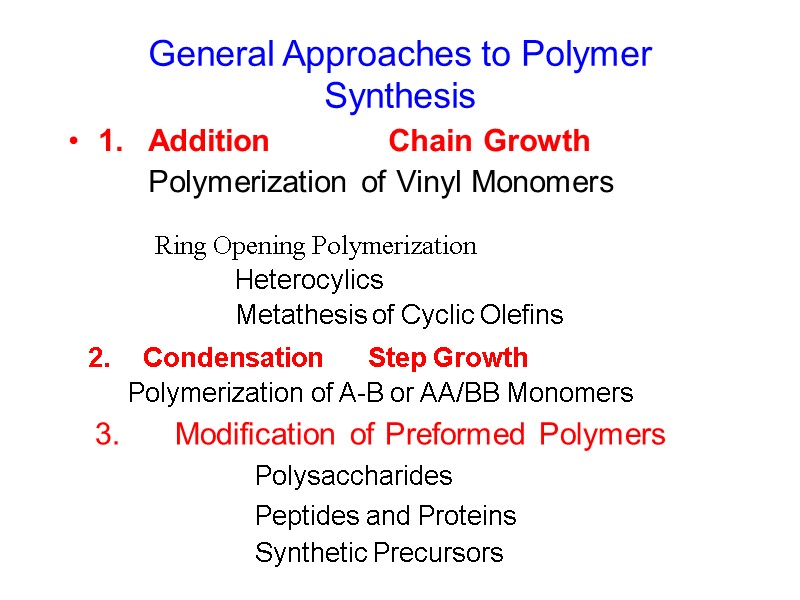
 General Approaches to Polymer Synthesis 1. Addition Chain Growth Polymerization of Vinyl Monomers Ring Opening Polymerization Heterocylics Metathesis of Cyclic Olefins 2. Condensation Step Growth Polymerization of A-B or AA/BB Monomers 3. Modification of Preformed Polymers Polysaccharides Peptides and Proteins Synthetic Precursors
General Approaches to Polymer Synthesis 1. Addition Chain Growth Polymerization of Vinyl Monomers Ring Opening Polymerization Heterocylics Metathesis of Cyclic Olefins 2. Condensation Step Growth Polymerization of A-B or AA/BB Monomers 3. Modification of Preformed Polymers Polysaccharides Peptides and Proteins Synthetic Precursors
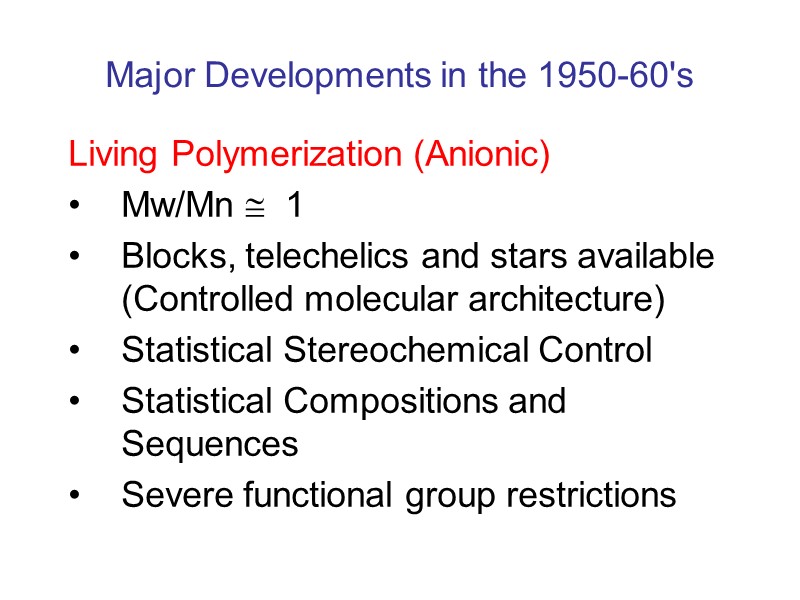
 Major Developments in the 1950-60's Living Polymerization (Anionic) Mw/Mn 1 Blocks, telechelics and stars available (Controlled molecular architecture) Statistical Stereochemical Control Statistical Compositions and Sequences Severe functional group restrictions
Major Developments in the 1950-60's Living Polymerization (Anionic) Mw/Mn 1 Blocks, telechelics and stars available (Controlled molecular architecture) Statistical Stereochemical Control Statistical Compositions and Sequences Severe functional group restrictions
 Ziegler-Natta (Metal-Coordinated) Polymerization Stereochemical Control Polydisperse products Statistical Compositions and Sequences Limited set of useful monomers, i.e. olefins SINGLE SITE CATALYSTS
Ziegler-Natta (Metal-Coordinated) Polymerization Stereochemical Control Polydisperse products Statistical Compositions and Sequences Limited set of useful monomers, i.e. olefins SINGLE SITE CATALYSTS
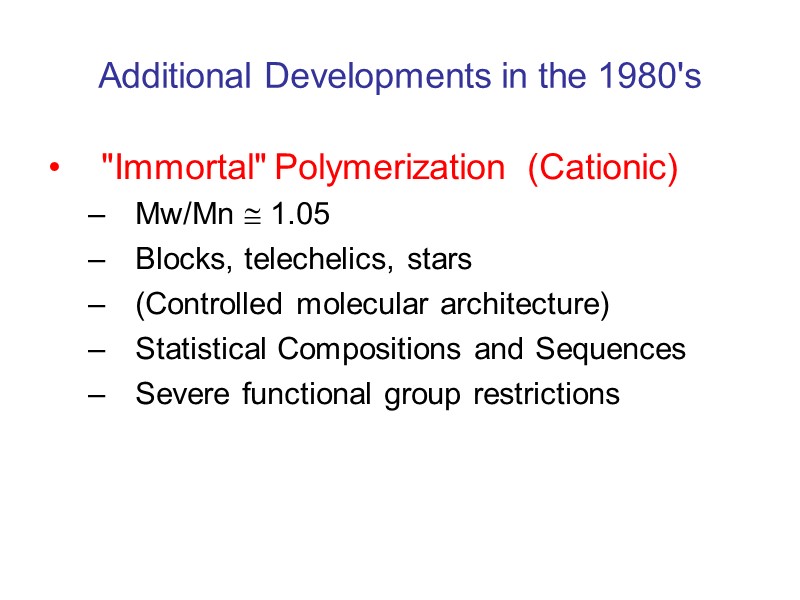
