Coordination Polymerization Ziegler Natta Processes Stereoregular Polymerization Cationic


39680-ch5_coordinationpolymerization_daly.ppt
- Количество слайдов: 31
 Coordination Polymerization Ziegler Natta Processes
Coordination Polymerization Ziegler Natta Processes
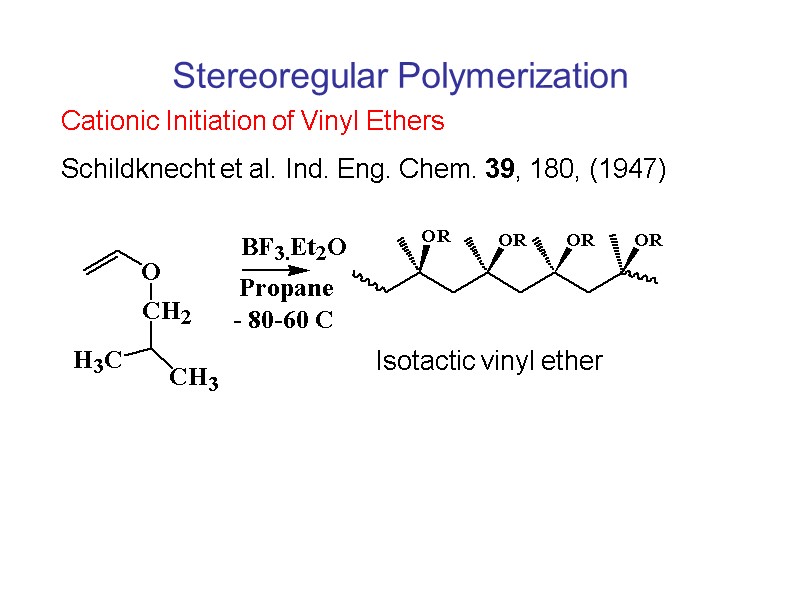
 Stereoregular Polymerization Cationic Initiation of Vinyl Ethers Schildknecht et al. Ind. Eng. Chem. 39, 180, (1947) Isotactic vinyl ether
Stereoregular Polymerization Cationic Initiation of Vinyl Ethers Schildknecht et al. Ind. Eng. Chem. 39, 180, (1947) Isotactic vinyl ether
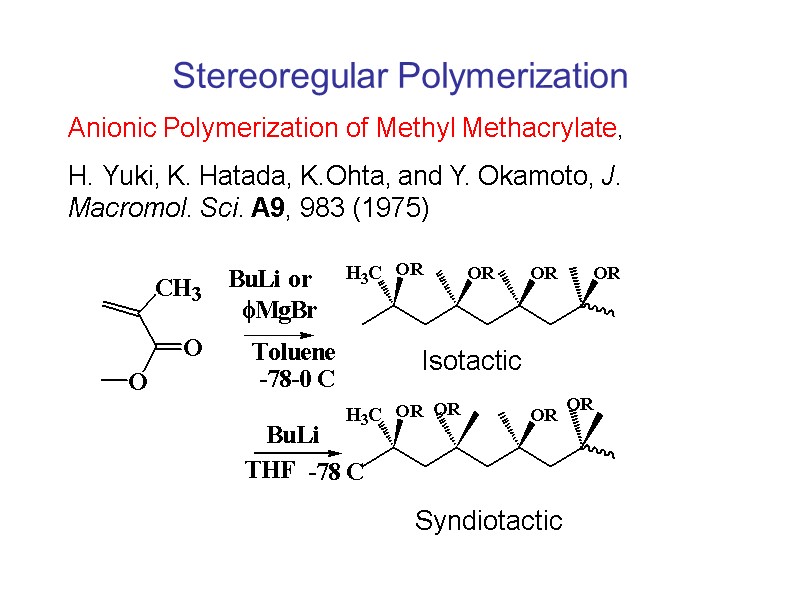
 Stereoregular Polymerization Anionic Polymerization of Methyl Methacrylate, H. Yuki, K. Hatada, K.Ohta, and Y. Okamoto, J. Macromol. Sci. A9, 983 (1975) Isotactic Syndiotactic
Stereoregular Polymerization Anionic Polymerization of Methyl Methacrylate, H. Yuki, K. Hatada, K.Ohta, and Y. Okamoto, J. Macromol. Sci. A9, 983 (1975) Isotactic Syndiotactic
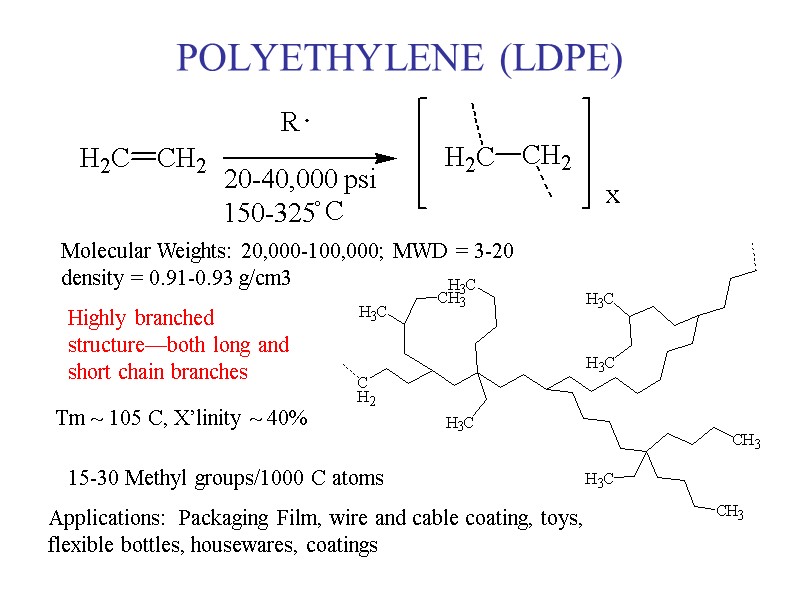
 POLYETHYLENE (LDPE) Molecular Weights: 20,000-100,000; MWD = 3-20 density = 0.91-0.93 g/cm3 Highly branched structure—both long and short chain branches 15-30 Methyl groups/1000 C atoms Tm ~ 105 C, X’linity ~ 40% Applications: Packaging Film, wire and cable coating, toys, flexible bottles, housewares, coatings
POLYETHYLENE (LDPE) Molecular Weights: 20,000-100,000; MWD = 3-20 density = 0.91-0.93 g/cm3 Highly branched structure—both long and short chain branches 15-30 Methyl groups/1000 C atoms Tm ~ 105 C, X’linity ~ 40% Applications: Packaging Film, wire and cable coating, toys, flexible bottles, housewares, coatings
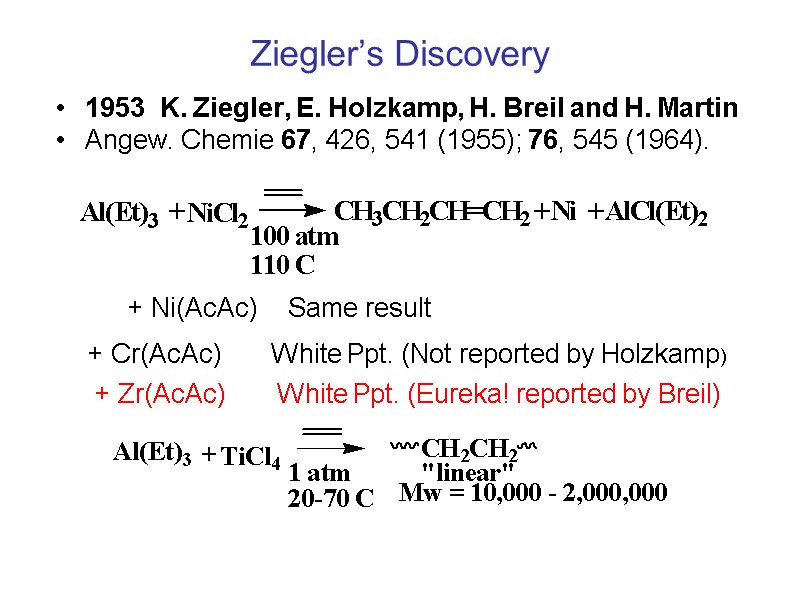
 Ziegler’s Discovery 1953 K. Ziegler, E. Holzkamp, H. Breil and H. Martin Angew. Chemie 67, 426, 541 (1955); 76, 545 (1964). + Ni(AcAc) Same result + Cr(AcAc) White Ppt. (Not reported by Holzkamp) + Zr(AcAc) White Ppt. (Eureka! reported by Breil)
