Computational Materials Science: Multiscale Modeling of Atomic Layer


![[1] Ref. [5] gives an unreasonable value of the threshold (more than 90 kcal/mol), [1] Ref. [5] gives an unreasonable value of the threshold (more than 90 kcal/mol),](https://present5.com/presentacii-2/20171213\35794-ald_film.ppt\35794-ald_film_31.jpg)


35794-ald_film.ppt
- Количество слайдов: 35
 Computational Materials Science: Multiscale Modeling of Atomic Layer Deposition of Thin Films Andrey Knizhnik Kinetic Technologies Ltd, Moscow RRC “Kurchatov Institute”, Moscow
Computational Materials Science: Multiscale Modeling of Atomic Layer Deposition of Thin Films Andrey Knizhnik Kinetic Technologies Ltd, Moscow RRC “Kurchatov Institute”, Moscow
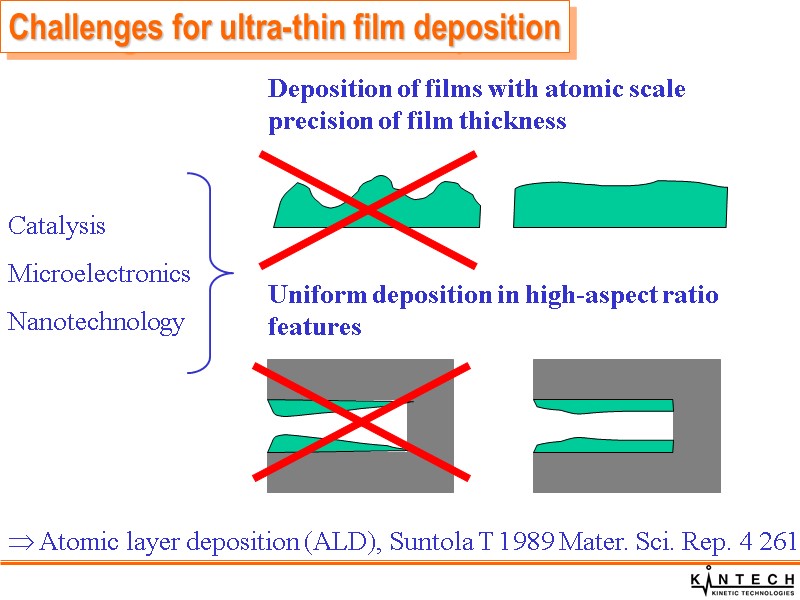
 Challenges for ultra-thin film deposition Deposition of films with atomic scale precision of film thickness Uniform deposition in high-aspect ratio features Catalysis Microelectronics Nanotechnology Atomic layer deposition (ALD), Suntola T 1989 Mater. Sci. Rep. 4 261
Challenges for ultra-thin film deposition Deposition of films with atomic scale precision of film thickness Uniform deposition in high-aspect ratio features Catalysis Microelectronics Nanotechnology Atomic layer deposition (ALD), Suntola T 1989 Mater. Sci. Rep. 4 261
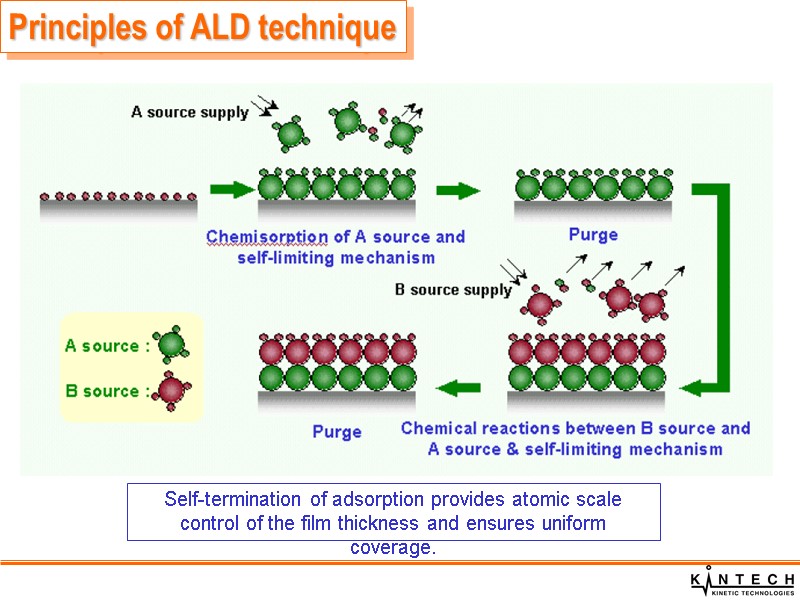
 Principles of ALD technique Self-termination of adsorption provides atomic scale control of the film thickness and ensures uniform coverage.
