d8b26eb8fa3d20b68c8f3fc9c5e7d115.ppt
- Количество слайдов: 34
 Coronary Stents: Understanding Differences in Design, Material, Drug, Usage Alexandra Lansky, MD Yale School of Medicine University College of London Director Interventional Cardiology Research New Haven, CT
Coronary Stents: Understanding Differences in Design, Material, Drug, Usage Alexandra Lansky, MD Yale School of Medicine University College of London Director Interventional Cardiology Research New Haven, CT
 Alexandra J. Lansky, MD I have no real or apparent conflicts of interest to report.
Alexandra J. Lansky, MD I have no real or apparent conflicts of interest to report.
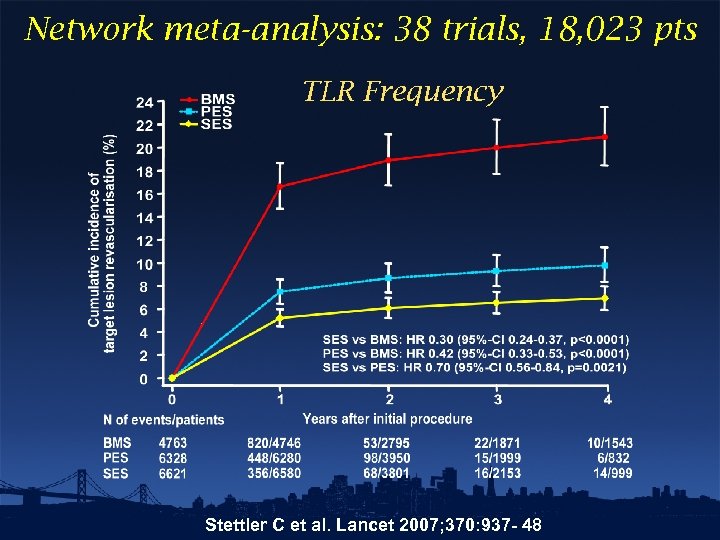 Network meta-analysis: 38 trials, 18, 023 pts TLR Frequency Stettler C et al. Lancet 2007; 370: 937 - 48
Network meta-analysis: 38 trials, 18, 023 pts TLR Frequency Stettler C et al. Lancet 2007; 370: 937 - 48
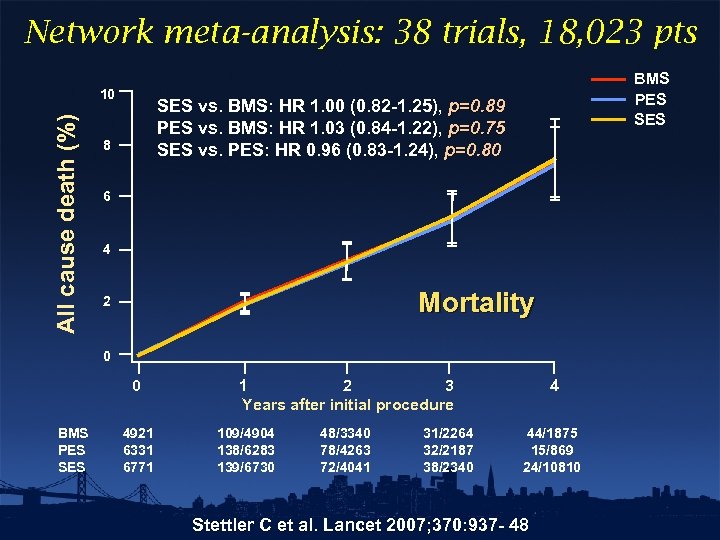 Network meta-analysis: 38 trials, 18, 023 pts All cause death (%) 10 BMS PES SES vs. BMS: HR 1. 00 (0. 82 -1. 25), p=0. 89 PES vs. BMS: HR 1. 03 (0. 84 -1. 22), p=0. 75 SES vs. PES: HR 0. 96 (0. 83 -1. 24), p=0. 80 8 6 4 Mortality 2 0 0 BMS PES SES 4921 6331 6771 1 2 3 Years after initial procedure 109/4904 138/6283 139/6730 48/3340 78/4263 72/4041 31/2264 32/2187 38/2340 4 44/1875 15/869 24/10810 Stettler C et al. Lancet 2007; 370: 937 - 48
Network meta-analysis: 38 trials, 18, 023 pts All cause death (%) 10 BMS PES SES vs. BMS: HR 1. 00 (0. 82 -1. 25), p=0. 89 PES vs. BMS: HR 1. 03 (0. 84 -1. 22), p=0. 75 SES vs. PES: HR 0. 96 (0. 83 -1. 24), p=0. 80 8 6 4 Mortality 2 0 0 BMS PES SES 4921 6331 6771 1 2 3 Years after initial procedure 109/4904 138/6283 139/6730 48/3340 78/4263 72/4041 31/2264 32/2187 38/2340 4 44/1875 15/869 24/10810 Stettler C et al. Lancet 2007; 370: 937 - 48
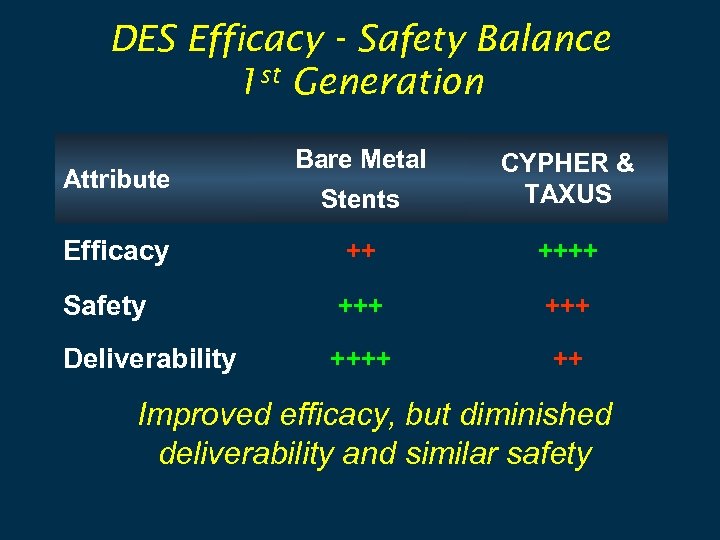 DES Efficacy - Safety Balance 1 st Generation Bare Metal Stents CYPHER & TAXUS Efficacy ++ ++++ Safety +++ Deliverability ++++ ++ Attribute Improved efficacy, but diminished deliverability and similar safety
DES Efficacy - Safety Balance 1 st Generation Bare Metal Stents CYPHER & TAXUS Efficacy ++ ++++ Safety +++ Deliverability ++++ ++ Attribute Improved efficacy, but diminished deliverability and similar safety
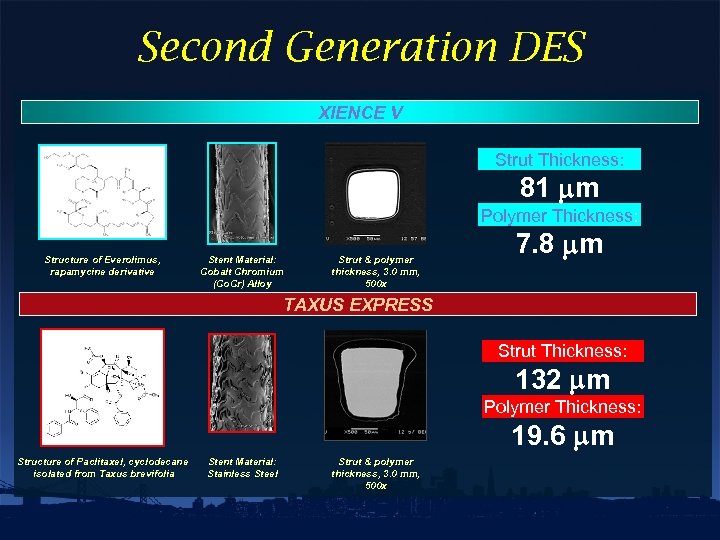 Second Generation DES XIENCE V Strut Thickness: 81 mm Polymer Thickness: Structure of Everolimus, rapamycine derivative Stent Material: Cobalt Chromium (Co. Cr) Alloy Strut & polymer thickness, 3. 0 mm, 500 x 7. 8 mm TAXUS EXPRESS Strut Thickness: 132 mm Polymer Thickness: 19. 6 mm Structure of Paclitaxel, cyclodecane isolated from Taxus brevifolia Stent Material: Stainless Steel Strut & polymer thickness, 3. 0 mm, 500 x
Second Generation DES XIENCE V Strut Thickness: 81 mm Polymer Thickness: Structure of Everolimus, rapamycine derivative Stent Material: Cobalt Chromium (Co. Cr) Alloy Strut & polymer thickness, 3. 0 mm, 500 x 7. 8 mm TAXUS EXPRESS Strut Thickness: 132 mm Polymer Thickness: 19. 6 mm Structure of Paclitaxel, cyclodecane isolated from Taxus brevifolia Stent Material: Stainless Steel Strut & polymer thickness, 3. 0 mm, 500 x