 Additional Developments in the 1980's "Immortal" Polymerization (Cationic) Mw/Mn 1.05 Blocks, telechelics, stars (Controlled molecular architecture) Statistical Compositions and Sequences Severe functional group restrictions
Additional Developments in the 1980's "Immortal" Polymerization (Cationic) Mw/Mn 1.05 Blocks, telechelics, stars (Controlled molecular architecture) Statistical Compositions and Sequences Severe functional group restrictions
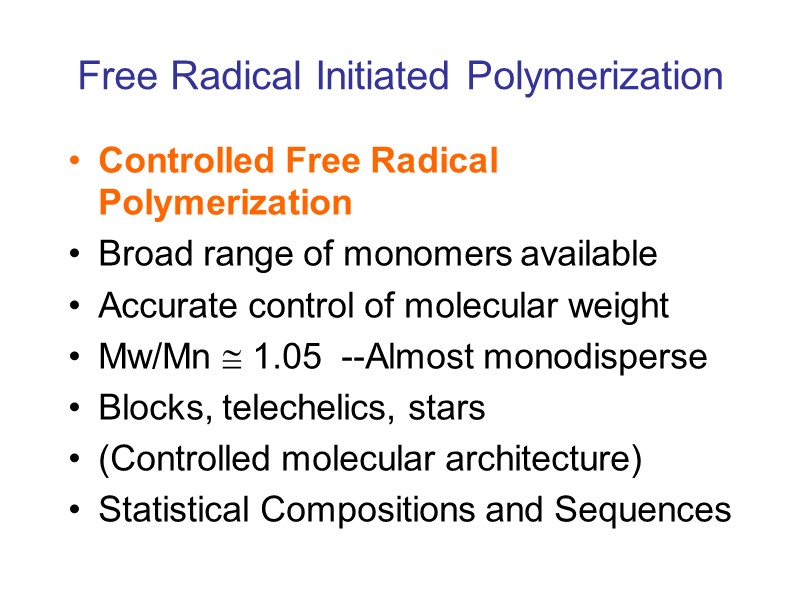
 Free Radical Initiated Polymerization Controlled Free Radical Polymerization Broad range of monomers available Accurate control of molecular weight Mw/Mn 1.05 --Almost monodisperse Blocks, telechelics, stars (Controlled molecular architecture) Statistical Compositions and Sequences
Free Radical Initiated Polymerization Controlled Free Radical Polymerization Broad range of monomers available Accurate control of molecular weight Mw/Mn 1.05 --Almost monodisperse Blocks, telechelics, stars (Controlled molecular architecture) Statistical Compositions and Sequences
 Current Strategies in Polymer Synthesis Objectives: Precise Macromolecular Design 1 . Control of: Molecular Weight Molecular Weight Distribution Composition Sequence of repeat units Stereochemistry 2. Versatility
Current Strategies in Polymer Synthesis Objectives: Precise Macromolecular Design 1 . Control of: Molecular Weight Molecular Weight Distribution Composition Sequence of repeat units Stereochemistry 2. Versatility
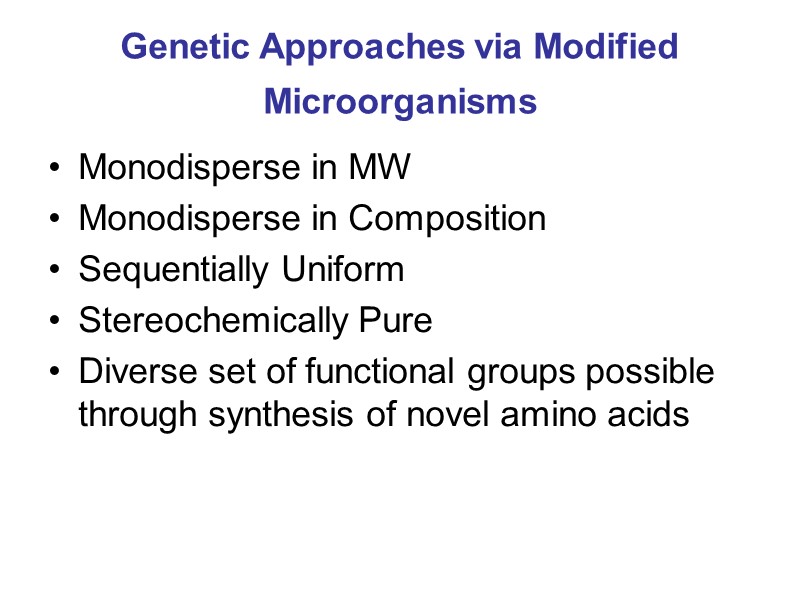
 Genetic Approaches via Modified Microorganisms Monodisperse in MW Monodisperse in Composition Sequentially Uniform Stereochemically Pure Diverse set of functional groups possible through synthesis of novel amino acids