Principles of ALD technique Self-termination of adsorption provides atomic scale control of the film thickness and ensures uniform coverage.
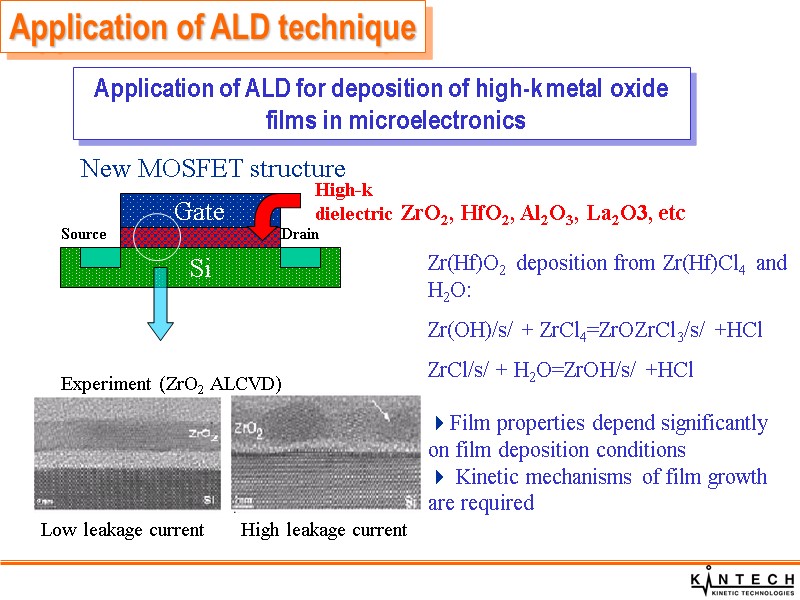
 Experiment (ZrO2 ALCVD) Zr(Hf)O2 deposition from Zr(Hf)Cl4 and H2O: Zr(OH)/s/ + ZrCl4=ZrOZrCl3/s/ +HCl ZrCl/s/ + H2O=ZrOH/s/ +HCl Film properties depend significantly on film deposition conditions Kinetic mechanisms of film growth are required Application of ALD technique Application of ALD for deposition of high-k metal oxide films in microelectronics ZrO2, HfO2, Al2O3, La2O3, etc
Experiment (ZrO2 ALCVD) Zr(Hf)O2 deposition from Zr(Hf)Cl4 and H2O: Zr(OH)/s/ + ZrCl4=ZrOZrCl3/s/ +HCl ZrCl/s/ + H2O=ZrOH/s/ +HCl Film properties depend significantly on film deposition conditions Kinetic mechanisms of film growth are required Application of ALD technique Application of ALD for deposition of high-k metal oxide films in microelectronics ZrO2, HfO2, Al2O3, La2O3, etc
 Maximum film growth rate Temperature dependence of film growth rate Residual impurities in as-deposited films Selection of precursors Film roughness Influence with initial support state Features of ALD technique Main features of atomic layer deposition
Maximum film growth rate Temperature dependence of film growth rate Residual impurities in as-deposited films Selection of precursors Film roughness Influence with initial support state Features of ALD technique Main features of atomic layer deposition