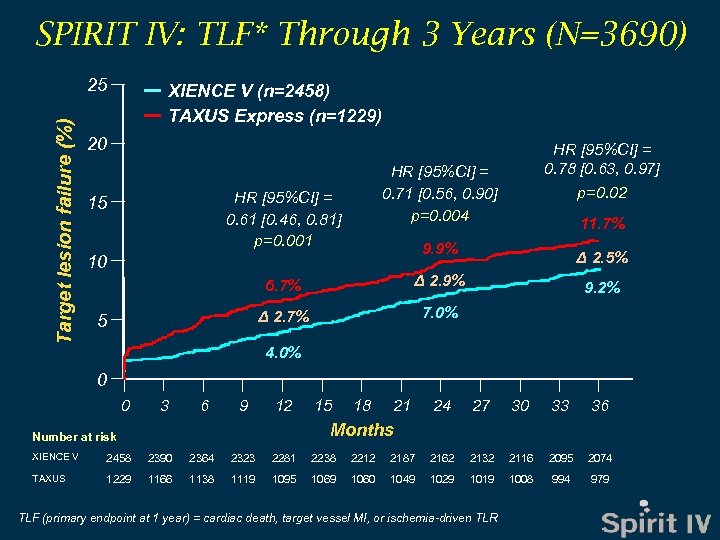 SPIRIT IV: TLF* Through 3 Years (N=3690) Target lesion failure (%) 25 XIENCE V (n=2458) TAXUS Express (n=1229) 20 HR [95%CI] = 0. 71 [0. 56, 0. 90] p=0. 004 HR [95%CI] = 0. 61 [0. 46, 0. 81] p=0. 001 15 11. 7% 9. 9% 10 6. 7% Δ 2. 5% Δ 2. 9% Δ 2. 7% 5 HR [95%CI] = 0. 78 [0. 63, 0. 97] p=0. 02 7. 0% 9. 2% 4. 0% 0 0 3 6 9 12 15 18 21 24 27 30 33 36 Months Number at risk XIENCE V 2458 2390 2364 2323 2281 2238 2212 2187 2162 2132 2116 2095 2074 TAXUS 1229 1166 1138 1119 1095 1069 1060 1049 1029 1019 1008 994 979 TLF (primary endpoint at 1 year) = cardiac death, target vessel MI, or ischemia-driven TLR
SPIRIT IV: TLF* Through 3 Years (N=3690) Target lesion failure (%) 25 XIENCE V (n=2458) TAXUS Express (n=1229) 20 HR [95%CI] = 0. 71 [0. 56, 0. 90] p=0. 004 HR [95%CI] = 0. 61 [0. 46, 0. 81] p=0. 001 15 11. 7% 9. 9% 10 6. 7% Δ 2. 5% Δ 2. 9% Δ 2. 7% 5 HR [95%CI] = 0. 78 [0. 63, 0. 97] p=0. 02 7. 0% 9. 2% 4. 0% 0 0 3 6 9 12 15 18 21 24 27 30 33 36 Months Number at risk XIENCE V 2458 2390 2364 2323 2281 2238 2212 2187 2162 2132 2116 2095 2074 TAXUS 1229 1166 1138 1119 1095 1069 1060 1049 1029 1019 1008 994 979 TLF (primary endpoint at 1 year) = cardiac death, target vessel MI, or ischemia-driven TLR
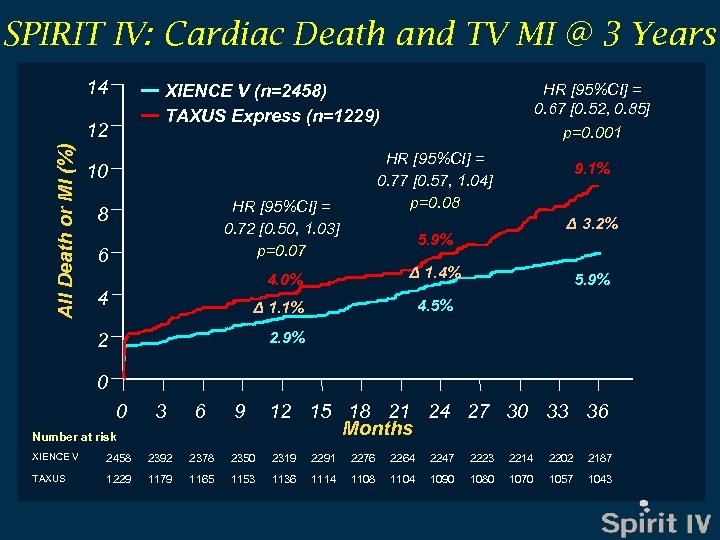 SPIRIT IV: Cardiac Death and TV MI @ 3 Years 14 XIENCE V (n=2458) TAXUS Express (n=1229) All Death or MI (%) 12 HR [95%CI] = 0. 77 [0. 57, 1. 04] p=0. 08 10 HR [95%CI] = 0. 72 [0. 50, 1. 03] p=0. 07 8 6 Δ 3. 2% Δ 1. 4% Δ 1. 1% 2 9. 1% 5. 9% 4. 0% 4 HR [95%CI] = 0. 67 [0. 52, 0. 85] p=0. 001 5. 9% 2. 9% 4. 5% 0 0 3 6 9 Number at risk 12 15 18 21 24 27 30 33 36 Months XIENCE V 2458 2392 2378 2350 2319 2291 2276 2264 2247 2223 2214 2202 2187 TAXUS 1229 1179 1165 1153 1136 1114 1108 1104 1090 1080 1070 1057 1043
SPIRIT IV: Cardiac Death and TV MI @ 3 Years 14 XIENCE V (n=2458) TAXUS Express (n=1229) All Death or MI (%) 12 HR [95%CI] = 0. 77 [0. 57, 1. 04] p=0. 08 10 HR [95%CI] = 0. 72 [0. 50, 1. 03] p=0. 07 8 6 Δ 3. 2% Δ 1. 4% Δ 1. 1% 2 9. 1% 5. 9% 4. 0% 4 HR [95%CI] = 0. 67 [0. 52, 0. 85] p=0. 001 5. 9% 2. 9% 4. 5% 0 0 3 6 9 Number at risk 12 15 18 21 24 27 30 33 36 Months XIENCE V 2458 2392 2378 2350 2319 2291 2276 2264 2247 2223 2214 2202 2187 TAXUS 1229 1179 1165 1153 1136 1114 1108 1104 1090 1080 1070 1057 1043
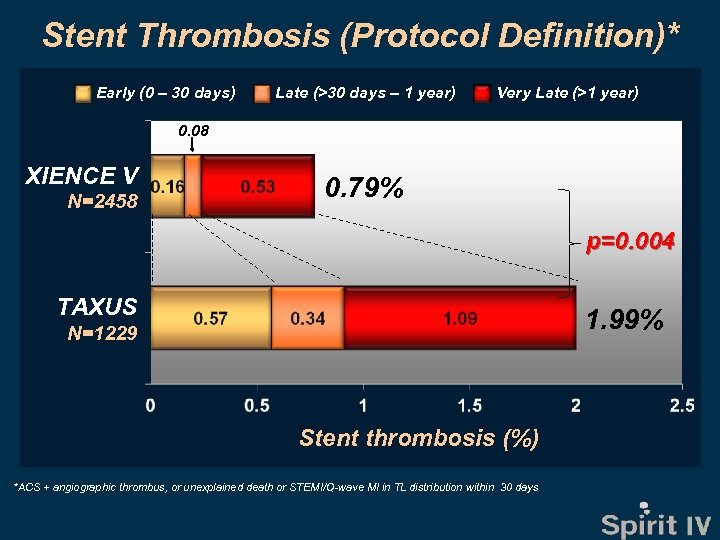 Stent Thrombosis (Protocol Definition)* Early (0 – 30 days) Late (>30 days – 1 year) Very Late (>1 year) 0. 08 XIENCE V N=2458 0. 79% p=0. 004 TAXUS 1. 99% N=1229 Stent thrombosis (%) *ACS + angiographic thrombus, or unexplained death or STEMI/Q-wave MI in TL distribution within 30 days
Stent Thrombosis (Protocol Definition)* Early (0 – 30 days) Late (>30 days – 1 year) Very Late (>1 year) 0. 08 XIENCE V N=2458 0. 79% p=0. 004 TAXUS 1. 99% N=1229 Stent thrombosis (%) *ACS + angiographic thrombus, or unexplained death or STEMI/Q-wave MI in TL distribution within 30 days
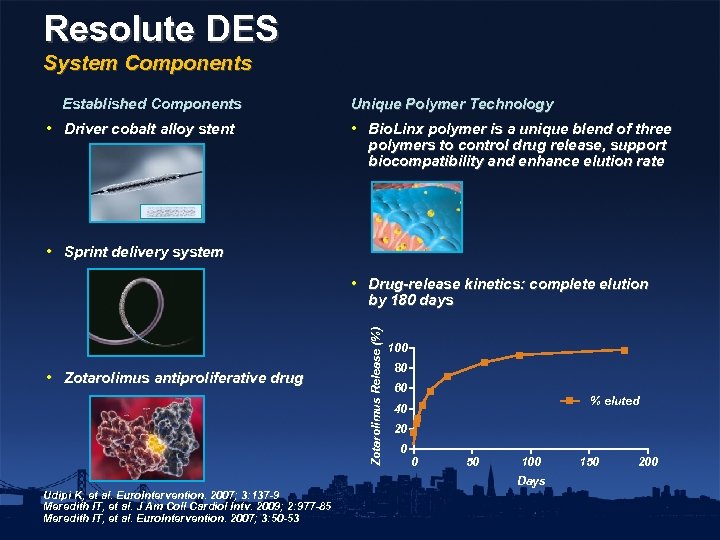 Resolute DES System Components Established Components • Driver cobalt alloy stent Unique Polymer Technology • Bio. Linx polymer is a unique blend of three polymers to control drug release, support biocompatibility and enhance elution rate • Sprint delivery system • Zotarolimus antiproliferative drug Zotarolimus Release (%) • Drug-release kinetics: complete elution by 180 days 100 80 60 % eluted 40 20 0 0 50 100 Days Udipi K, et al. Euro. Intervention. 2007; 3: 137 -9 Meredith IT, et al. J Am Coll Cardiol Intv. 2009; 2: 977 -85 Meredith IT, et al. Euro. Intervention. 2007; 3: 50 -53 150 200