Genetic Approaches via Modified Microorganisms Monodisperse in MW Monodisperse in Composition Sequentially Uniform Stereochemically Pure Diverse set of functional groups possible through synthesis of novel amino acids
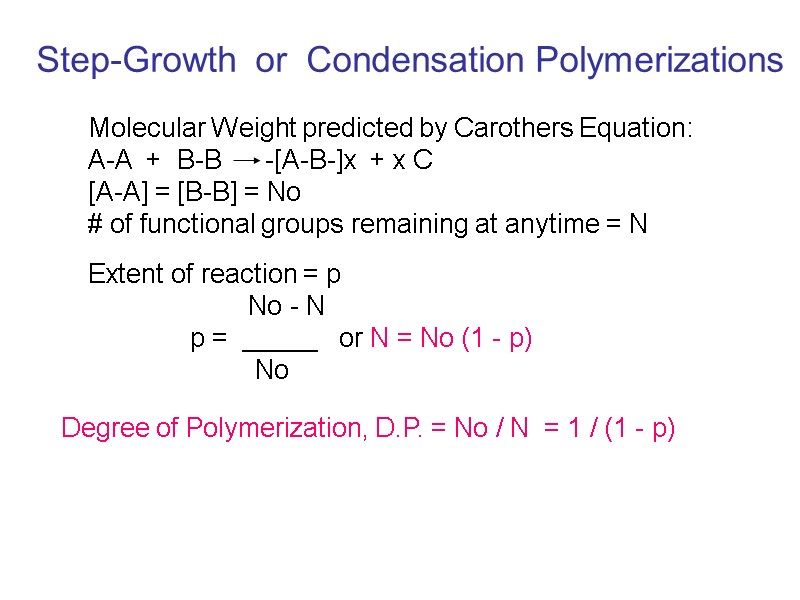
 Step-Growth or Condensation Polymerizations Molecular Weight predicted by Carothers Equation: A-A + B-B -[A-B-]x + x C [A-A] = [B-B] = No # of functional groups remaining at anytime = N Extent of reaction = p No - N p = _____ or N = No (1 - p) No Degree of Polymerization, D.P. = No / N = 1 / (1 - p)
Step-Growth or Condensation Polymerizations Molecular Weight predicted by Carothers Equation: A-A + B-B -[A-B-]x + x C [A-A] = [B-B] = No # of functional groups remaining at anytime = N Extent of reaction = p No - N p = _____ or N = No (1 - p) No Degree of Polymerization, D.P. = No / N = 1 / (1 - p)
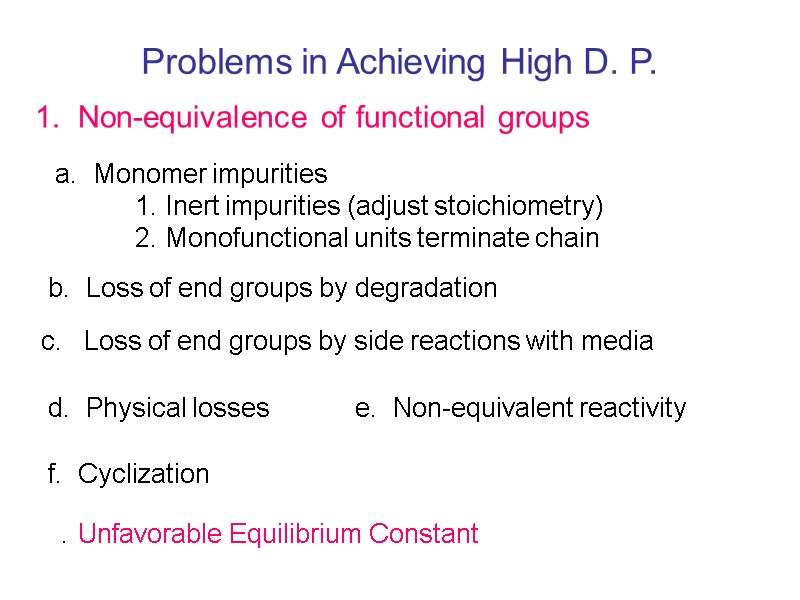
 Problems in Achieving High D. P. 1. Non-equivalence of functional groups a. Monomer impurities 1. Inert impurities (adjust stoichiometry) 2. Monofunctional units terminate chain b. Loss of end groups by degradation c. Loss of end groups by side reactions with media d. Physical losses e. Non-equivalent reactivity f. Cyclization . Unfavorable Equilibrium Constant
Problems in Achieving High D. P. 1. Non-equivalence of functional groups a. Monomer impurities 1. Inert impurities (adjust stoichiometry) 2. Monofunctional units terminate chain b. Loss of end groups by degradation c. Loss of end groups by side reactions with media d. Physical losses e. Non-equivalent reactivity f. Cyclization . Unfavorable Equilibrium Constant
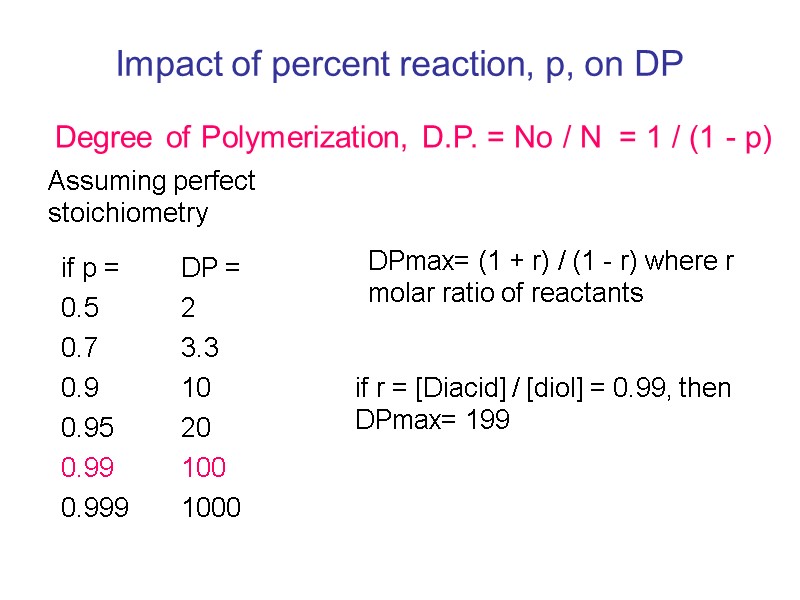