Ziegler’s Discovery 1953 K. Ziegler, E. Holzkamp, H. Breil and H. Martin Angew. Chemie 67, 426, 541 (1955); 76, 545 (1964). + Ni(AcAc) Same result + Cr(AcAc) White Ppt. (Not reported by Holzkamp) + Zr(AcAc) White Ppt. (Eureka! reported by Breil)
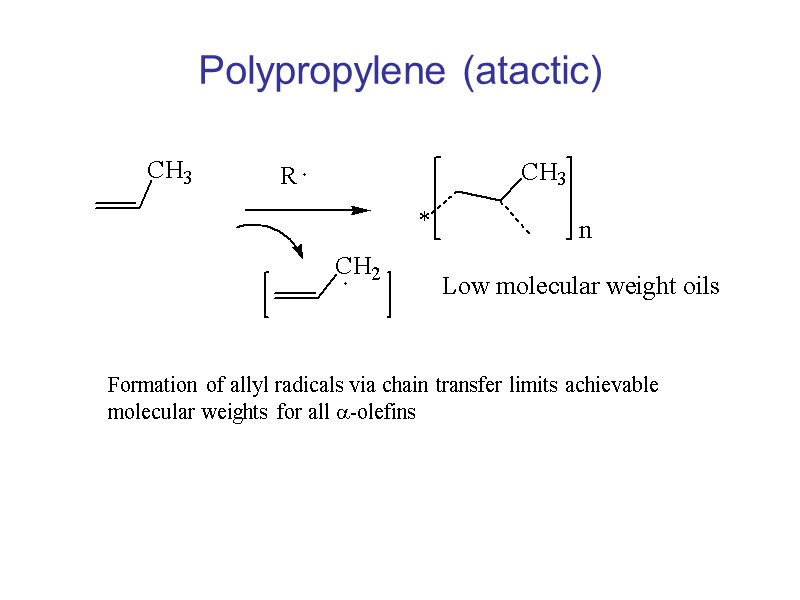
 Polypropylene (atactic) Formation of allyl radicals via chain transfer limits achievable molecular weights for all a-olefins
Polypropylene (atactic) Formation of allyl radicals via chain transfer limits achievable molecular weights for all a-olefins
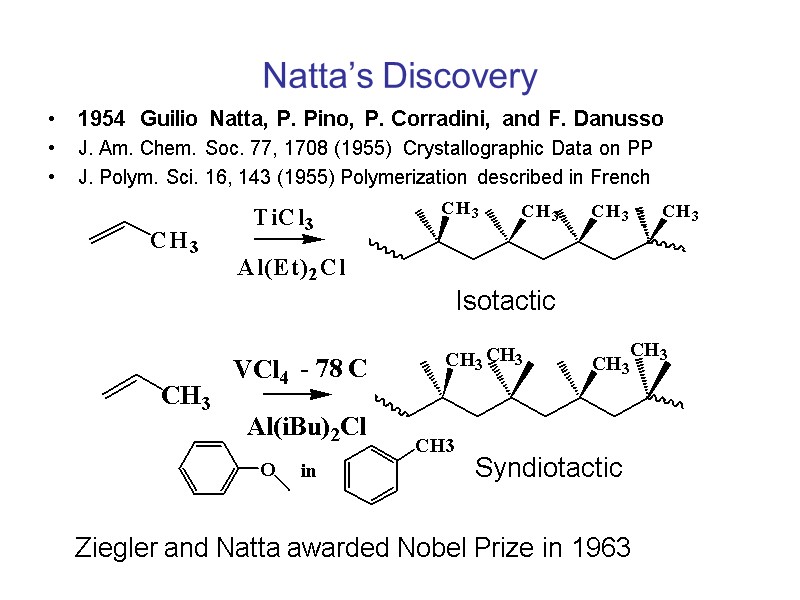
 Natta’s Discovery 1954 Guilio Natta, P. Pino, P. Corradini, and F. Danusso J. Am. Chem. Soc. 77, 1708 (1955) Crystallographic Data on PP J. Polym. Sci. 16, 143 (1955) Polymerization described in French Isotactic Syndiotactic Ziegler and Natta awarded Nobel Prize in 1963
Natta’s Discovery 1954 Guilio Natta, P. Pino, P. Corradini, and F. Danusso J. Am. Chem. Soc. 77, 1708 (1955) Crystallographic Data on PP J. Polym. Sci. 16, 143 (1955) Polymerization described in French Isotactic Syndiotactic Ziegler and Natta awarded Nobel Prize in 1963
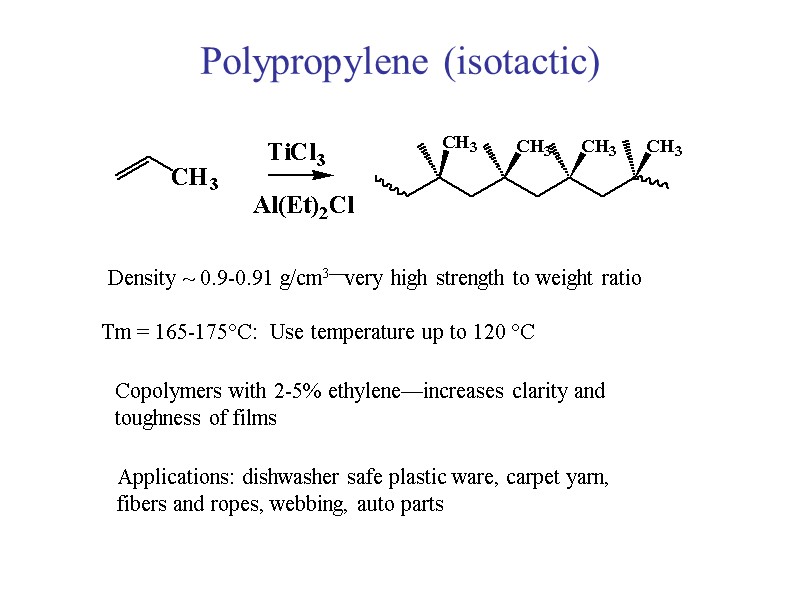
 Polypropylene (isotactic) Density ~ 0.9-0.91 g/cm3—very high strength to weight ratio Tm = 165-175C: Use temperature up to 120 C Copolymers with 2-5% ethylene—increases clarity and toughness of films Applications: dishwasher safe plastic ware, carpet yarn, fibers and ropes, webbing, auto parts
Polypropylene (isotactic) Density ~ 0.9-0.91 g/cm3—very high strength to weight ratio Tm = 165-175C: Use temperature up to 120 C Copolymers with 2-5% ethylene—increases clarity and toughness of films Applications: dishwasher safe plastic ware, carpet yarn, fibers and ropes, webbing, auto parts