Resolute DES System Components Established Components • Driver cobalt alloy stent Unique Polymer Technology • Bio. Linx polymer is a unique blend of three polymers to control drug release, support biocompatibility and enhance elution rate • Sprint delivery system • Zotarolimus antiproliferative drug Zotarolimus Release (%) • Drug-release kinetics: complete elution by 180 days 100 80 60 % eluted 40 20 0 0 50 100 Days Udipi K, et al. Euro. Intervention. 2007; 3: 137 -9 Meredith IT, et al. J Am Coll Cardiol Intv. 2009; 2: 977 -85 Meredith IT, et al. Euro. Intervention. 2007; 3: 50 -53 150 200
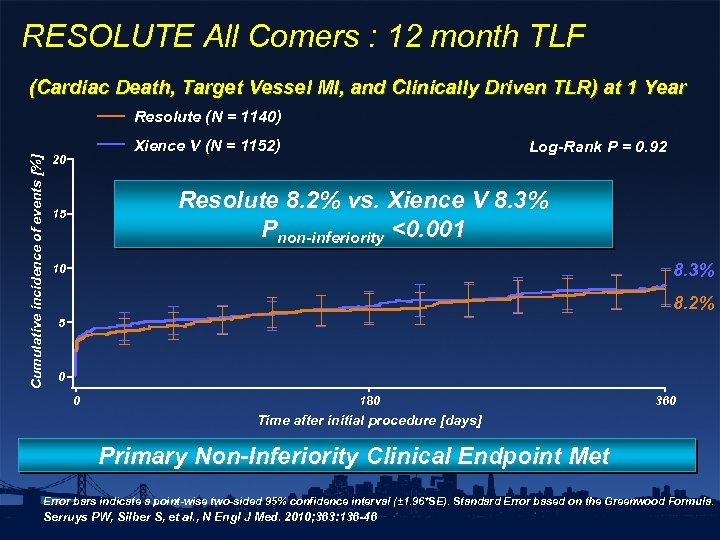 RESOLUTE All Comers : 12 month TLF (Cardiac Death, Target Vessel MI, and Clinically Driven TLR) at 1 Year Cumulative incidence of events [%] Resolute (N = 1140) Xience V (N = 1152) 20 Log-Rank P = 0. 92 Resolute 8. 2% vs. Xience V 8. 3% Pnon-inferiority <0. 001 15 8. 3% 10 8. 2% 5 0 0 180 360 Time after initial procedure [days] No. at risk ZES EES 0 1140 1152 30 1110 1123 60 1084 1088 90 1076 1080 120 1078 150 1062 1074 180 1068 210 1058 1061 240 1051 1047 270 1042 1046 300 1038 Primary Non-Inferiority Clinical Endpoint Met 330 1037 1032 360 1025 1019 Error bars indicate a point-wise two-sided 95% confidence interval (± 1. 96*SE). Standard Error based on the Greenwood Formula. Serruys PW, Silber S, et al. , N Engl J Med. 2010; 363: 136 -46
RESOLUTE All Comers : 12 month TLF (Cardiac Death, Target Vessel MI, and Clinically Driven TLR) at 1 Year Cumulative incidence of events [%] Resolute (N = 1140) Xience V (N = 1152) 20 Log-Rank P = 0. 92 Resolute 8. 2% vs. Xience V 8. 3% Pnon-inferiority <0. 001 15 8. 3% 10 8. 2% 5 0 0 180 360 Time after initial procedure [days] No. at risk ZES EES 0 1140 1152 30 1110 1123 60 1084 1088 90 1076 1080 120 1078 150 1062 1074 180 1068 210 1058 1061 240 1051 1047 270 1042 1046 300 1038 Primary Non-Inferiority Clinical Endpoint Met 330 1037 1032 360 1025 1019 Error bars indicate a point-wise two-sided 95% confidence interval (± 1. 96*SE). Standard Error based on the Greenwood Formula. Serruys PW, Silber S, et al. , N Engl J Med. 2010; 363: 136 -46
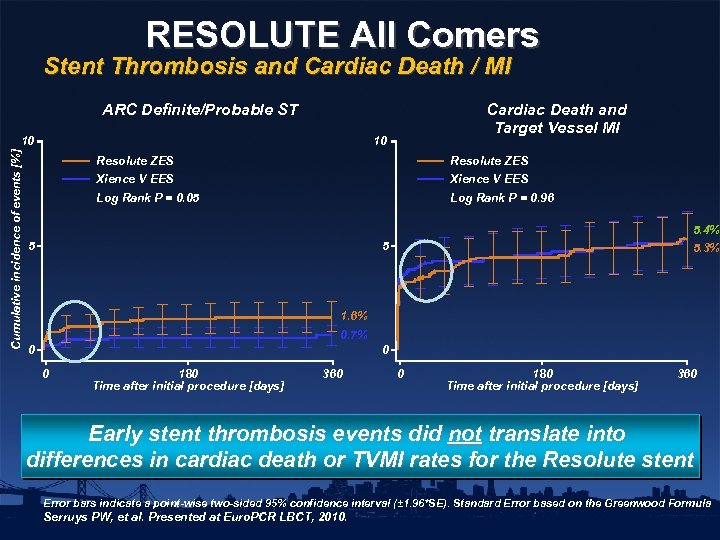 RESOLUTE All Comers Stent Thrombosis and Cardiac Death / MI ARC Definite/Probable ST Cumulative incidence of events [%] 10 Cardiac Death and Target Vessel MI 10 Resolute ZES Xience V EES Log Rank P = 0. 05 Log Rank P = 0. 96 5 5. 4% 5. 3% 5 1. 6% 0. 7% 0 0 0 180 Time after initial procedure [days] 360 Early stent thrombosis events did not translate into differences in cardiac death or TVMI rates for the Resolute stent Error bars indicate a point-wise two-sided 95% confidence interval (± 1. 96*SE). Standard Error based on the Greenwood Formula Serruys PW, et al. Presented at Euro. PCR LBCT, 2010.
RESOLUTE All Comers Stent Thrombosis and Cardiac Death / MI ARC Definite/Probable ST Cumulative incidence of events [%] 10 Cardiac Death and Target Vessel MI 10 Resolute ZES Xience V EES Log Rank P = 0. 05 Log Rank P = 0. 96 5 5. 4% 5. 3% 5 1. 6% 0. 7% 0 0 0 180 Time after initial procedure [days] 360 Early stent thrombosis events did not translate into differences in cardiac death or TVMI rates for the Resolute stent Error bars indicate a point-wise two-sided 95% confidence interval (± 1. 96*SE). Standard Error based on the Greenwood Formula Serruys PW, et al. Presented at Euro. PCR LBCT, 2010.