 Impact of percent reaction, p, on DP Degree of Polymerization, D.P. = No / N = 1 / (1 - p) Assuming perfect stoichiometry DPmax= (1 + r) / (1 - r) where r molar ratio of reactants if r = [Diacid] / [diol] = 0.99, then DPmax= 199
Impact of percent reaction, p, on DP Degree of Polymerization, D.P. = No / N = 1 / (1 - p) Assuming perfect stoichiometry DPmax= (1 + r) / (1 - r) where r molar ratio of reactants if r = [Diacid] / [diol] = 0.99, then DPmax= 199
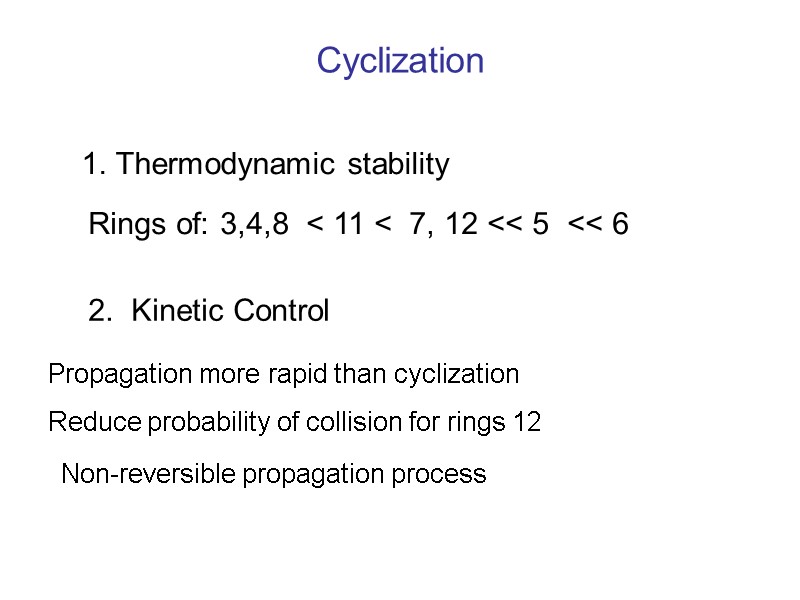
 Cyclization 1. Thermodynamic stability Rings of: 3,4,8 < 11 < 7, 12 << 5 << 6 2. Kinetic Control Propagation more rapid than cyclization Reduce probability of collision for rings 12 Non-reversible propagation process
Cyclization 1. Thermodynamic stability Rings of: 3,4,8 < 11 < 7, 12 << 5 << 6 2. Kinetic Control Propagation more rapid than cyclization Reduce probability of collision for rings 12 Non-reversible propagation process
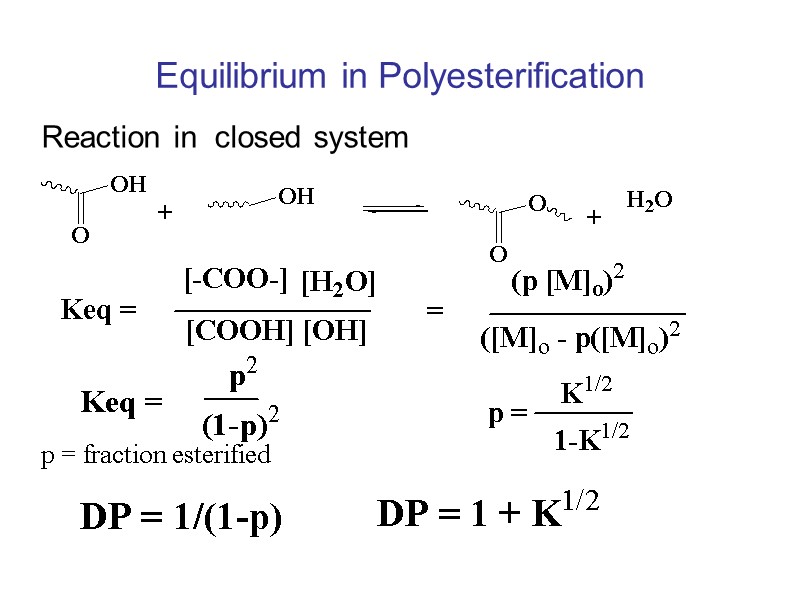
 Equilibrium in Polyesterification Reaction in closed system p = fraction esterified
Equilibrium in Polyesterification Reaction in closed system p = fraction esterified
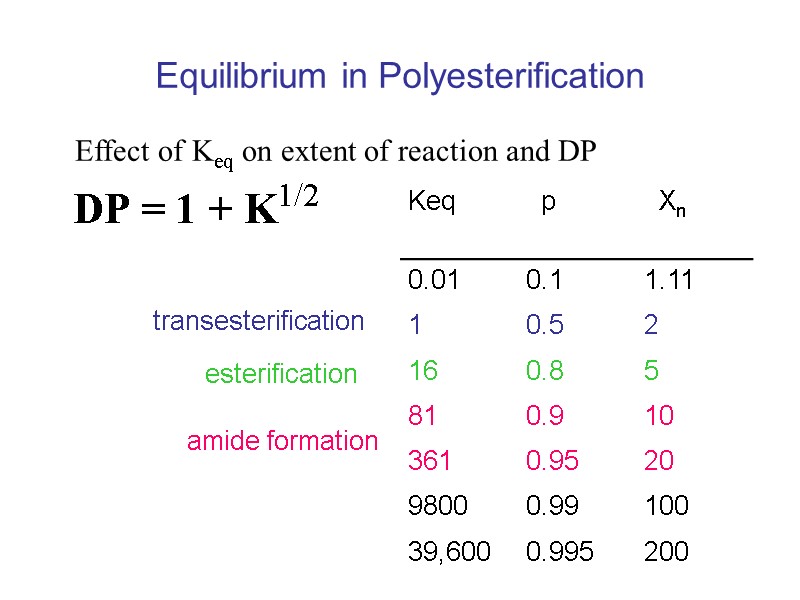
 Equilibrium in Polyesterification Effect of Keq on extent of reaction and DP transesterification esterification amide formation
Equilibrium in Polyesterification Effect of Keq on extent of reaction and DP transesterification esterification amide formation
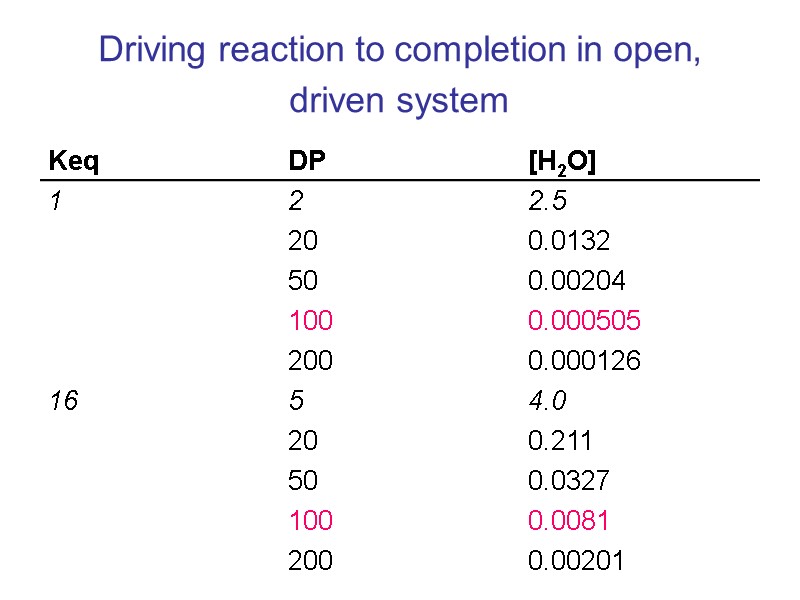
 Driving reaction to completion in open, driven system
Driving reaction to completion in open, driven system
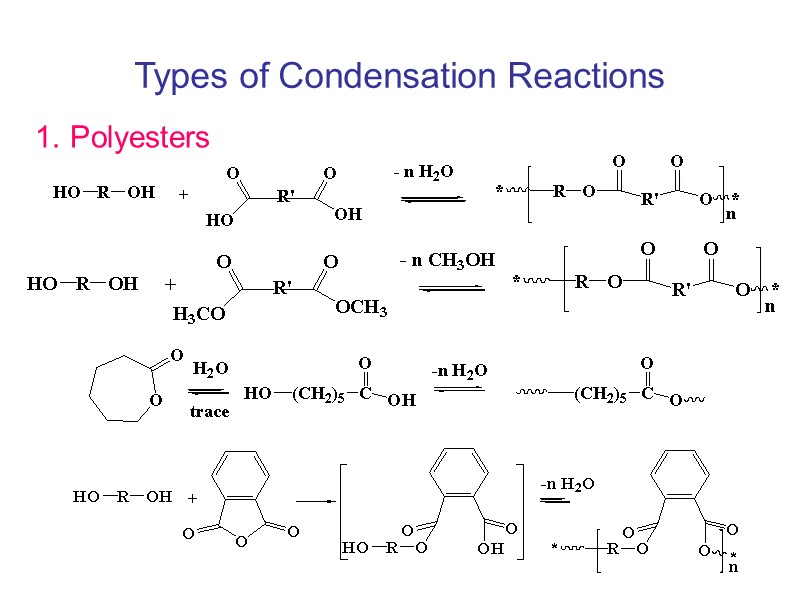
 Types of Condensation Reactions 1. Polyesters
Types of Condensation Reactions 1. Polyesters
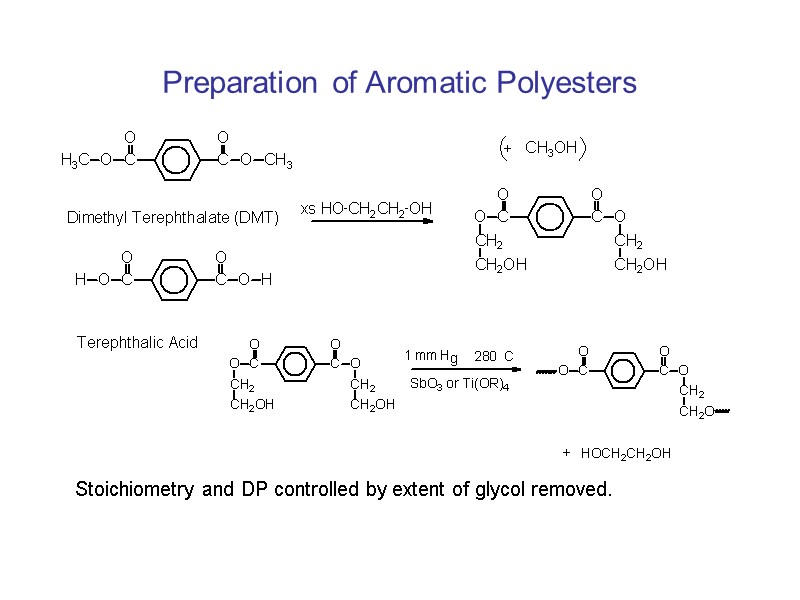
 Preparation of Aromatic Polyesters Stoichiometry and DP controlled by extent of glycol removed.