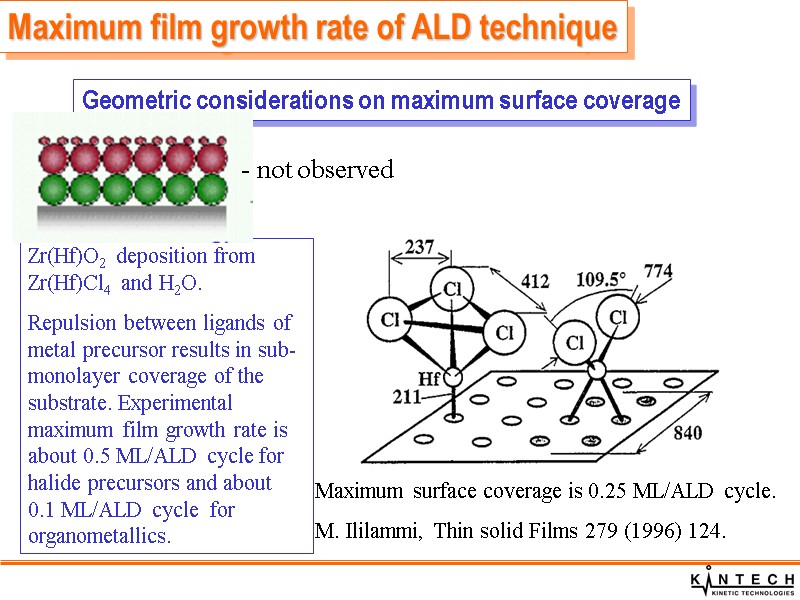
 Maximum film growth rate of ALD technique Maximum surface coverage is 0.25 ML/ALD cycle. M. Ililammi, Thin solid Films 279 (1996) 124. Geometric considerations on maximum surface coverage Zr(Hf)O2 deposition from Zr(Hf)Cl4 and H2O. Repulsion between ligands of metal precursor results in sub-monolayer coverage of the substrate. Experimental maximum film growth rate is about 0.5 ML/ALD cycle for halide precursors and about 0.1 ML/ALD cycle for organometallics. - not observed
Maximum film growth rate of ALD technique Maximum surface coverage is 0.25 ML/ALD cycle. M. Ililammi, Thin solid Films 279 (1996) 124. Geometric considerations on maximum surface coverage Zr(Hf)O2 deposition from Zr(Hf)Cl4 and H2O. Repulsion between ligands of metal precursor results in sub-monolayer coverage of the substrate. Experimental maximum film growth rate is about 0.5 ML/ALD cycle for halide precursors and about 0.1 ML/ALD cycle for organometallics. - not observed
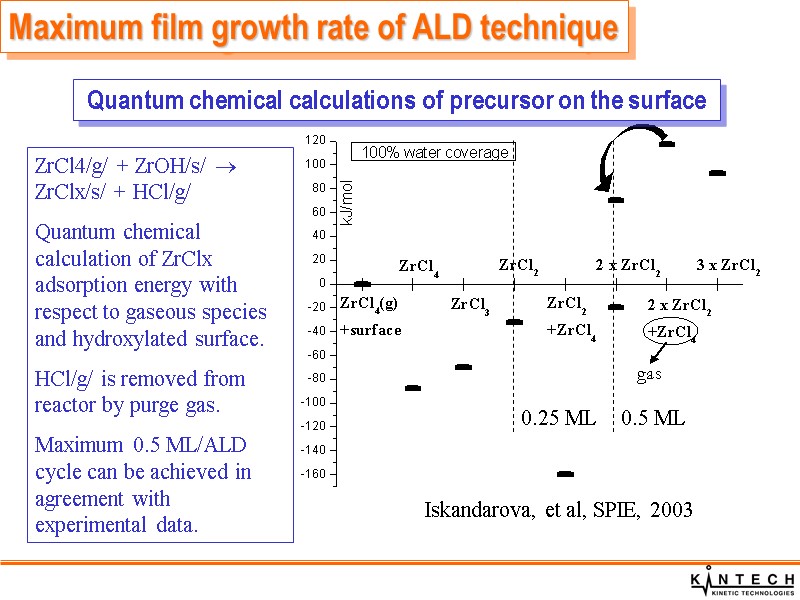
 Maximum film growth rate of ALD technique Quantum chemical calculations of precursor on the surface Iskandarova, et al, SPIE, 2003 ZrCl4/g/ + ZrOH/s/ ZrClx/s/ + HCl/g/ Quantum chemical calculation of ZrClx adsorption energy with respect to gaseous species and hydroxylated surface. HCl/g/ is removed from reactor by purge gas. Maximum 0.5 ML/ALD cycle can be achieved in agreement with experimental data. 0.5 ML 0.25 ML