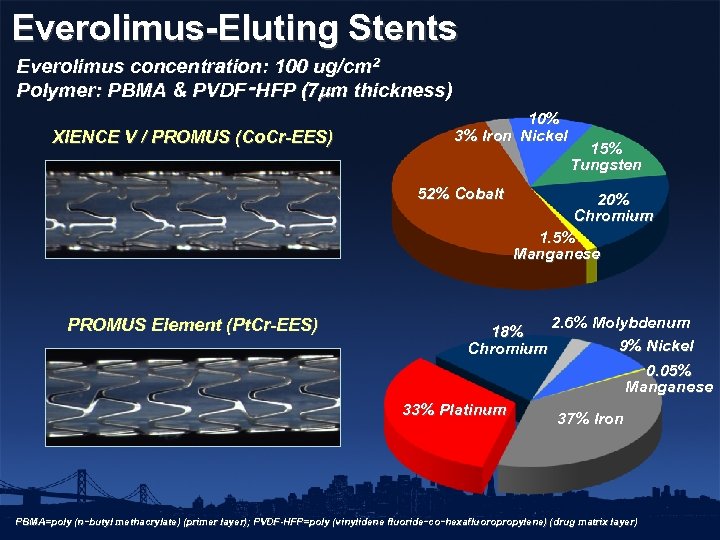 Everolimus-Eluting Stents Everolimus concentration: 100 ug/cm 2 Polymer: PBMA & PVDF‑HFP (7 m thickness) XIENCE V / PROMUS (Co. Cr-EES) 10% 3% Iron Nickel 52% Cobalt PROMUS Element (Pt. Cr-EES) 15% Tungsten 20% Chromium 1. 5% Manganese 18% Chromium 2. 6% Molybdenum 9% Nickel 0. 05% Manganese 33% Platinum 37% Iron PBMA=poly (n‑butyl methacrylate) (primer layer); PVDF-HFP=poly (vinylidene fluoride‑co‑hexafluoropropylene) (drug matrix layer)
Everolimus-Eluting Stents Everolimus concentration: 100 ug/cm 2 Polymer: PBMA & PVDF‑HFP (7 m thickness) XIENCE V / PROMUS (Co. Cr-EES) 10% 3% Iron Nickel 52% Cobalt PROMUS Element (Pt. Cr-EES) 15% Tungsten 20% Chromium 1. 5% Manganese 18% Chromium 2. 6% Molybdenum 9% Nickel 0. 05% Manganese 33% Platinum 37% Iron PBMA=poly (n‑butyl methacrylate) (primer layer); PVDF-HFP=poly (vinylidene fluoride‑co‑hexafluoropropylene) (drug matrix layer)
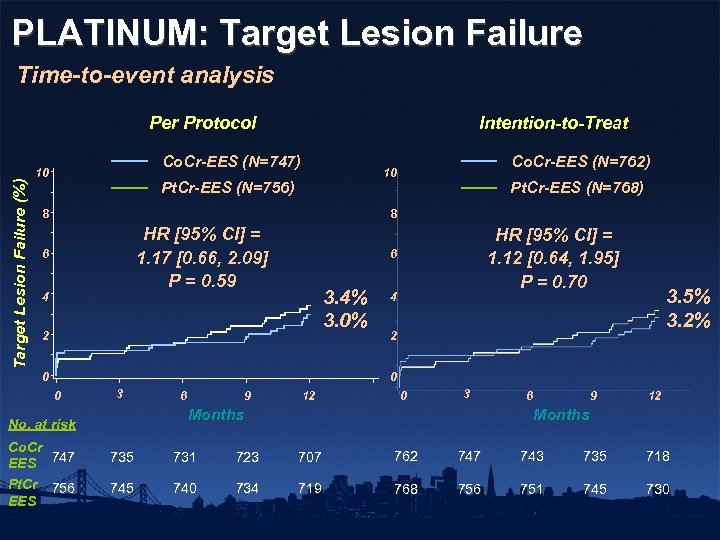 PLATINUM: Target Lesion Failure Time-to-event analysis Target Lesion Failure (%) Per Protocol Intention-to-Treat Co. Cr-EES (N=747) 10 Co. Cr-EES (N=762) 10 Pt. Cr-EES (N=756) 8 Pt. Cr-EES (N=768) 8 HR [95% CI] = 1. 17 [0. 66, 2. 09] P = 0. 59 6 4 HR [95% CI] = 1. 12 [0. 64, 1. 95] P = 0. 70 6 3. 4% 3. 0% 2 0 4 3. 5% 3. 2% 2 0 0 3 9 12 0 3 Months No. at risk Co. Cr EES 747 Pt. Cr 756 EES 6 6 9 12 Months 735 731 723 707 762 747 743 735 718 745 740 734 719 768 756 751 745 730
PLATINUM: Target Lesion Failure Time-to-event analysis Target Lesion Failure (%) Per Protocol Intention-to-Treat Co. Cr-EES (N=747) 10 Co. Cr-EES (N=762) 10 Pt. Cr-EES (N=756) 8 Pt. Cr-EES (N=768) 8 HR [95% CI] = 1. 17 [0. 66, 2. 09] P = 0. 59 6 4 HR [95% CI] = 1. 12 [0. 64, 1. 95] P = 0. 70 6 3. 4% 3. 0% 2 0 4 3. 5% 3. 2% 2 0 0 3 9 12 0 3 Months No. at risk Co. Cr EES 747 Pt. Cr 756 EES 6 6 9 12 Months 735 731 723 707 762 747 743 735 718 745 740 734 719 768 756 751 745 730
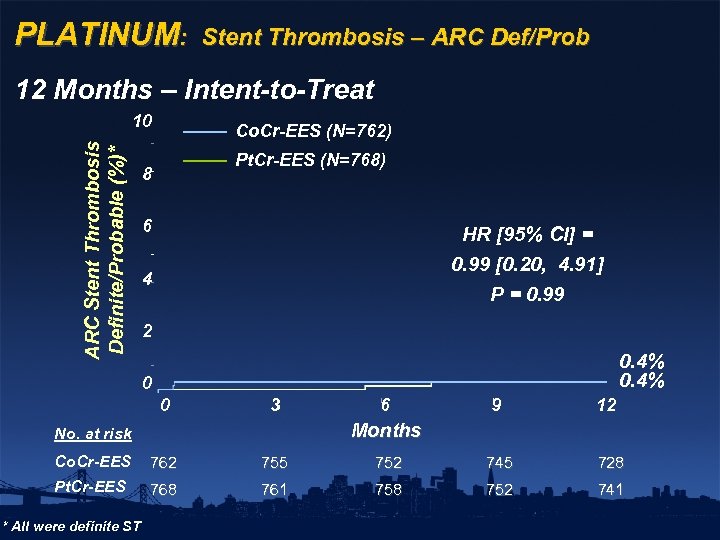 PLATINUM: Stent Thrombosis – ARC Def/Prob 12 Months – Intent-to-Treat ARC Stent Thrombosis Definite/Probable (%)* 10 Co. Cr-EES (N=762) Pt. Cr-EES (N=768) 8 6 HR [95% CI] = 0. 99 [0. 20, 4. 91] 4 P = 0. 99 2 0. 4% 0 0 3 6 9 12 Months No. at risk Co. Cr-EES 762 755 752 745 728 Pt. Cr-EES 768 761 758 752 741 * All were definite ST
PLATINUM: Stent Thrombosis – ARC Def/Prob 12 Months – Intent-to-Treat ARC Stent Thrombosis Definite/Probable (%)* 10 Co. Cr-EES (N=762) Pt. Cr-EES (N=768) 8 6 HR [95% CI] = 0. 99 [0. 20, 4. 91] 4 P = 0. 99 2 0. 4% 0 0 3 6 9 12 Months No. at risk Co. Cr-EES 762 755 752 745 728 Pt. Cr-EES 768 761 758 752 741 * All were definite ST
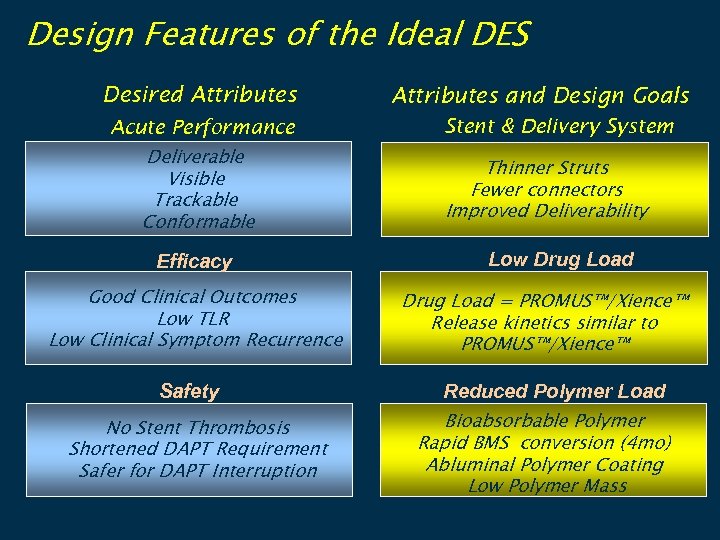 Design Features of the Ideal DES Desired Attributes Acute Performance Deliverable Visible Trackable Conformable Efficacy Good Clinical Outcomes Low TLR Low Clinical Symptom Recurrence Safety No Stent Thrombosis Shortened DAPT Requirement Safer for DAPT Interruption Attributes and Design Goals Stent & Delivery System Thinner Struts Fewer connectors Improved Deliverability Low Drug Load = PROMUS™/Xience™ Release kinetics similar to PROMUS™/Xience™ Reduced Polymer Load Bioabsorbable Polymer Rapid BMS conversion (4 mo) Abluminal Polymer Coating Low Polymer Mass
Design Features of the Ideal DES Desired Attributes Acute Performance Deliverable Visible Trackable Conformable Efficacy Good Clinical Outcomes Low TLR Low Clinical Symptom Recurrence Safety No Stent Thrombosis Shortened DAPT Requirement Safer for DAPT Interruption Attributes and Design Goals Stent & Delivery System Thinner Struts Fewer connectors Improved Deliverability Low Drug Load = PROMUS™/Xience™ Release kinetics similar to PROMUS™/Xience™ Reduced Polymer Load Bioabsorbable Polymer Rapid BMS conversion (4 mo) Abluminal Polymer Coating Low Polymer Mass
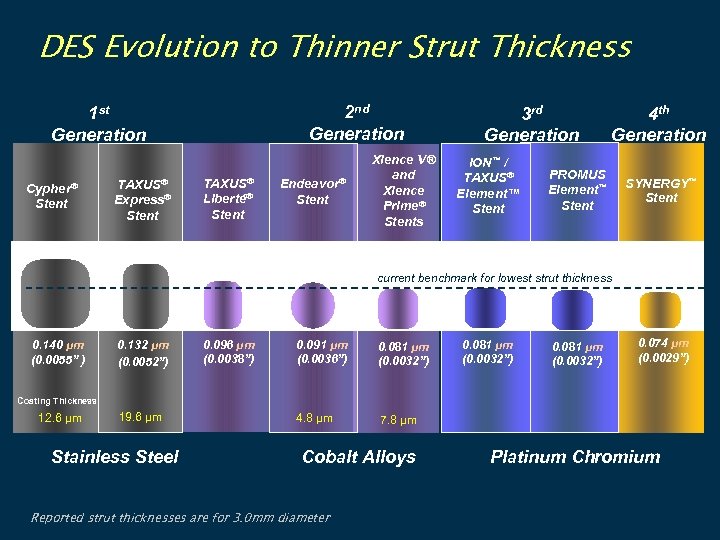 DES Evolution to Thinner Strut Thickness 2 nd Generation 1 st Generation Cypher® Stent TAXUS® Express® Stent TAXUS® Liberté® Stent Endeavor® Stent Xience V® and Xience Prime® Stents 3 rd Generation ION™ / TAXUS® Element™ Stent 4 th Generation PROMUS Element™ Stent SYNERGY™ Stent current benchmark for lowest strut thickness 0. 140 μm (0. 0055” ) 0. 132 μm (0. 0052”) 0. 096 μm (0. 0038”) 0. 091 μm (0. 0036”) 0. 081 μm (0. 0032”) 4. 8 µm 0. 081 μm (0. 0032”) 0. 074 μm (0. 0029”) 7. 8 µm 0. 081 μm (0. 0032”) Coating Thickness: 12. 6 µm 19. 6 µm Stainless Steel Cobalt Alloys Reported strut thicknesses are for 3. 0 mm diameter Platinum Chromium