Preparation of Aromatic Polyesters Stoichiometry and DP controlled by extent of glycol removed.
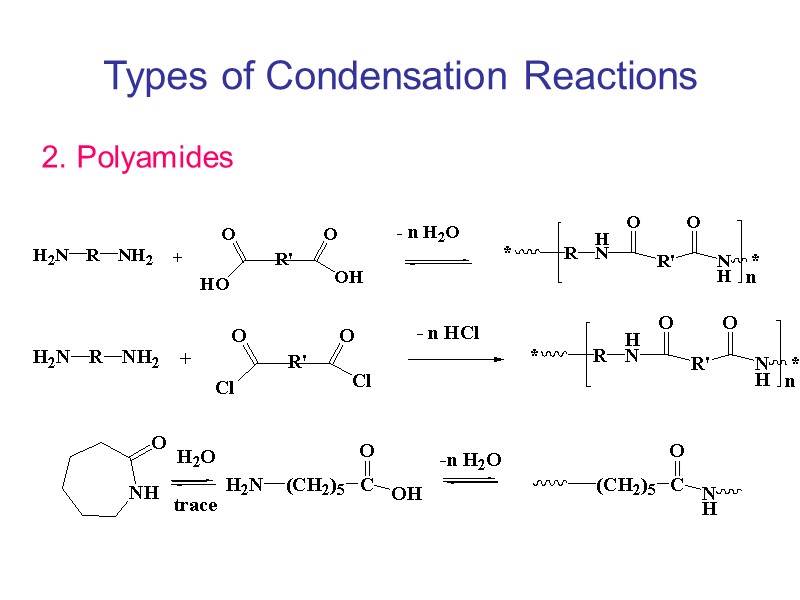
 Types of Condensation Reactions 2. Polyamides
Types of Condensation Reactions 2. Polyamides
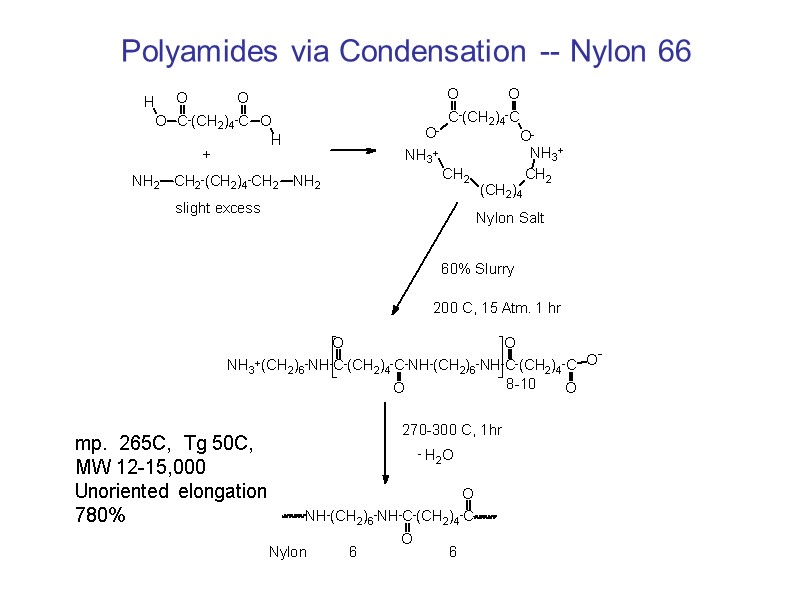
 Polyamides via Condensation -- Nylon 66 mp. 265C, Tg 50C, MW 12-15,000 Unoriented elongation 780%
Polyamides via Condensation -- Nylon 66 mp. 265C, Tg 50C, MW 12-15,000 Unoriented elongation 780%
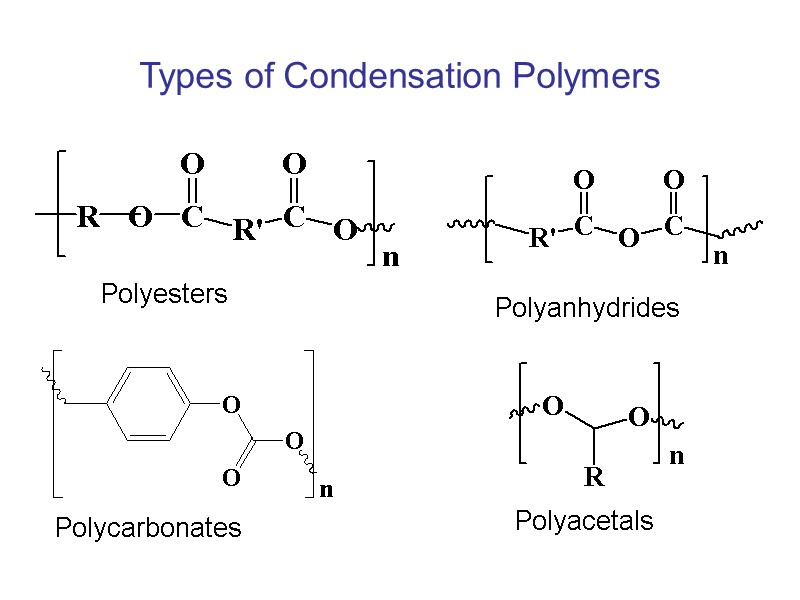
 Types of Condensation Polymers Polyesters Polycarbonates Polyanhydrides Polyacetals
Types of Condensation Polymers Polyesters Polycarbonates Polyanhydrides Polyacetals
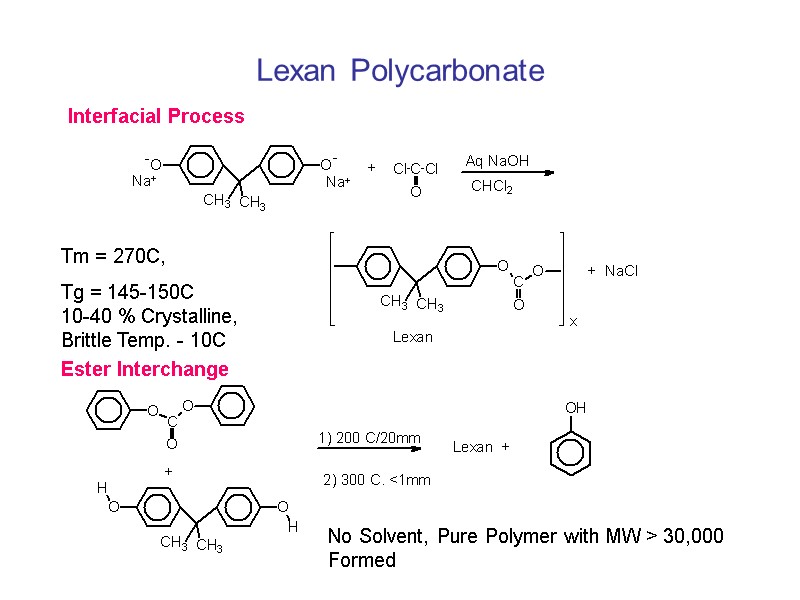
 Lexan Polycarbonate Interfacial Process Tm = 270C, Tg = 145-150C 10-40 % Crystalline, Brittle Temp. - 10C Ester Interchange No Solvent, Pure Polymer with MW > 30,000 Formed
Lexan Polycarbonate Interfacial Process Tm = 270C, Tg = 145-150C 10-40 % Crystalline, Brittle Temp. - 10C Ester Interchange No Solvent, Pure Polymer with MW > 30,000 Formed
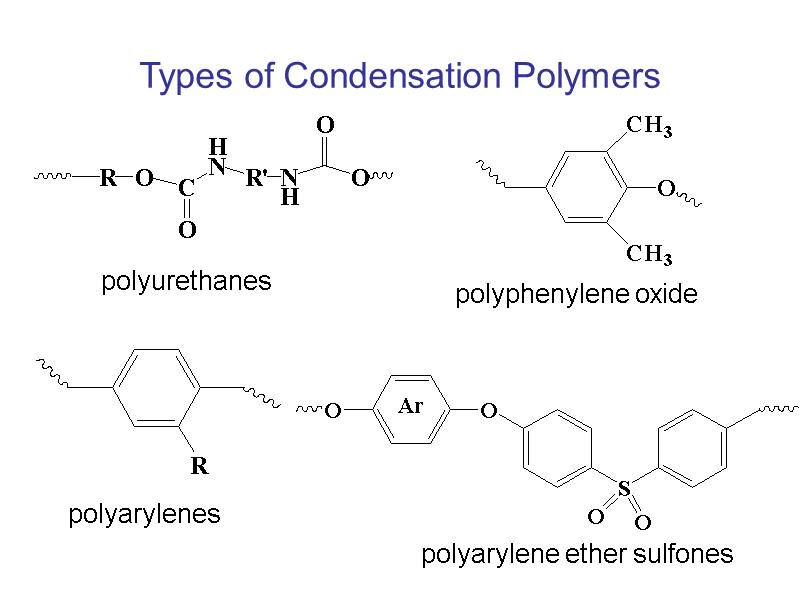
 Types of Condensation Polymers polyurethanes polyphenylene oxide polyarylenes polyarylene ether sulfones
Types of Condensation Polymers polyurethanes polyphenylene oxide polyarylenes polyarylene ether sulfones
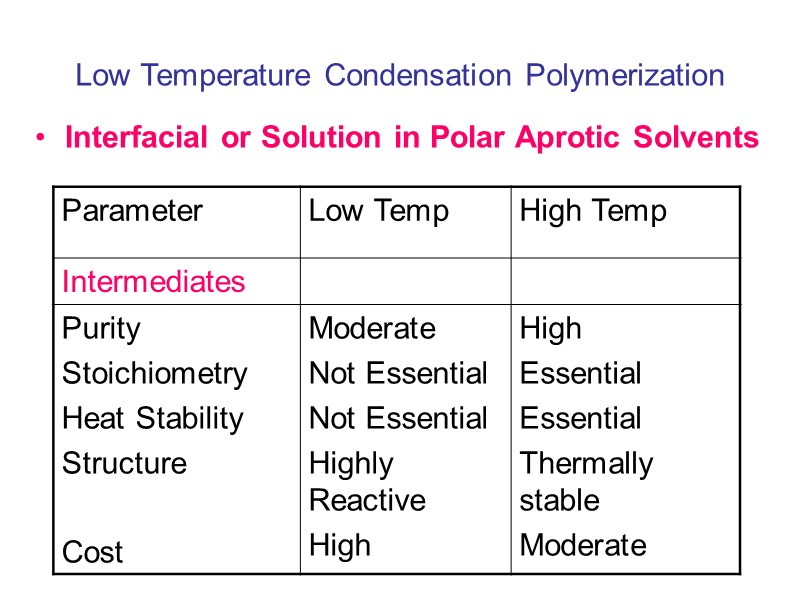