Maximum film growth rate of ALD technique Quantum chemical calculations of precursor on the surface Iskandarova, et al, SPIE, 2003 ZrCl4/g/ + ZrOH/s/ ZrClx/s/ + HCl/g/ Quantum chemical calculation of ZrClx adsorption energy with respect to gaseous species and hydroxylated surface. HCl/g/ is removed from reactor by purge gas. Maximum 0.5 ML/ALD cycle can be achieved in agreement with experimental data. 0.5 ML 0.25 ML
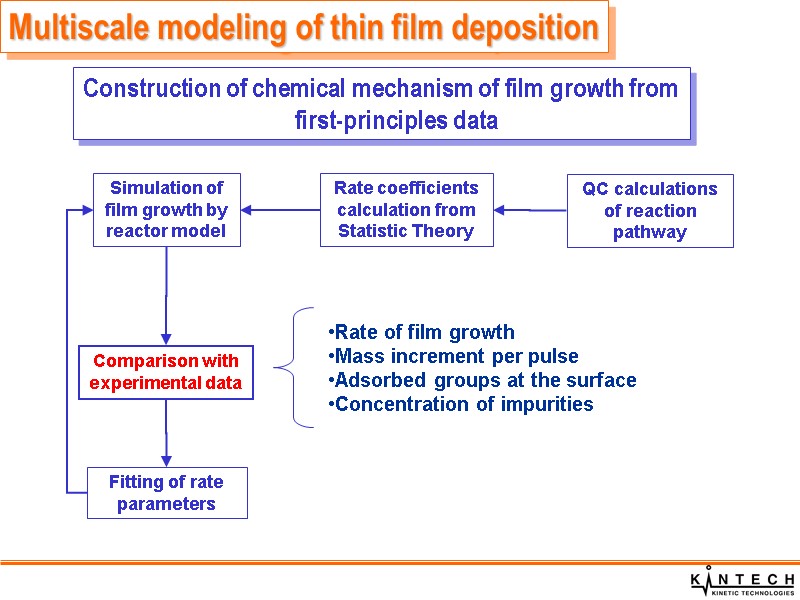
 QC calculations of reaction pathway Rate coefficients calculation from Statistic Theory Simulation of film growth by reactor model Comparison with experimental data Fitting of rate parameters Multiscale modeling of thin film deposition Construction of chemical mechanism of film growth from first-principles data Rate of film growth Mass increment per pulse Adsorbed groups at the surface Concentration of impurities
QC calculations of reaction pathway Rate coefficients calculation from Statistic Theory Simulation of film growth by reactor model Comparison with experimental data Fitting of rate parameters Multiscale modeling of thin film deposition Construction of chemical mechanism of film growth from first-principles data Rate of film growth Mass increment per pulse Adsorbed groups at the surface Concentration of impurities