DES Evolution to Thinner Strut Thickness 2 nd Generation 1 st Generation Cypher® Stent TAXUS® Express® Stent TAXUS® Liberté® Stent Endeavor® Stent Xience V® and Xience Prime® Stents 3 rd Generation ION™ / TAXUS® Element™ Stent 4 th Generation PROMUS Element™ Stent SYNERGY™ Stent current benchmark for lowest strut thickness 0. 140 μm (0. 0055” ) 0. 132 μm (0. 0052”) 0. 096 μm (0. 0038”) 0. 091 μm (0. 0036”) 0. 081 μm (0. 0032”) 4. 8 µm 0. 081 μm (0. 0032”) 0. 074 μm (0. 0029”) 7. 8 µm 0. 081 μm (0. 0032”) Coating Thickness: 12. 6 µm 19. 6 µm Stainless Steel Cobalt Alloys Reported strut thicknesses are for 3. 0 mm diameter Platinum Chromium
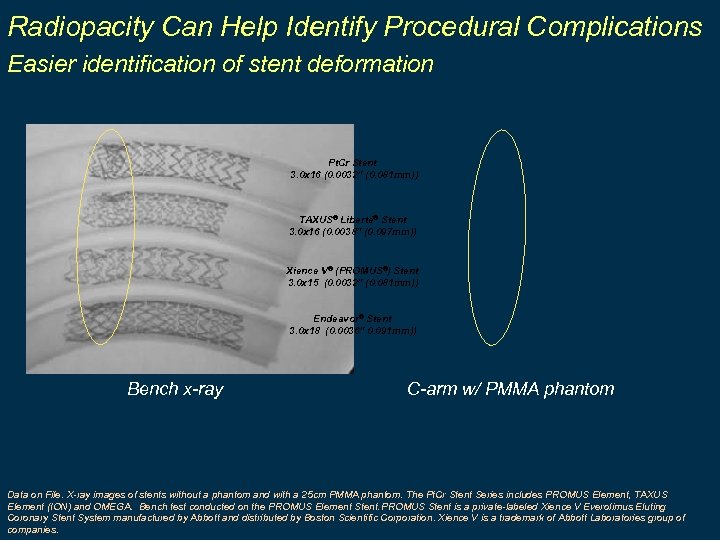 Radiopacity Can Help Identify Procedural Complications Easier identification of stent deformation Pt. Cr Stent 3. 0 x 16 (0. 0032” (0. 081 mm)) TAXUS® Liberté® Stent 3. 0 x 16 (0. 0038” (0. 097 mm)) Xience V® (PROMUS®) Stent 3. 0 x 15 (0. 0032” (0. 081 mm)) Endeavor® Stent 3. 0 x 18 (0. 0036” 0. 091 mm)) Bench x-ray C-arm w/ PMMA phantom Data on File. X-ray images of stents without a phantom and with a 25 cm PMMA phantom. The Pt. Cr Stent Series includes PROMUS Element, TAXUS Element (ION) and OMEGA. Bench test conducted on the PROMUS Element Stent. PROMUS Stent is a private-labeled Xience V Everolimus Eluting Coronary Stent System manufactured by Abbott and distributed by Boston Scientific Corporation. Xience V is a trademark of Abbott Laboratories group of companies.
Radiopacity Can Help Identify Procedural Complications Easier identification of stent deformation Pt. Cr Stent 3. 0 x 16 (0. 0032” (0. 081 mm)) TAXUS® Liberté® Stent 3. 0 x 16 (0. 0038” (0. 097 mm)) Xience V® (PROMUS®) Stent 3. 0 x 15 (0. 0032” (0. 081 mm)) Endeavor® Stent 3. 0 x 18 (0. 0036” 0. 091 mm)) Bench x-ray C-arm w/ PMMA phantom Data on File. X-ray images of stents without a phantom and with a 25 cm PMMA phantom. The Pt. Cr Stent Series includes PROMUS Element, TAXUS Element (ION) and OMEGA. Bench test conducted on the PROMUS Element Stent. PROMUS Stent is a private-labeled Xience V Everolimus Eluting Coronary Stent System manufactured by Abbott and distributed by Boston Scientific Corporation. Xience V is a trademark of Abbott Laboratories group of companies.
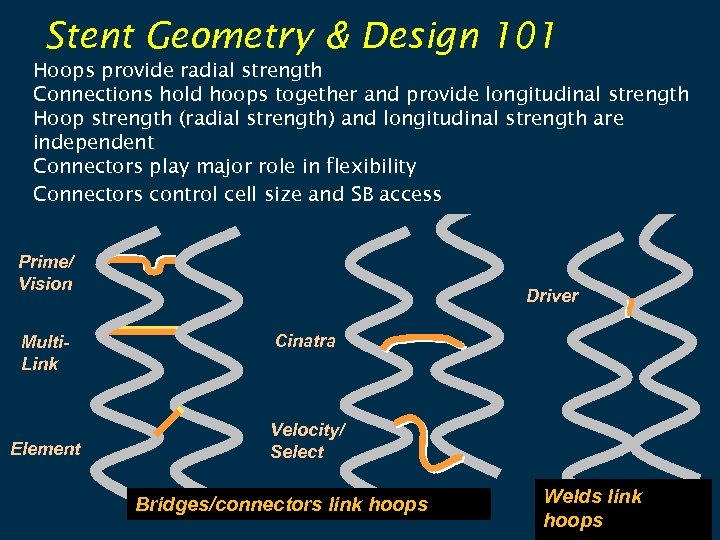 Stent Geometry & Design 101 Hoops provide radial strength Connections hold hoops together and provide longitudinal strength Hoop strength (radial strength) and longitudinal strength are independent Connectors play major role in flexibility Connectors control cell size and SB access Prime/ Vision Multi. Link Element Driver Cinatra Velocity/ Select Bridges/connectors link hoops Welds link hoops
Stent Geometry & Design 101 Hoops provide radial strength Connections hold hoops together and provide longitudinal strength Hoop strength (radial strength) and longitudinal strength are independent Connectors play major role in flexibility Connectors control cell size and SB access Prime/ Vision Multi. Link Element Driver Cinatra Velocity/ Select Bridges/connectors link hoops Welds link hoops
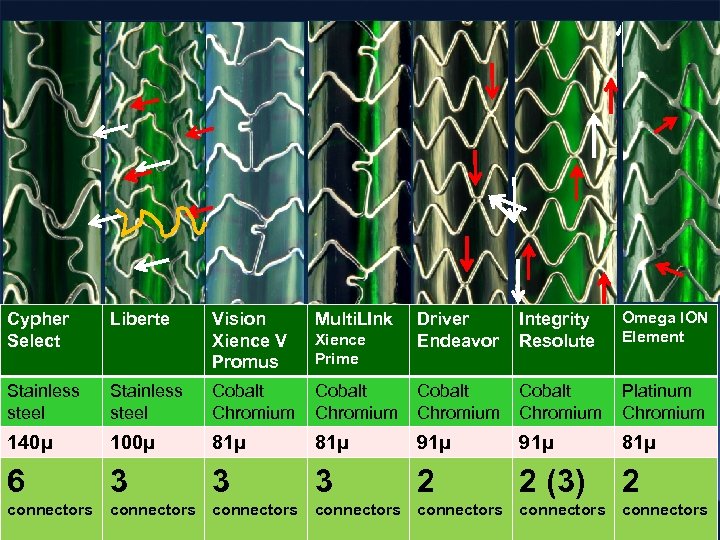 Driver Endeavor Integrity Resolute Omega ION Element Cobalt Chromium Platinum Chromium 81µ 91µ 81µ 3 3 3 2 2 (3) 2 connectors connectors Cypher Select Liberte Vision Xience V Promus Multi. LInk Stainless steel Cobalt Chromium 140µ 100µ 6 connectors Xience Prime
Driver Endeavor Integrity Resolute Omega ION Element Cobalt Chromium Platinum Chromium 81µ 91µ 81µ 3 3 3 2 2 (3) 2 connectors connectors Cypher Select Liberte Vision Xience V Promus Multi. LInk Stainless steel Cobalt Chromium 140µ 100µ 6 connectors Xience Prime
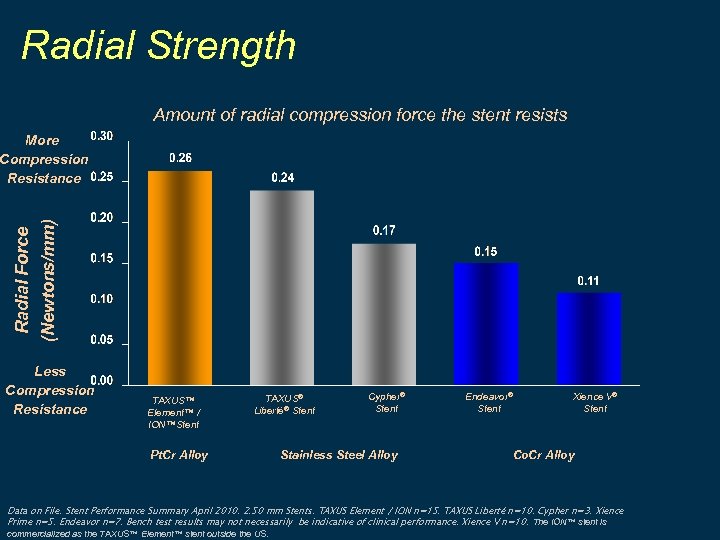 Radial Strength Amount of radial compression force the stent resists Radial Force (Newtons/mm) More Compression Resistance Less Compression Resistance TAXUS™ Element™ / ION™Stent TAXUS® Liberté® Stent Pt. Cr Alloy Cypher® Stent Stainless Steel Alloy Endeavor® Stent Xience V® Stent Co. Cr Alloy Data on File. Stent Performance Summary April 2010. 2. 50 mm Stents. TAXUS Element / ION n=15. TAXUS Liberté n=10. Cypher n=3. Xience Prime n=5. Endeavor n=7. Bench test results may not necessarily be indicative of clinical performance. Xience V n=10. The ION™ stent is commercialized as the TAXUS™ Element™ stent outside the US.