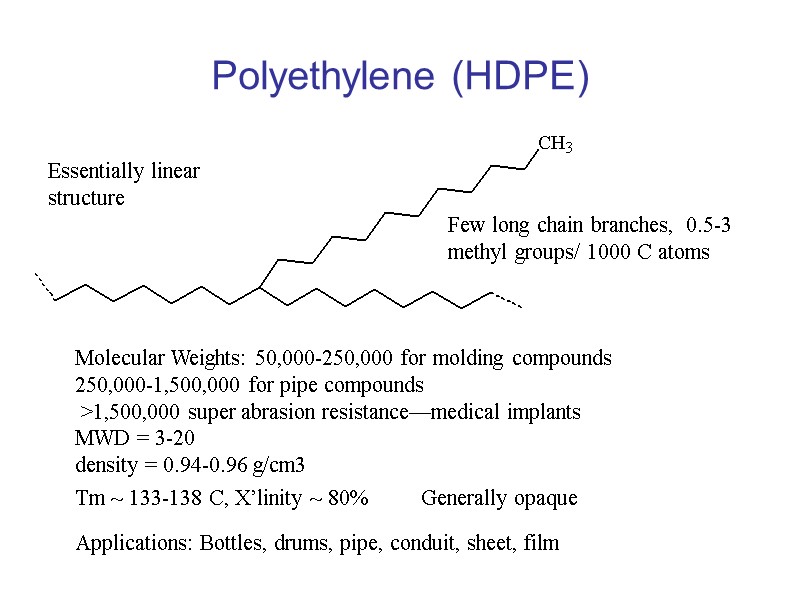
 Polyethylene (HDPE) Essentially linear structure Few long chain branches, 0.5-3 methyl groups/ 1000 C atoms Molecular Weights: 50,000-250,000 for molding compounds 250,000-1,500,000 for pipe compounds >1,500,000 super abrasion resistance—medical implants MWD = 3-20 density = 0.94-0.96 g/cm3 Tm ~ 133-138 C, X’linity ~ 80% Applications: Bottles, drums, pipe, conduit, sheet, film Generally opaque
Polyethylene (HDPE) Essentially linear structure Few long chain branches, 0.5-3 methyl groups/ 1000 C atoms Molecular Weights: 50,000-250,000 for molding compounds 250,000-1,500,000 for pipe compounds >1,500,000 super abrasion resistance—medical implants MWD = 3-20 density = 0.94-0.96 g/cm3 Tm ~ 133-138 C, X’linity ~ 80% Applications: Bottles, drums, pipe, conduit, sheet, film Generally opaque
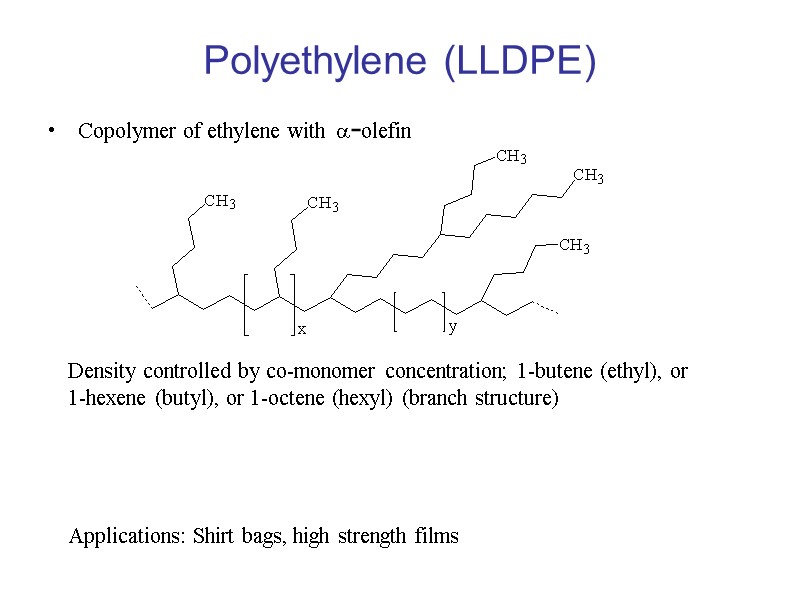
 Polyethylene (LLDPE) Copolymer of ethylene with a-olefin Density controlled by co-monomer concentration; 1-butene (ethyl), or 1-hexene (butyl), or 1-octene (hexyl) (branch structure) Applications: Shirt bags, high strength films
Polyethylene (LLDPE) Copolymer of ethylene with a-olefin Density controlled by co-monomer concentration; 1-butene (ethyl), or 1-hexene (butyl), or 1-octene (hexyl) (branch structure) Applications: Shirt bags, high strength films
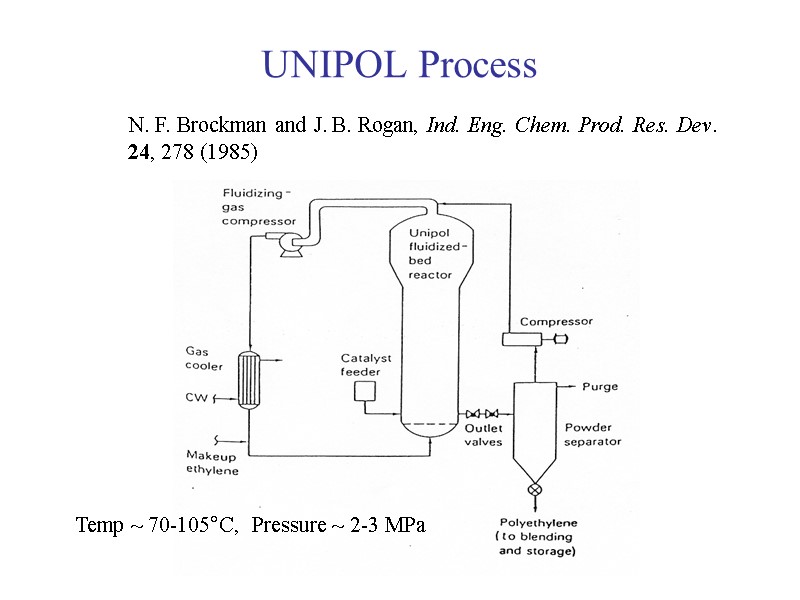
 UNIPOL Process N. F. Brockman and J. B. Rogan, Ind. Eng. Chem. Prod. Res. Dev. 24, 278 (1985) Temp ~ 70-105°C, Pressure ~ 2-3 MPa
UNIPOL Process N. F. Brockman and J. B. Rogan, Ind. Eng. Chem. Prod. Res. Dev. 24, 278 (1985) Temp ~ 70-105°C, Pressure ~ 2-3 MPa
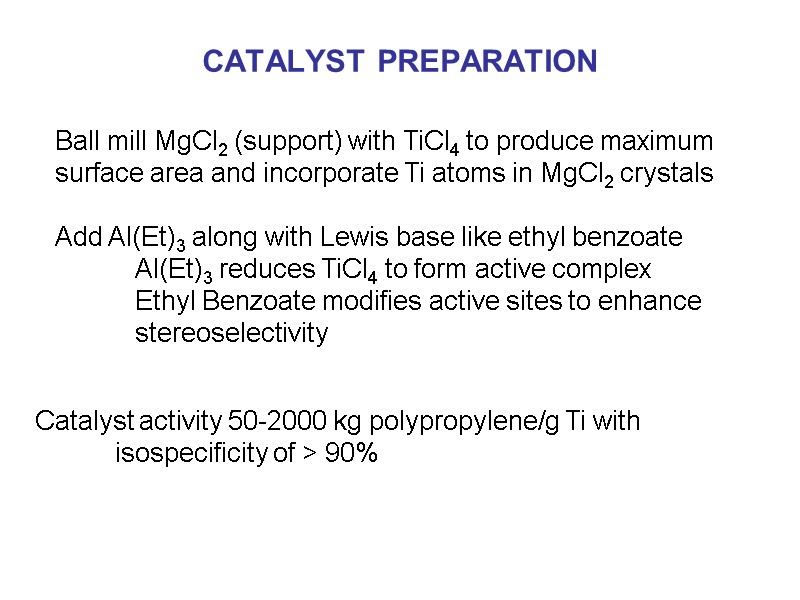