 Low Temperature Condensation Polymerization Interfacial or Solution in Polar Aprotic Solvents
Low Temperature Condensation Polymerization Interfacial or Solution in Polar Aprotic Solvents
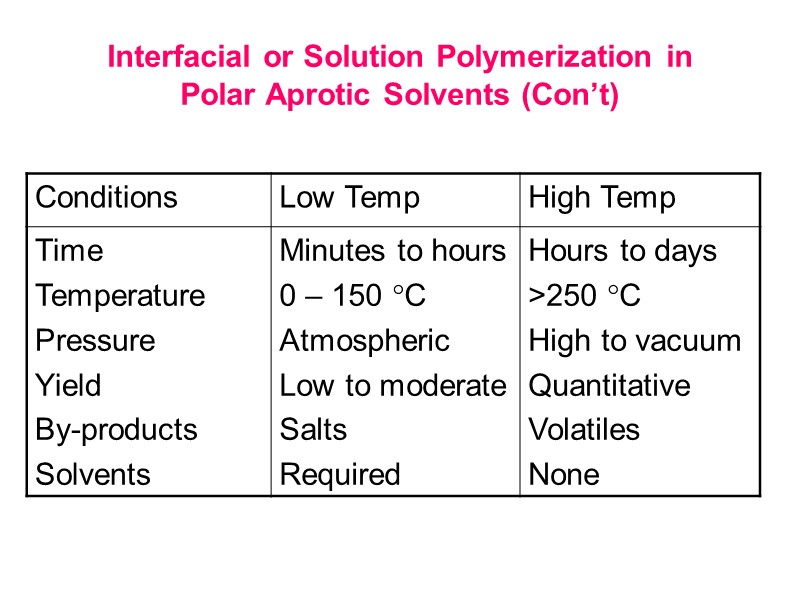
 Interfacial or Solution Polymerization in Polar Aprotic Solvents (Con’t)
Interfacial or Solution Polymerization in Polar Aprotic Solvents (Con’t)
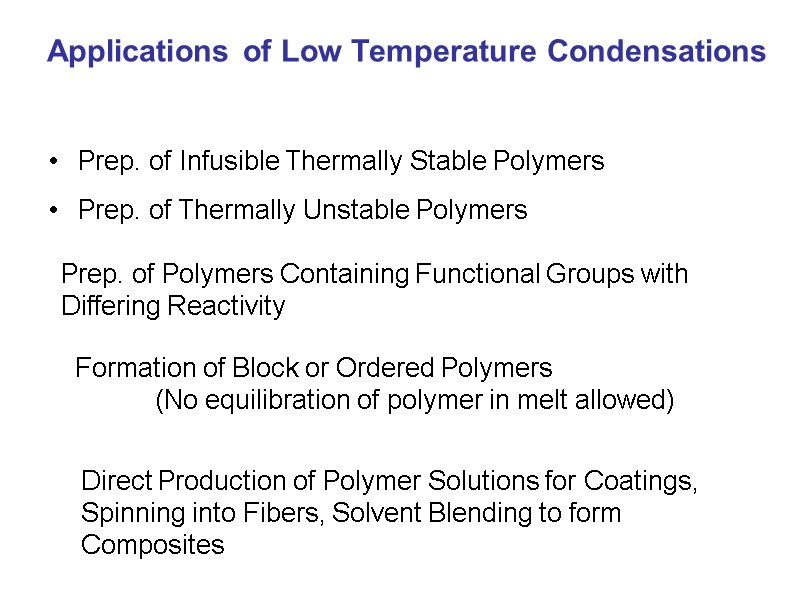
 Applications of Low Temperature Condensations Prep. of Infusible Thermally Stable Polymers Prep. of Thermally Unstable Polymers Prep. of Polymers Containing Functional Groups with Differing Reactivity Formation of Block or Ordered Polymers (No equilibration of polymer in melt allowed) Direct Production of Polymer Solutions for Coatings, Spinning into Fibers, Solvent Blending to form Composites
Applications of Low Temperature Condensations Prep. of Infusible Thermally Stable Polymers Prep. of Thermally Unstable Polymers Prep. of Polymers Containing Functional Groups with Differing Reactivity Formation of Block or Ordered Polymers (No equilibration of polymer in melt allowed) Direct Production of Polymer Solutions for Coatings, Spinning into Fibers, Solvent Blending to form Composites
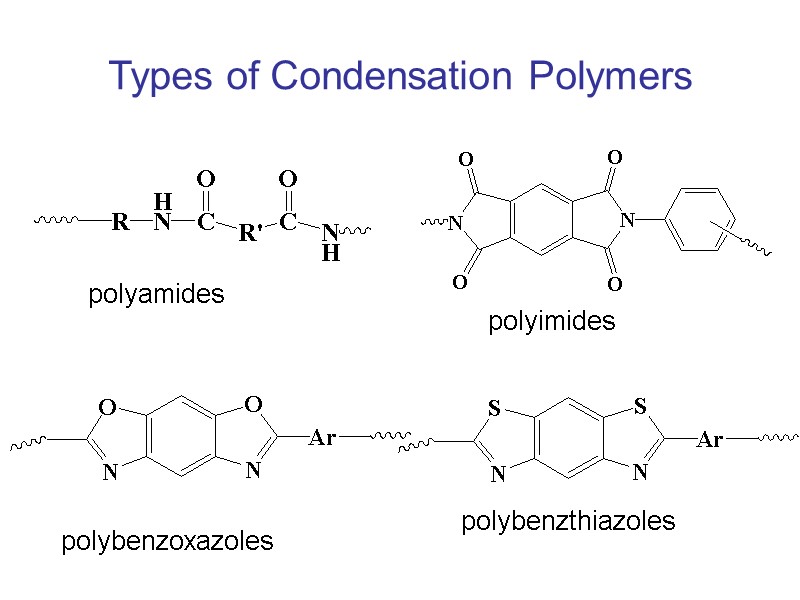
 Types of Condensation Polymers polyamides polyimides polybenzoxazoles polybenzthiazoles
Types of Condensation Polymers polyamides polyimides polybenzoxazoles polybenzthiazoles