Radial Strength Amount of radial compression force the stent resists Radial Force (Newtons/mm) More Compression Resistance Less Compression Resistance TAXUS™ Element™ / ION™Stent TAXUS® Liberté® Stent Pt. Cr Alloy Cypher® Stent Stainless Steel Alloy Endeavor® Stent Xience V® Stent Co. Cr Alloy Data on File. Stent Performance Summary April 2010. 2. 50 mm Stents. TAXUS Element / ION n=15. TAXUS Liberté n=10. Cypher n=3. Xience Prime n=5. Endeavor n=7. Bench test results may not necessarily be indicative of clinical performance. Xience V n=10. The ION™ stent is commercialized as the TAXUS™ Element™ stent outside the US.
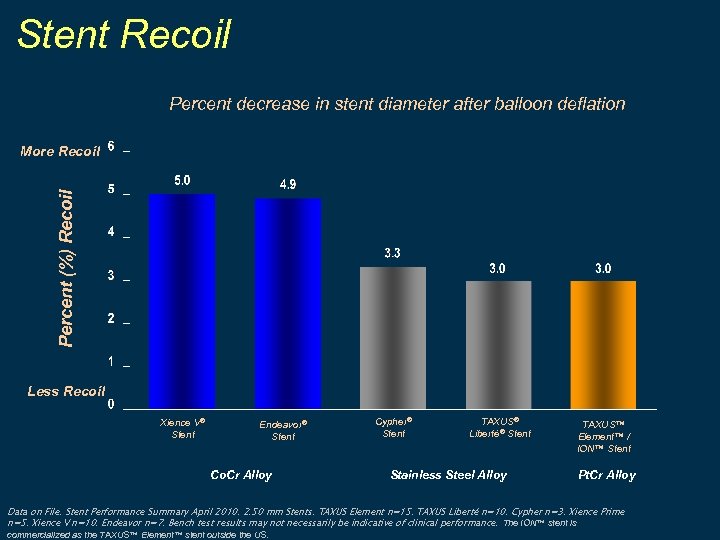 Stent Recoil Percent decrease in stent diameter after balloon deflation Percent (%) Recoil More Recoil Less Recoil Xience V® Stent Endeavor® Stent Co. Cr Alloy Cypher® Stent TAXUS® Liberté® Stent Stainless Steel Alloy TAXUS™ Element™ / ION™ Stent Pt. Cr Alloy Data on File. Stent Performance Summary April 2010. 2. 50 mm Stents. TAXUS Element n=15. TAXUS Liberté n=10. Cypher n=3. Xience Prime n=5. Xience V n=10. Endeavor n=7. Bench test results may not necessarily be indicative of clinical performance. The ION™ stent is commercialized as the TAXUS™ Element™ stent outside the US.
Stent Recoil Percent decrease in stent diameter after balloon deflation Percent (%) Recoil More Recoil Less Recoil Xience V® Stent Endeavor® Stent Co. Cr Alloy Cypher® Stent TAXUS® Liberté® Stent Stainless Steel Alloy TAXUS™ Element™ / ION™ Stent Pt. Cr Alloy Data on File. Stent Performance Summary April 2010. 2. 50 mm Stents. TAXUS Element n=15. TAXUS Liberté n=10. Cypher n=3. Xience Prime n=5. Xience V n=10. Endeavor n=7. Bench test results may not necessarily be indicative of clinical performance. The ION™ stent is commercialized as the TAXUS™ Element™ stent outside the US.
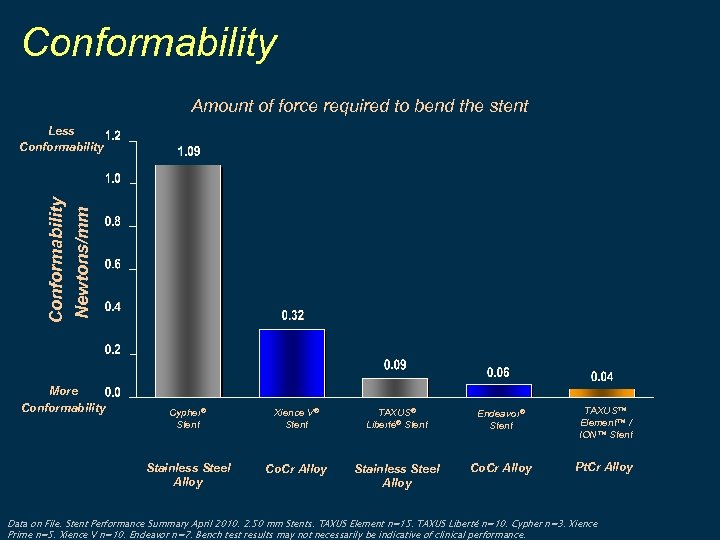 Conformability Amount of force required to bend the stent Conformability Newtons/mm Less Conformability More Conformability Cypher® Stent Xience V® Stent TAXUS® Liberté® Stent Endeavor® Stent TAXUS™ Element™ / ION™ Stent Stainless Steel Alloy Co. Cr Alloy Pt. Cr Alloy Data on File. Stent Performance Summary April 2010. 2. 50 mm Stents. TAXUS Element n=15. TAXUS Liberté n=10. Cypher n=3. Xience Prime n=5. Xience V n=10. Endeavor n=7. Bench test results may not necessarily be indicative of clinical performance.
Conformability Amount of force required to bend the stent Conformability Newtons/mm Less Conformability More Conformability Cypher® Stent Xience V® Stent TAXUS® Liberté® Stent Endeavor® Stent TAXUS™ Element™ / ION™ Stent Stainless Steel Alloy Co. Cr Alloy Pt. Cr Alloy Data on File. Stent Performance Summary April 2010. 2. 50 mm Stents. TAXUS Element n=15. TAXUS Liberté n=10. Cypher n=3. Xience Prime n=5. Xience V n=10. Endeavor n=7. Bench test results may not necessarily be indicative of clinical performance.