 CATALYST PREPARATION Ball mill MgCl2 (support) with TiCl4 to produce maximum surface area and incorporate Ti atoms in MgCl2 crystals Add Al(Et)3 along with Lewis base like ethyl benzoate Al(Et)3 reduces TiCl4 to form active complex Ethyl Benzoate modifies active sites to enhance stereoselectivity Catalyst activity 50-2000 kg polypropylene/g Ti with isospecificity of > 90%
CATALYST PREPARATION Ball mill MgCl2 (support) with TiCl4 to produce maximum surface area and incorporate Ti atoms in MgCl2 crystals Add Al(Et)3 along with Lewis base like ethyl benzoate Al(Et)3 reduces TiCl4 to form active complex Ethyl Benzoate modifies active sites to enhance stereoselectivity Catalyst activity 50-2000 kg polypropylene/g Ti with isospecificity of > 90%
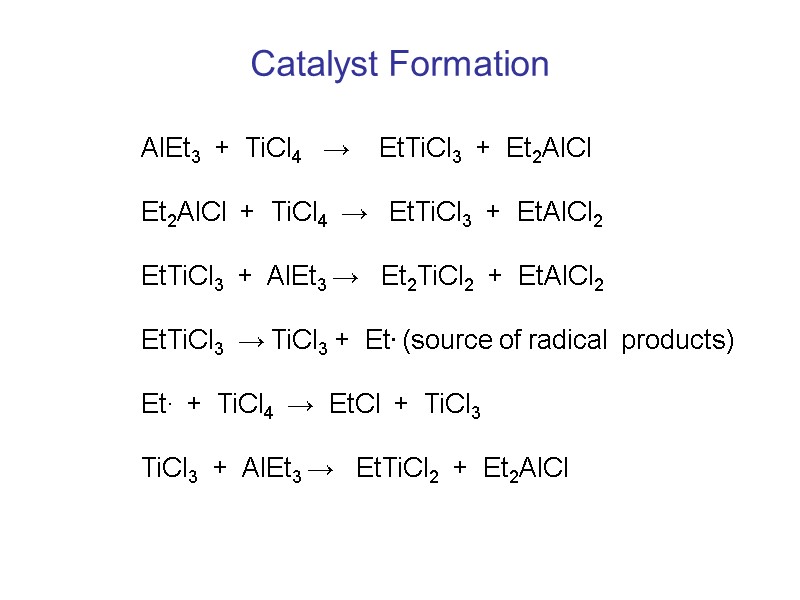
 Catalyst Formation AlEt3 + TiCl4 → EtTiCl3 + Et2AlCl Et2AlCl + TiCl4 → EtTiCl3 + EtAlCl2 EtTiCl3 + AlEt3 → Et2TiCl2 + EtAlCl2 EtTiCl3 → TiCl3 + Et. (source of radical products) Et. + TiCl4 → EtCl + TiCl3 TiCl3 + AlEt3 → EtTiCl2 + Et2AlCl
Catalyst Formation AlEt3 + TiCl4 → EtTiCl3 + Et2AlCl Et2AlCl + TiCl4 → EtTiCl3 + EtAlCl2 EtTiCl3 + AlEt3 → Et2TiCl2 + EtAlCl2 EtTiCl3 → TiCl3 + Et. (source of radical products) Et. + TiCl4 → EtCl + TiCl3 TiCl3 + AlEt3 → EtTiCl2 + Et2AlCl
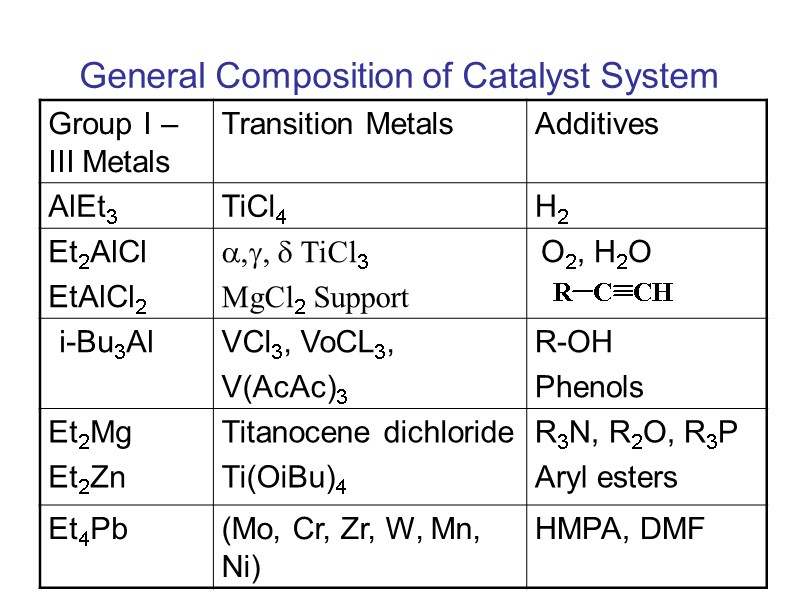
 General Composition of Catalyst System
General Composition of Catalyst System
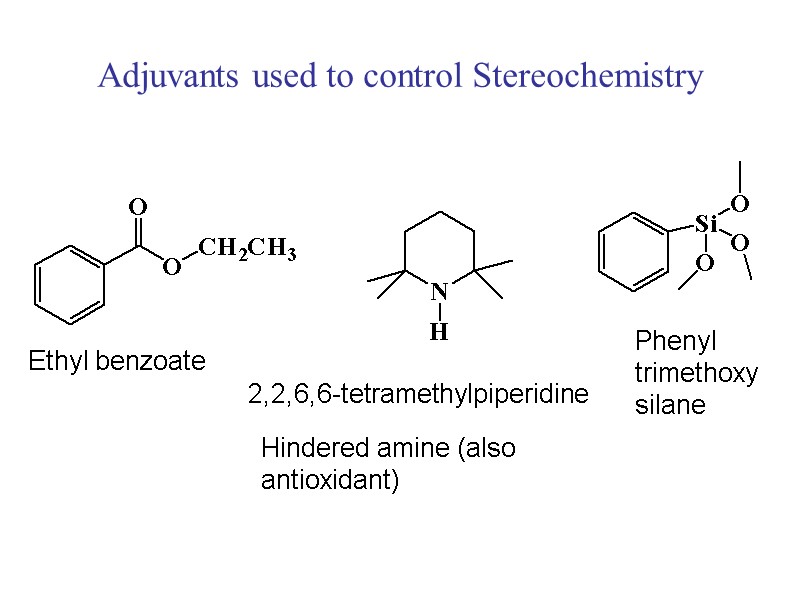
 Adjuvants used to control Stereochemistry Ethyl benzoate 2,2,6,6-tetramethylpiperidine Hindered amine (also antioxidant) Phenyl trimethoxy silane
Adjuvants used to control Stereochemistry Ethyl benzoate 2,2,6,6-tetramethylpiperidine Hindered amine (also antioxidant) Phenyl trimethoxy silane
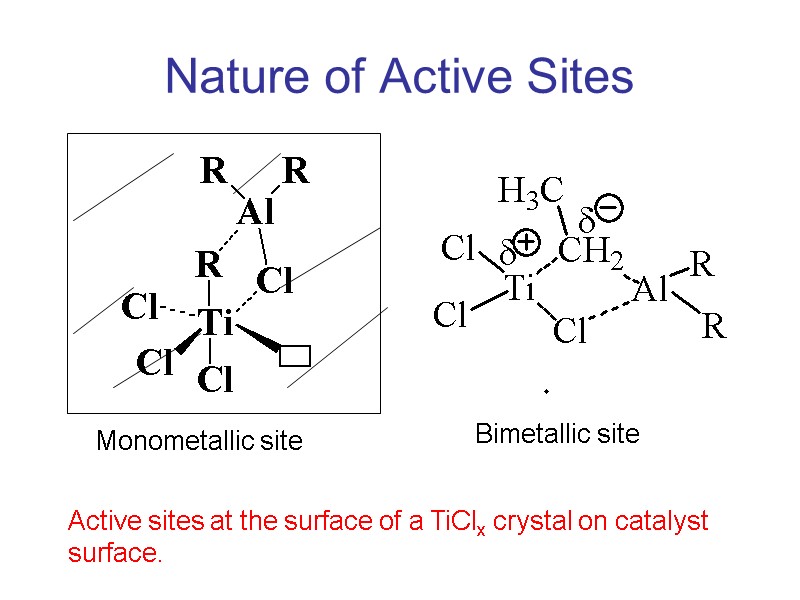
 Nature of Active Sites Monometallic site Bimetallic site Active sites at the surface of a TiClx crystal on catalyst surface.