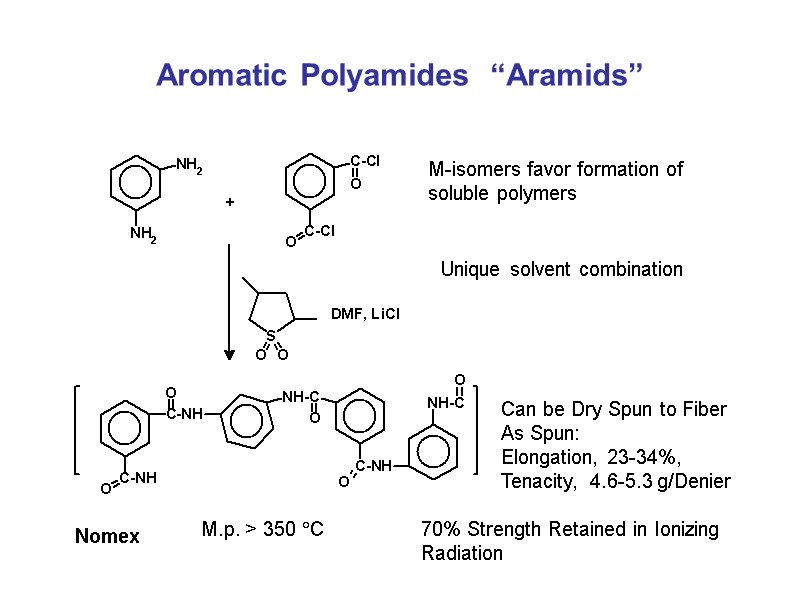
 Aromatic Polyamides “Aramids” Can be Dry Spun to Fiber As Spun: Elongation, 23-34%, Tenacity, 4.6-5.3 g/Denier 70% Strength Retained in Ionizing Radiation Nomex M.p. > 350 C Unique solvent combination M-isomers favor formation of soluble polymers
Aromatic Polyamides “Aramids” Can be Dry Spun to Fiber As Spun: Elongation, 23-34%, Tenacity, 4.6-5.3 g/Denier 70% Strength Retained in Ionizing Radiation Nomex M.p. > 350 C Unique solvent combination M-isomers favor formation of soluble polymers
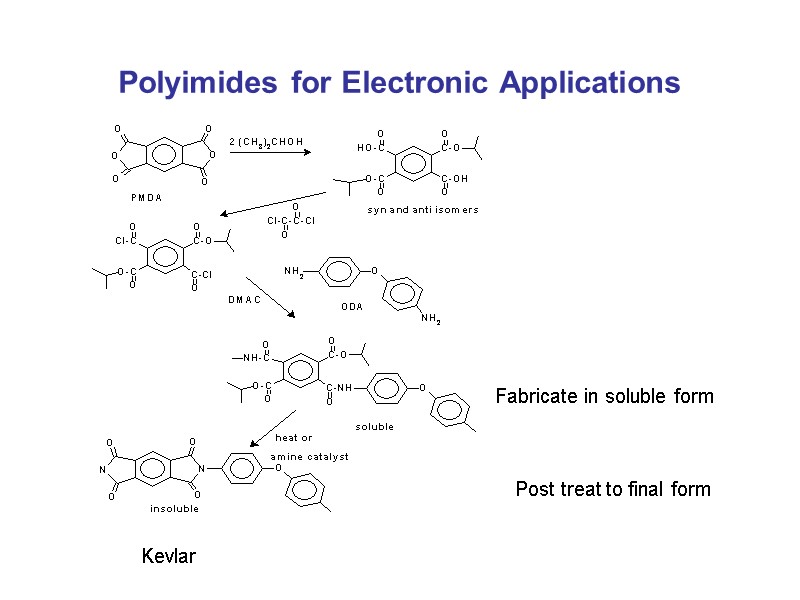
 Polyimides for Electronic Applications Kevlar Fabricate in soluble form Post treat to final form
Polyimides for Electronic Applications Kevlar Fabricate in soluble form Post treat to final form
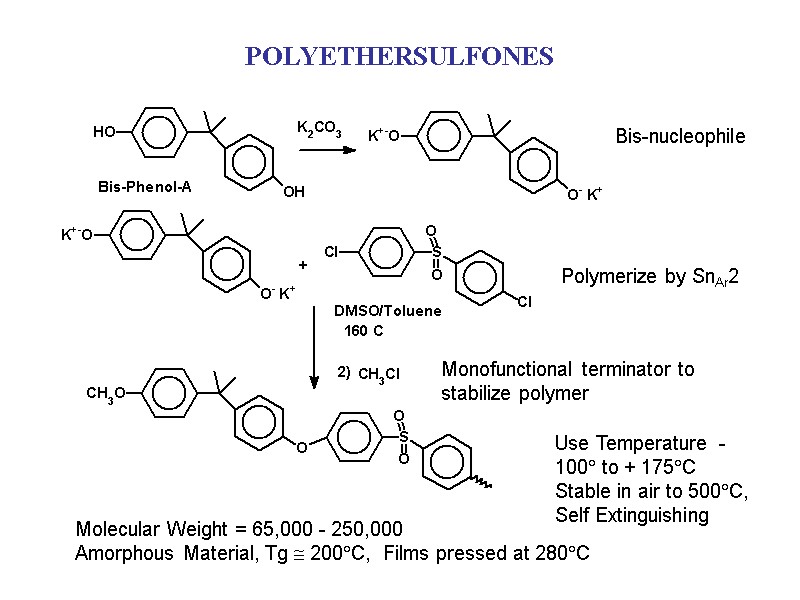
 POLYETHERSULFONES Molecular Weight = 65,000 - 250,000 Amorphous Material, Tg 200C, Films pressed at 280C Use Temperature -100 to + 175C Stable in air to 500C, Self Extinguishing Bis-nucleophile Polymerize by SnAr2 Monofunctional terminator to stabilize polymer
POLYETHERSULFONES Molecular Weight = 65,000 - 250,000 Amorphous Material, Tg 200C, Films pressed at 280C Use Temperature -100 to + 175C Stable in air to 500C, Self Extinguishing Bis-nucleophile Polymerize by SnAr2 Monofunctional terminator to stabilize polymer
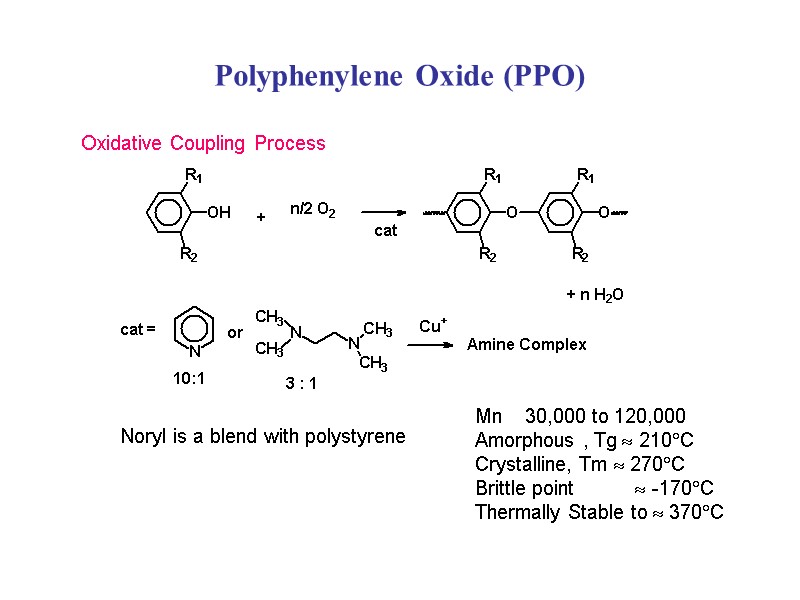