 How far are stents compressed with 0. 5 N force ? • The Cypher Select did not shorten • The Element shortened 5 mm • Xience 1 mm
How far are stents compressed with 0. 5 N force ? • The Cypher Select did not shorten • The Element shortened 5 mm • Xience 1 mm
 Profound differences in longitudinal strength of contemporary DES • Performance Characteristics: • Flexibility and longitudinal strength are trade off, mostly function of # connectors ¡ Consequences: stent compression, elongation and malapposition • Radial Strength and recoil considerations • Device Selection and Strategies to limit distortion: ¡ Stent selection in ostial and highly calcified lesions ¡ Meticulous Technique to avoid deep guide engagement, full balloon deflation prior to withdrawal
Profound differences in longitudinal strength of contemporary DES • Performance Characteristics: • Flexibility and longitudinal strength are trade off, mostly function of # connectors ¡ Consequences: stent compression, elongation and malapposition • Radial Strength and recoil considerations • Device Selection and Strategies to limit distortion: ¡ Stent selection in ostial and highly calcified lesions ¡ Meticulous Technique to avoid deep guide engagement, full balloon deflation prior to withdrawal
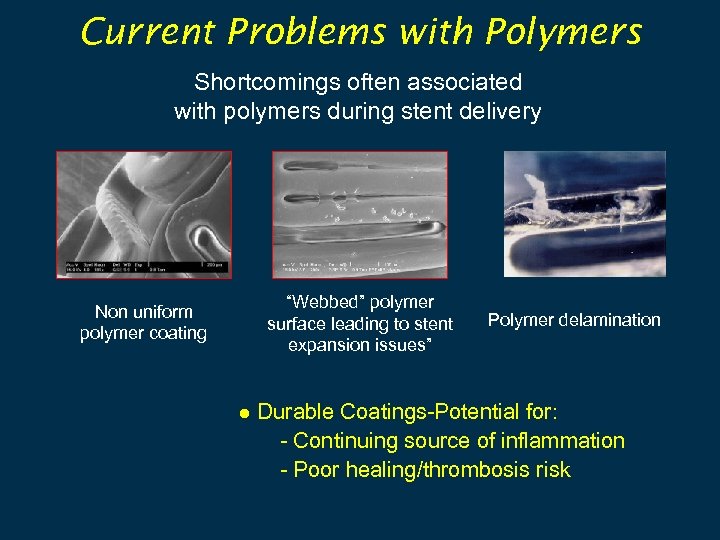 Current Problems with Polymers Shortcomings often associated with polymers during stent delivery Non uniform polymer coating “Webbed” polymer surface leading to stent expansion issues” Polymer delamination ● Durable Coatings-Potential for: - Continuing source of inflammation - Poor healing/thrombosis risk
Current Problems with Polymers Shortcomings often associated with polymers during stent delivery Non uniform polymer coating “Webbed” polymer surface leading to stent expansion issues” Polymer delamination ● Durable Coatings-Potential for: - Continuing source of inflammation - Poor healing/thrombosis risk
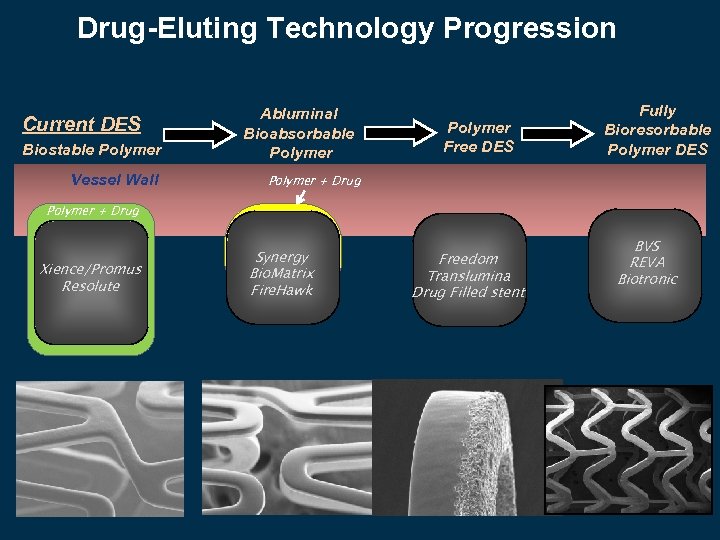 Drug-Eluting Technology Progression Current DES Biostable Polymer Vessel Wall Abluminal Bioabsorbable Polymer Free DES Fully Bioresorbable Polymer DES Polymer + Drug Xience/Promus Resolute Synergy Bio. Matrix Fire. Hawk Freedom Translumina Drug Filled stent BVS REVA Biotronic
Drug-Eluting Technology Progression Current DES Biostable Polymer Vessel Wall Abluminal Bioabsorbable Polymer Free DES Fully Bioresorbable Polymer DES Polymer + Drug Xience/Promus Resolute Synergy Bio. Matrix Fire. Hawk Freedom Translumina Drug Filled stent BVS REVA Biotronic
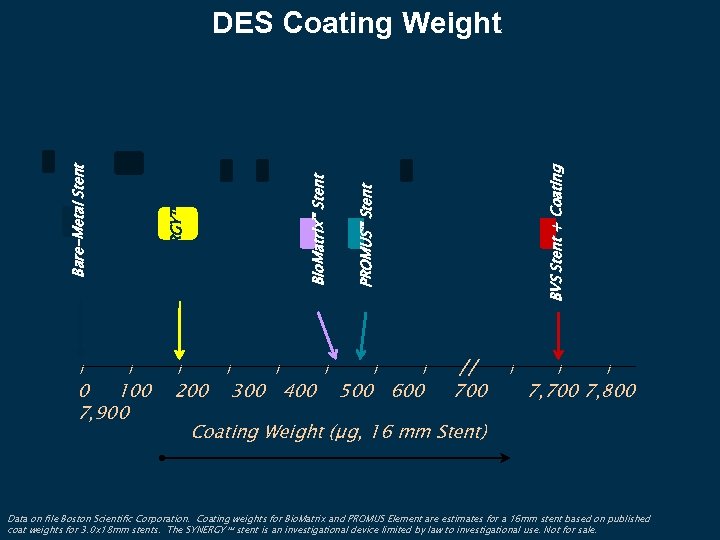 l 0 100 7, 900 l 200 l l BVS Stent + Coating PROMUS™ Stent l Bio. Matrix™ Stent Bare-Metal Stent SYNERGY™ Stent* DES Coating Weight 300 400 l 500 600 // 700 l l l 7, 700 7, 800 Coating Weight (µg, 16 mm Stent) Data on file Boston Scientific Corporation. Coating weights for Bio. Matrix and PROMUS Element are estimates for a 16 mm stent based on published coat weights for 3. 0 x 18 mm stents. The SYNERGY stent is an investigational device limited by law to investigational use. Not for sale.
l 0 100 7, 900 l 200 l l BVS Stent + Coating PROMUS™ Stent l Bio. Matrix™ Stent Bare-Metal Stent SYNERGY™ Stent* DES Coating Weight 300 400 l 500 600 // 700 l l l 7, 700 7, 800 Coating Weight (µg, 16 mm Stent) Data on file Boston Scientific Corporation. Coating weights for Bio. Matrix and PROMUS Element are estimates for a 16 mm stent based on published coat weights for 3. 0 x 18 mm stents. The SYNERGY stent is an investigational device limited by law to investigational use. Not for sale.