Nature of Active Sites Monometallic site Bimetallic site Active sites at the surface of a TiClx crystal on catalyst surface.
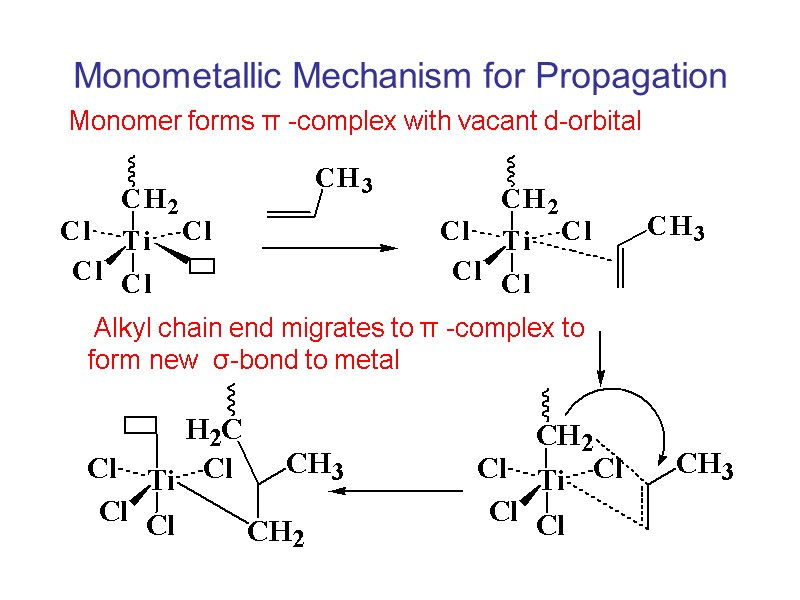
 Monometallic Mechanism for Propagation Monomer forms π -complex with vacant d-orbital Alkyl chain end migrates to π -complex to form new σ-bond to metal
Monometallic Mechanism for Propagation Monomer forms π -complex with vacant d-orbital Alkyl chain end migrates to π -complex to form new σ-bond to metal
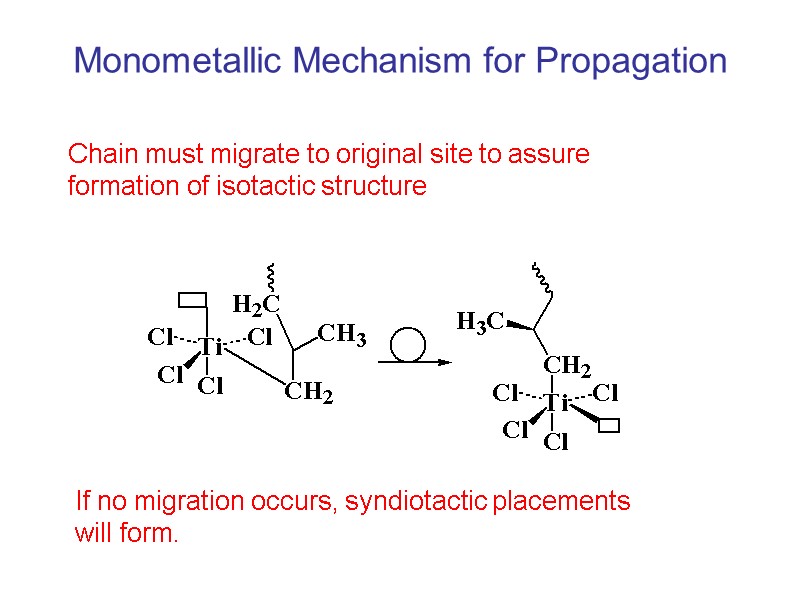
 Monometallic Mechanism for Propagation Chain must migrate to original site to assure formation of isotactic structure If no migration occurs, syndiotactic placements will form.
Monometallic Mechanism for Propagation Chain must migrate to original site to assure formation of isotactic structure If no migration occurs, syndiotactic placements will form.
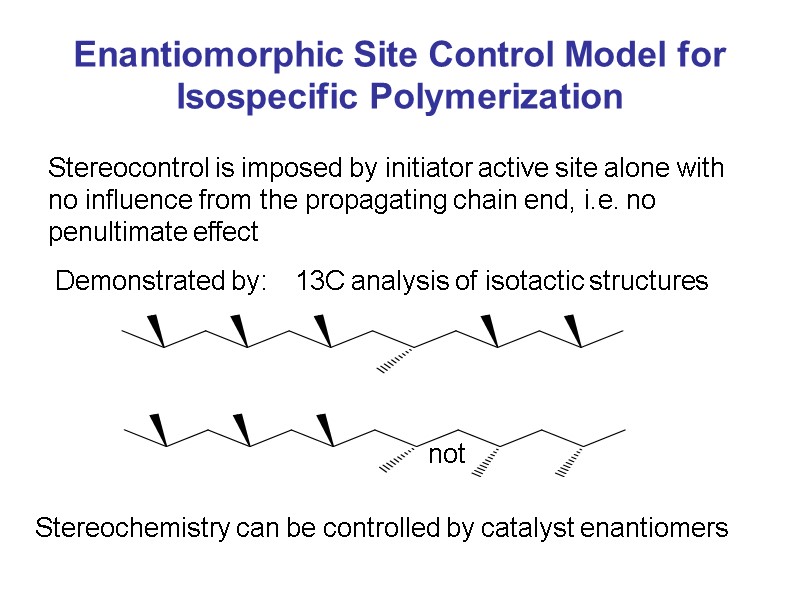
 Enantiomorphic Site Control Model for Isospecific Polymerization Stereocontrol is imposed by initiator active site alone with no influence from the propagating chain end, i.e. no penultimate effect Demonstrated by: 13C analysis of isotactic structures not Stereochemistry can be controlled by catalyst enantiomers
Enantiomorphic Site Control Model for Isospecific Polymerization Stereocontrol is imposed by initiator active site alone with no influence from the propagating chain end, i.e. no penultimate effect Demonstrated by: 13C analysis of isotactic structures not Stereochemistry can be controlled by catalyst enantiomers
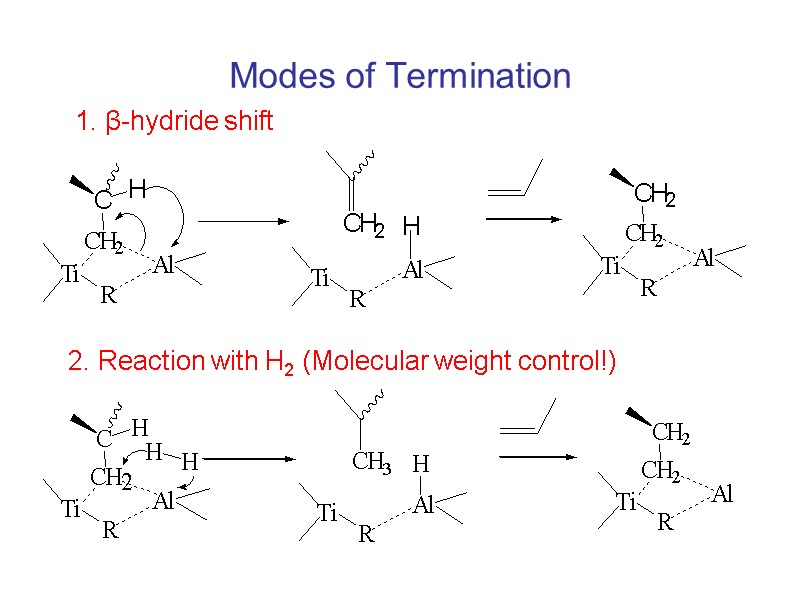
 Modes of Termination 1. β-hydride shift 2. Reaction with H2 (Molecular weight control!)