 Polyphenylene Oxide (PPO) Noryl is a blend with polystyrene Oxidative Coupling Process Mn 30,000 to 120,000 Amorphous , Tg 210C Crystalline, Tm 270C Brittle point -170C Thermally Stable to 370C
Polyphenylene Oxide (PPO) Noryl is a blend with polystyrene Oxidative Coupling Process Mn 30,000 to 120,000 Amorphous , Tg 210C Crystalline, Tm 270C Brittle point -170C Thermally Stable to 370C
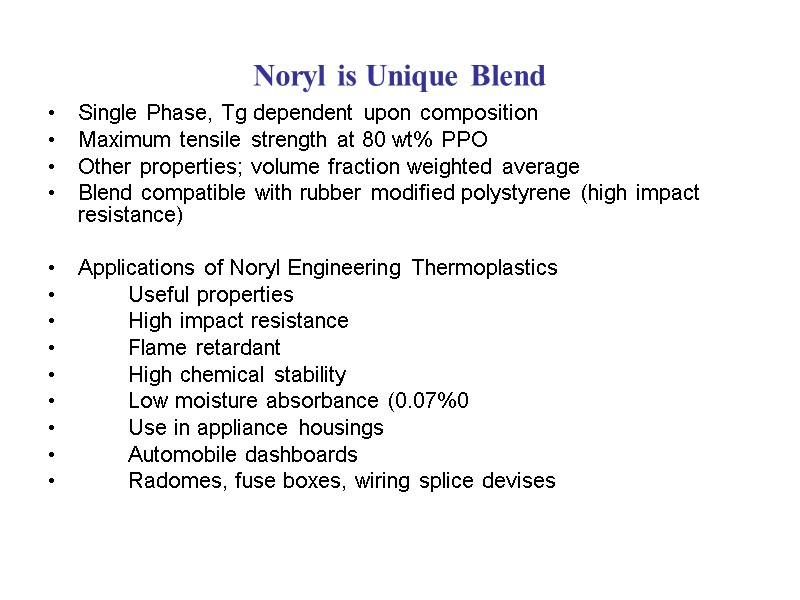
 Noryl is Unique Blend Single Phase, Tg dependent upon composition Maximum tensile strength at 80 wt% PPO Other properties; volume fraction weighted average Blend compatible with rubber modified polystyrene (high impact resistance) Applications of Noryl Engineering Thermoplastics Useful properties High impact resistance Flame retardant High chemical stability Low moisture absorbance (0.07%0 Use in appliance housings Automobile dashboards Radomes, fuse boxes, wiring splice devises
Noryl is Unique Blend Single Phase, Tg dependent upon composition Maximum tensile strength at 80 wt% PPO Other properties; volume fraction weighted average Blend compatible with rubber modified polystyrene (high impact resistance) Applications of Noryl Engineering Thermoplastics Useful properties High impact resistance Flame retardant High chemical stability Low moisture absorbance (0.07%0 Use in appliance housings Automobile dashboards Radomes, fuse boxes, wiring splice devises