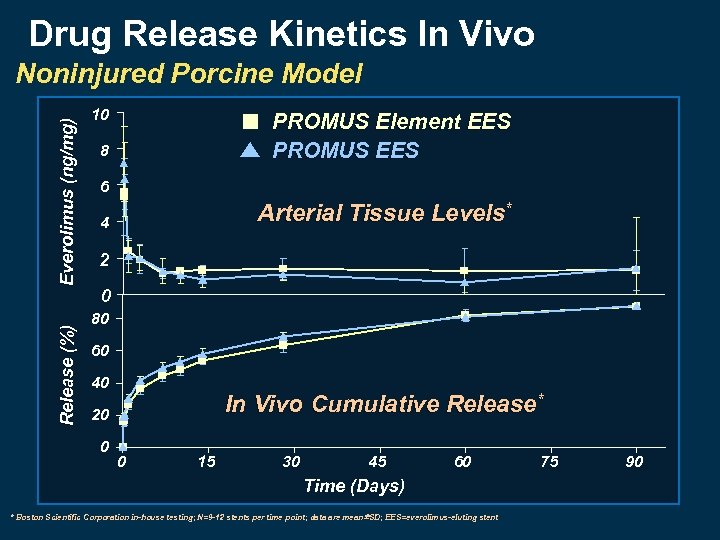 Drug Release Kinetics In Vivo Release (%) Everolimus (ng/mg) Noninjured Porcine Model 10 PROMUS Element EES PROMUS EES 8 6 Arterial Tissue Levels* 4 2 0 80 60 40 In Vivo Cumulative Release* 20 0 0 15 30 45 60 Time (Days) * Boston Scientific Corporation in-house testing; N=9 -12 stents per time point; data are mean SD; EES=everolimus-eluting stent 75 90
Drug Release Kinetics In Vivo Release (%) Everolimus (ng/mg) Noninjured Porcine Model 10 PROMUS Element EES PROMUS EES 8 6 Arterial Tissue Levels* 4 2 0 80 60 40 In Vivo Cumulative Release* 20 0 0 15 30 45 60 Time (Days) * Boston Scientific Corporation in-house testing; N=9 -12 stents per time point; data are mean SD; EES=everolimus-eluting stent 75 90
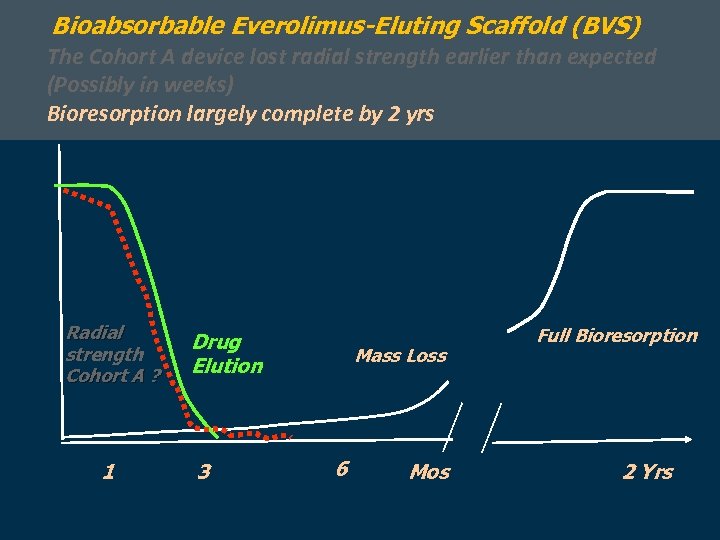 Bioabsorbable Everolimus-Eluting Scaffold (BVS) The Cohort A device lost radial strength earlier than expected (Possibly in weeks) Bioresorption largely complete by 2 yrs Radial strength Cohort A ? 1 Drug Elution 3 Mass Loss 6 Mos Full Bioresorption 2 Yrs
Bioabsorbable Everolimus-Eluting Scaffold (BVS) The Cohort A device lost radial strength earlier than expected (Possibly in weeks) Bioresorption largely complete by 2 yrs Radial strength Cohort A ? 1 Drug Elution 3 Mass Loss 6 Mos Full Bioresorption 2 Yrs
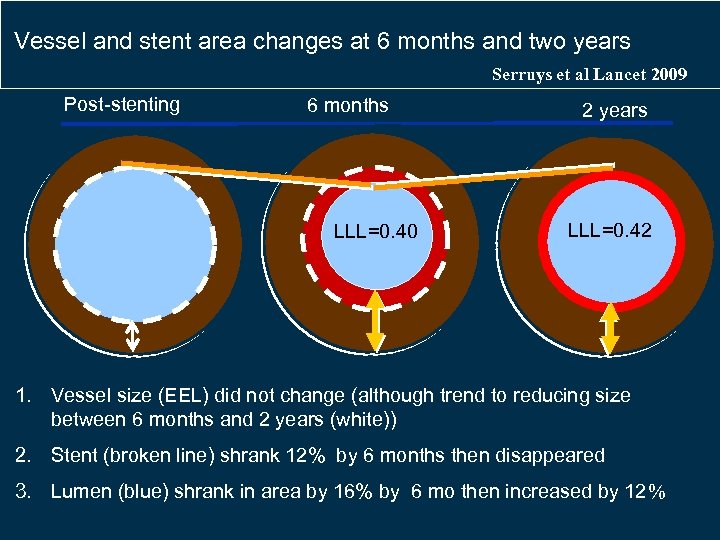 Vessel and stent area changes at 6 months and two years Serruys et al Lancet 2009 Post-stenting 6 months LLL=0. 40 2 years LLL=0. 42 1. Vessel size (EEL) did not change (although trend to reducing size between 6 months and 2 years (white)) 2. Stent (broken line) shrank 12% by 6 months then disappeared 3. Lumen (blue) shrank in area by 16% by 6 mo then increased by 12%
Vessel and stent area changes at 6 months and two years Serruys et al Lancet 2009 Post-stenting 6 months LLL=0. 40 2 years LLL=0. 42 1. Vessel size (EEL) did not change (although trend to reducing size between 6 months and 2 years (white)) 2. Stent (broken line) shrank 12% by 6 months then disappeared 3. Lumen (blue) shrank in area by 16% by 6 mo then increased by 12%
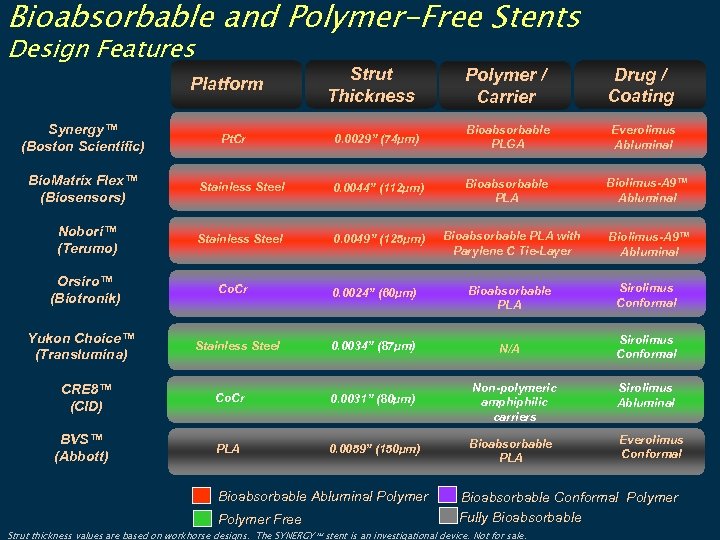 Bioabsorbable and Polymer-Free Stents Design Features Synergy™ (Boston Scientific) Bio. Matrix Flex™ (Biosensors) Nobori™ (Terumo) Orsiro™ (Biotronik) Yukon Choice™ (Translumina) CRE 8™ (CID) BVS™ (Abbott) Strut Thickness Polymer / Carrier Drug / Coating 0. 0029” (74µm) Bioabsorbable PLGA Everolimus Abluminal Stainless Steel 0. 0044” (112µm) Bioabsorbable PLA Biolimus-A 9™ Abluminal Stainless Steel 0. 0049” (125µm) Platform Pt. Cr Co. Cr Stainless Steel Bioabsorbable PLA with Parylene C Tie-Layer Biolimus-A 9™ Abluminal 0. 0024” (60µm) Bioabsorbable PLA Sirolimus Conformal 0. 0034” (87µm) N/A Sirolimus Conformal Co. Cr 0. 0031” (80µm) Non-polymeric amphiphilic carriers Sirolimus Abluminal PLA 0. 0059” (150µm) Bioabsorbable PLA Everolimus Conformal Bioabsorbable Abluminal Polymer Free Bioabsorbable Conformal Polymer Fully Bioabsorbable Strut thickness values are based on workhorse designs. The SYNERGY stent is an investigational device. Not for sale.
Bioabsorbable and Polymer-Free Stents Design Features Synergy™ (Boston Scientific) Bio. Matrix Flex™ (Biosensors) Nobori™ (Terumo) Orsiro™ (Biotronik) Yukon Choice™ (Translumina) CRE 8™ (CID) BVS™ (Abbott) Strut Thickness Polymer / Carrier Drug / Coating 0. 0029” (74µm) Bioabsorbable PLGA Everolimus Abluminal Stainless Steel 0. 0044” (112µm) Bioabsorbable PLA Biolimus-A 9™ Abluminal Stainless Steel 0. 0049” (125µm) Platform Pt. Cr Co. Cr Stainless Steel Bioabsorbable PLA with Parylene C Tie-Layer Biolimus-A 9™ Abluminal 0. 0024” (60µm) Bioabsorbable PLA Sirolimus Conformal 0. 0034” (87µm) N/A Sirolimus Conformal Co. Cr 0. 0031” (80µm) Non-polymeric amphiphilic carriers Sirolimus Abluminal PLA 0. 0059” (150µm) Bioabsorbable PLA Everolimus Conformal Bioabsorbable Abluminal Polymer Free Bioabsorbable Conformal Polymer Fully Bioabsorbable Strut thickness values are based on workhorse designs. The SYNERGY stent is an investigational device. Not for sale.
 Future DES Summary Conclusions… 1 • Next generation DES (Endeavor, Xience V/Promus, Element) have already demonstrated superior performance characteristics, including enhanced deliverability, improved safety, and increased antirestenosis efficacy. • Future DES are focusing largely on drug carrier enhancements to reduce safety concerns ¡ Bioabsorbable polymer drug delivery – many versions, much promise, insufficient long-term clinical data to assess incremental value
Future DES Summary Conclusions… 1 • Next generation DES (Endeavor, Xience V/Promus, Element) have already demonstrated superior performance characteristics, including enhanced deliverability, improved safety, and increased antirestenosis efficacy. • Future DES are focusing largely on drug carrier enhancements to reduce safety concerns ¡ Bioabsorbable polymer drug delivery – many versions, much promise, insufficient long-term clinical data to assess incremental value
 Future DES Summary Conclusions… 2 ¡ Polymer-free drug delivery – more difficult to achieve optimal drug elution profiles, best chance for “BMS-like” safety profile, BUT almost no clinical data thus far • Bioabsorbable stents have important potential advantages, each system is unique (all will require iterative stent designs and drug elution), but early proof-of-concept has already been accomplished (BVS) suggesting a more biocompatible solution with excellent safety and efficacy; may represent a breakthrough DES technology in the future!
Future DES Summary Conclusions… 2 ¡ Polymer-free drug delivery – more difficult to achieve optimal drug elution profiles, best chance for “BMS-like” safety profile, BUT almost no clinical data thus far • Bioabsorbable stents have important potential advantages, each system is unique (all will require iterative stent designs and drug elution), but early proof-of-concept has already been accomplished (BVS) suggesting a more biocompatible solution with excellent safety and efficacy; may represent a breakthrough DES technology in the future!