Modes of Termination 1. β-hydride shift 2. Reaction with H2 (Molecular weight control!)
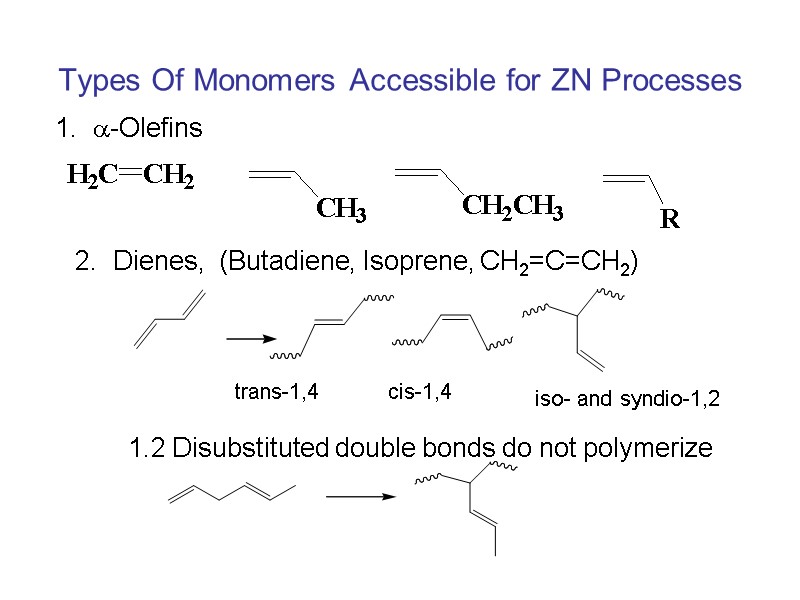
 Types Of Monomers Accessible for ZN Processes 1. -Olefins 2. Dienes, (Butadiene, Isoprene, CH2=C=CH2) 1.2 Disubstituted double bonds do not polymerize trans-1,4 cis-1,4 iso- and syndio-1,2
Types Of Monomers Accessible for ZN Processes 1. -Olefins 2. Dienes, (Butadiene, Isoprene, CH2=C=CH2) 1.2 Disubstituted double bonds do not polymerize trans-1,4 cis-1,4 iso- and syndio-1,2
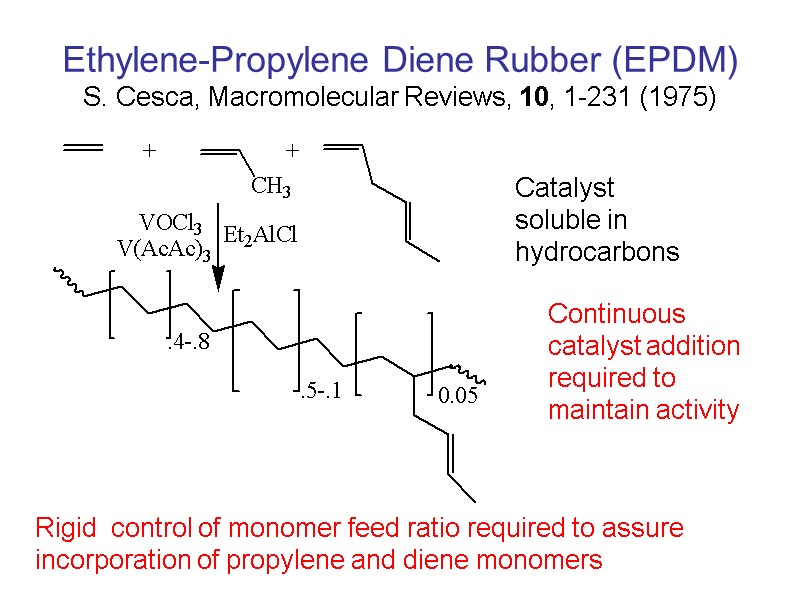
 Ethylene-Propylene Diene Rubber (EPDM) S. Cesca, Macromolecular Reviews, 10, 1-231 (1975) Catalyst soluble in hydrocarbons Continuous catalyst addition required to maintain activity Rigid control of monomer feed ratio required to assure incorporation of propylene and diene monomers
Ethylene-Propylene Diene Rubber (EPDM) S. Cesca, Macromolecular Reviews, 10, 1-231 (1975) Catalyst soluble in hydrocarbons Continuous catalyst addition required to maintain activity Rigid control of monomer feed ratio required to assure incorporation of propylene and diene monomers
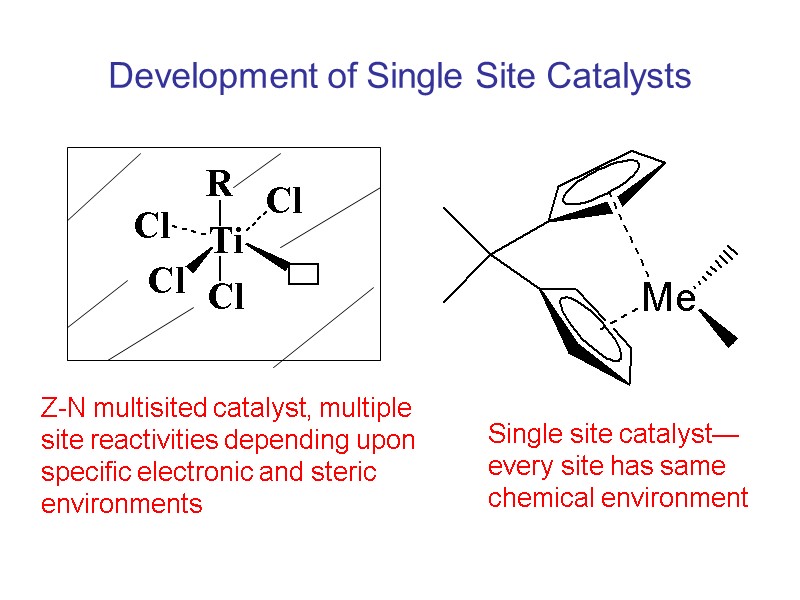
 Development of Single Site Catalysts Z-N multisited catalyst, multiple site reactivities depending upon specific electronic and steric environments Single site catalyst—every site has same chemical environment
Development of Single Site Catalysts Z-N multisited catalyst, multiple site reactivities depending upon specific electronic and steric environments Single site catalyst—every site has same chemical environment
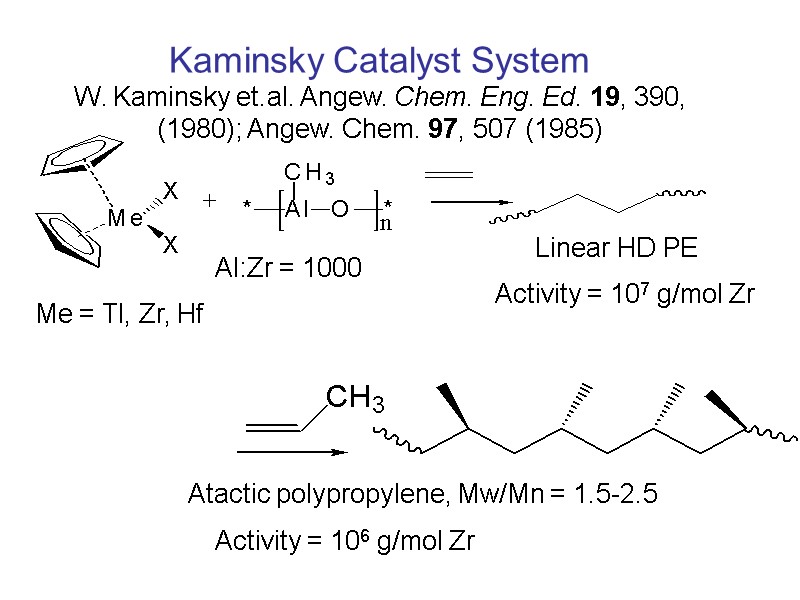
 Al:Zr = 1000 Me = Tl, Zr, Hf Linear HD PE Activity = 107 g/mol Zr Atactic polypropylene, Mw/Mn = 1.5-2.5 Activity = 106 g/mol Zr Kaminsky Catalyst System W. Kaminsky et.al. Angew. Chem. Eng. Ed. 19, 390, (1980); Angew. Chem. 97, 507 (1985)
Al:Zr = 1000 Me = Tl, Zr, Hf Linear HD PE Activity = 107 g/mol Zr Atactic polypropylene, Mw/Mn = 1.5-2.5 Activity = 106 g/mol Zr Kaminsky Catalyst System W. Kaminsky et.al. Angew. Chem. Eng. Ed. 19, 390, (1980); Angew. Chem. 97, 507 (1985)
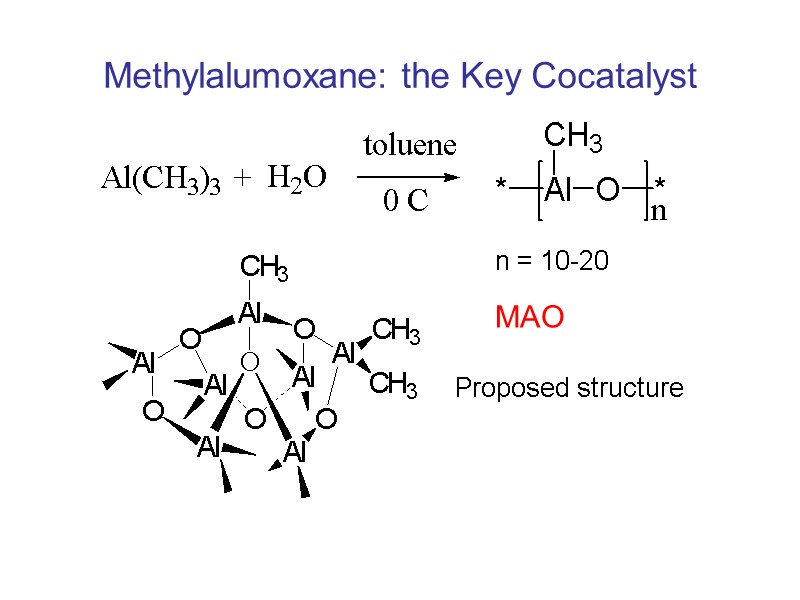
 Methylalumoxane: the Key Cocatalyst n = 10-20 Proposed structure MAO
Methylalumoxane: the Key Cocatalyst n = 10-20 Proposed structure MAO
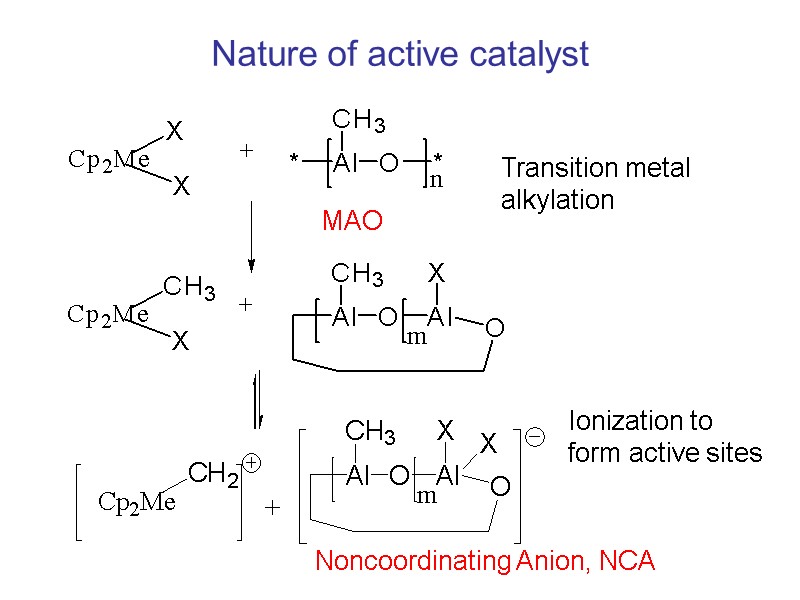
 Nature of active catalyst Transition metal alkylation Ionization to form active sites MAO Noncoordinating Anion, NCA
Nature of active catalyst Transition metal alkylation Ionization to form active sites MAO Noncoordinating Anion, NCA
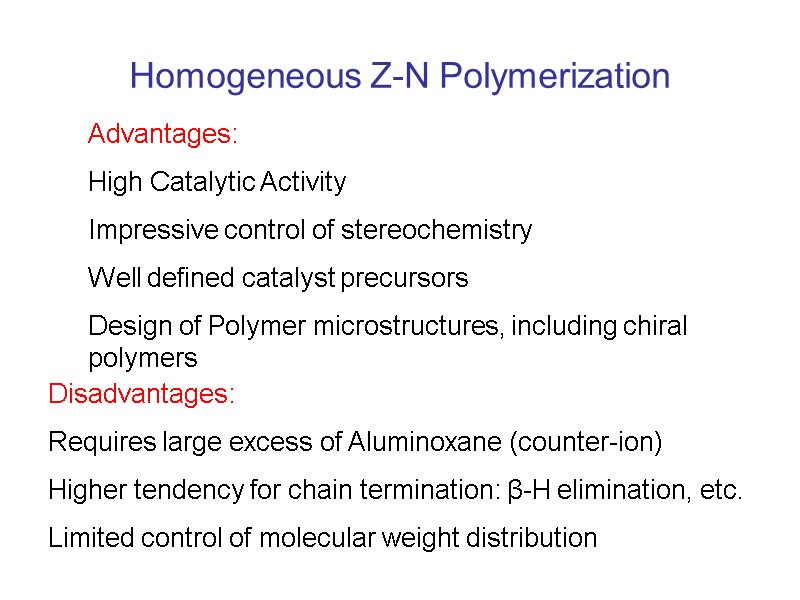
 Homogeneous Z-N Polymerization Advantages: High Catalytic Activity Impressive control of stereochemistry Well defined catalyst precursors Design of Polymer microstructures, including chiral polymers Disadvantages: Requires large excess of Aluminoxane (counter-ion) Higher tendency for chain termination: β-H elimination, etc. Limited control of molecular weight distribution
Homogeneous Z-N Polymerization Advantages: High Catalytic Activity Impressive control of stereochemistry Well defined catalyst precursors Design of Polymer microstructures, including chiral polymers Disadvantages: Requires large excess of Aluminoxane (counter-ion) Higher tendency for chain termination: β-H elimination, etc. Limited control of molecular weight distribution
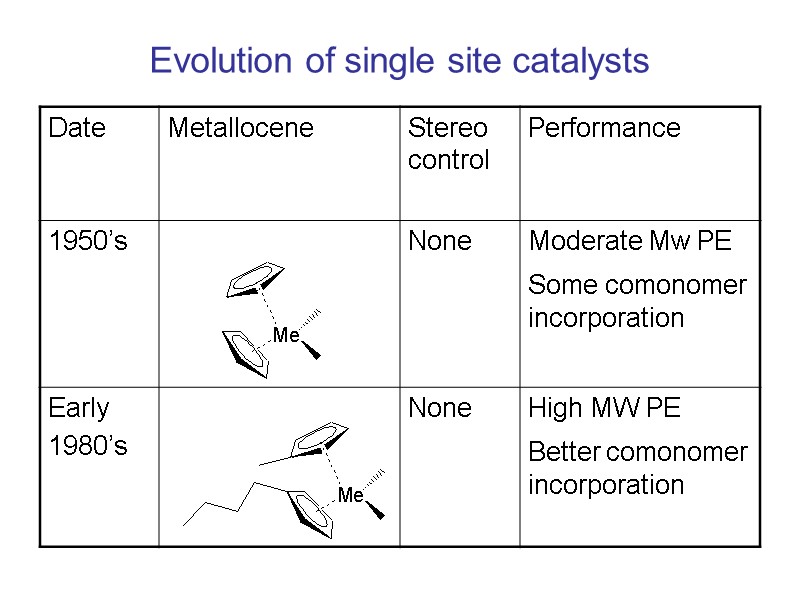
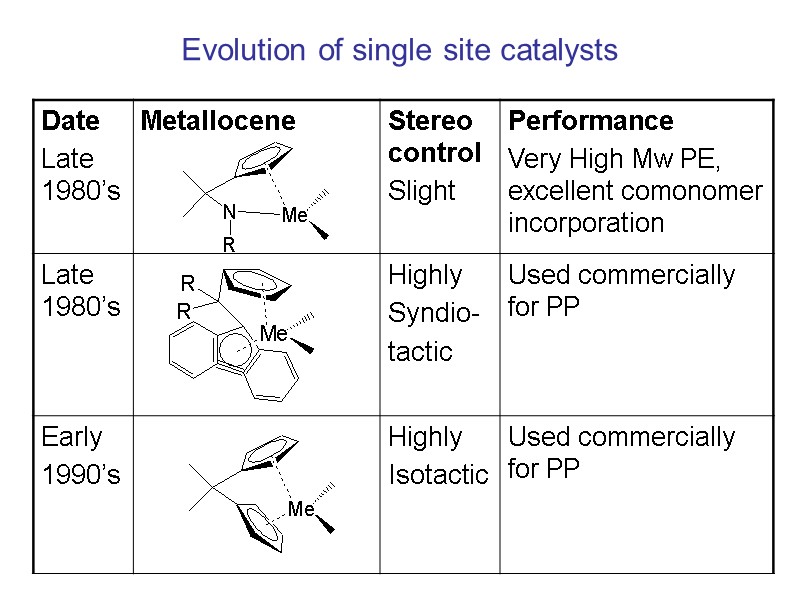
 Evolution of single site catalysts
Evolution of single site catalysts
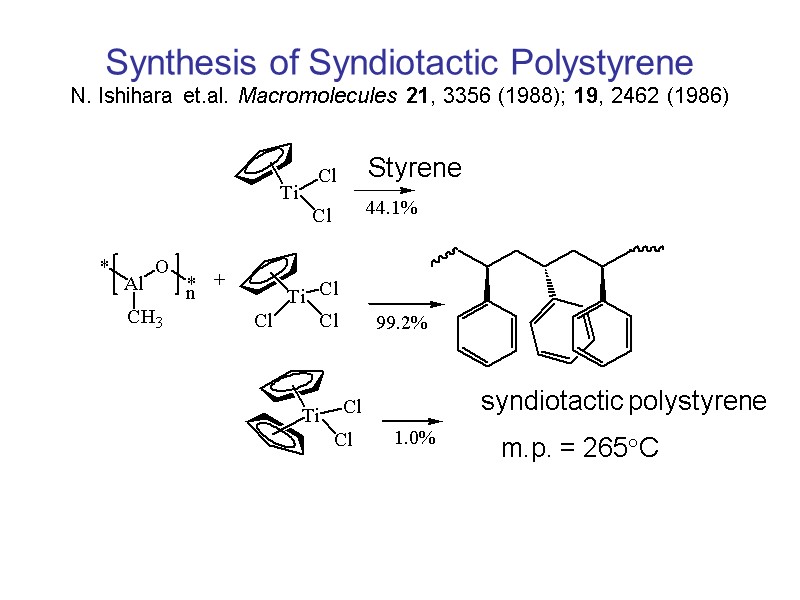
 Synthesis of Syndiotactic Polystyrene N. Ishihara et.al. Macromolecules 21, 3356 (1988); 19, 2462 (1986) syndiotactic polystyrene m.p. = 265C Styrene
Synthesis of Syndiotactic Polystyrene N. Ishihara et.al. Macromolecules 21, 3356 (1988); 19, 2462 (1986) syndiotactic polystyrene m.p. = 265C Styrene
 Evolution of single site catalysts
Evolution of single site catalysts
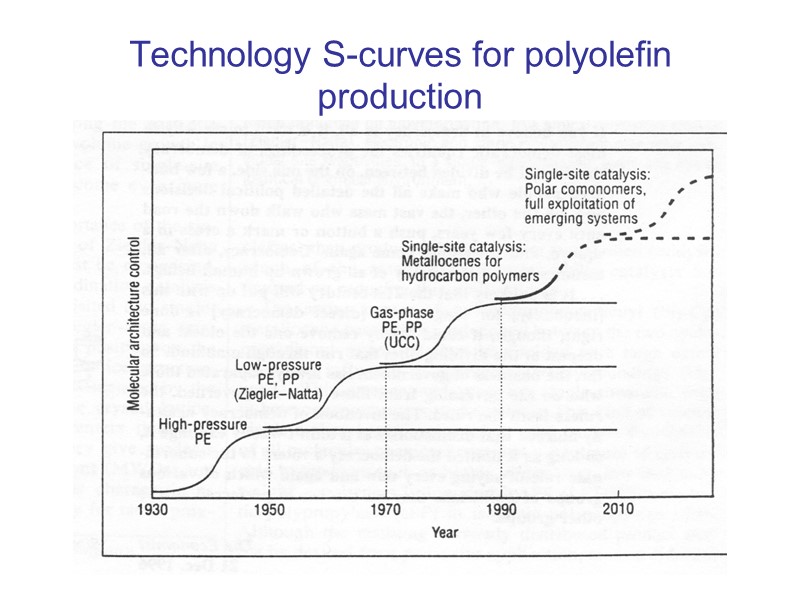
 Technology S-curves for polyolefin production
Technology S-curves for polyolefin production