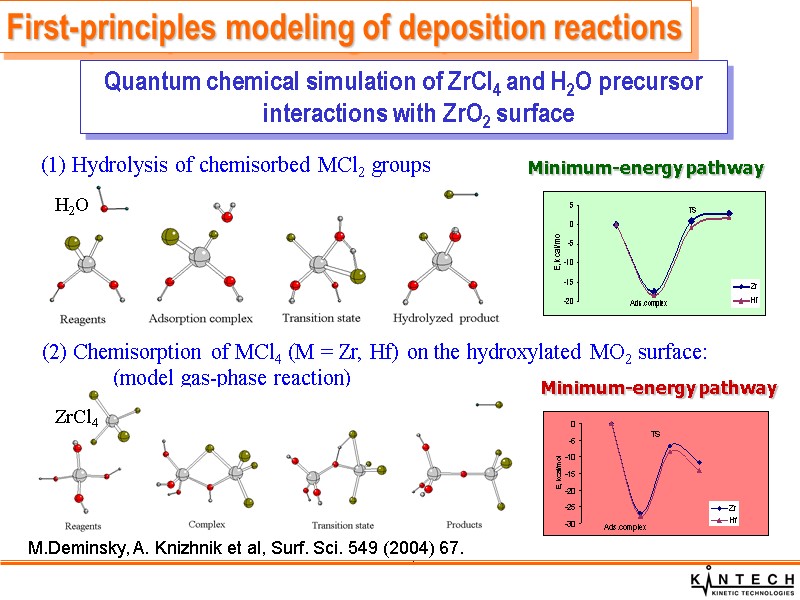
 (1) Hydrolysis of chemisorbed MCl2 groups . (2) Chemisorption of MCl4 (M = Zr, Hf) on the hydroxylated MO2 surface: (model gas-phase reaction) Minimum-energy pathway Minimum-energy pathway First-principles modeling of deposition reactions Quantum chemical simulation of ZrCl4 and H2O precursor interactions with ZrO2 surface M.Deminsky, A. Knizhnik et al, Surf. Sci. 549 (2004) 67. H2O ZrCl4
(1) Hydrolysis of chemisorbed MCl2 groups . (2) Chemisorption of MCl4 (M = Zr, Hf) on the hydroxylated MO2 surface: (model gas-phase reaction) Minimum-energy pathway Minimum-energy pathway First-principles modeling of deposition reactions Quantum chemical simulation of ZrCl4 and H2O precursor interactions with ZrO2 surface M.Deminsky, A. Knizhnik et al, Surf. Sci. 549 (2004) 67. H2O ZrCl4
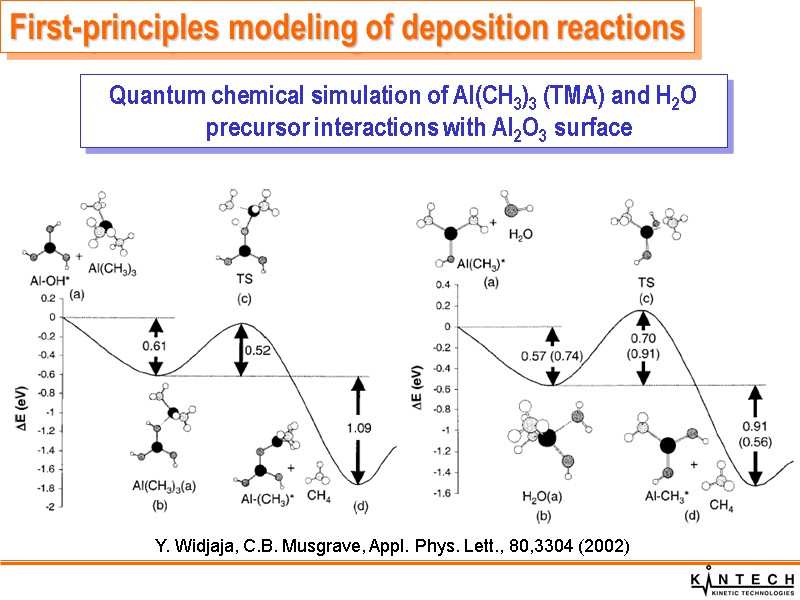
 Y. Widjaja, C.B. Musgrave, Appl. Phys. Lett., 80,3304 (2002) Quantum chemical simulation of Al(CH3)3 (TMA) and H2O precursor interactions with Al2O3 surface First-principles modeling of deposition reactions
Y. Widjaja, C.B. Musgrave, Appl. Phys. Lett., 80,3304 (2002) Quantum chemical simulation of Al(CH3)3 (TMA) and H2O precursor interactions with Al2O3 surface First-principles modeling of deposition reactions
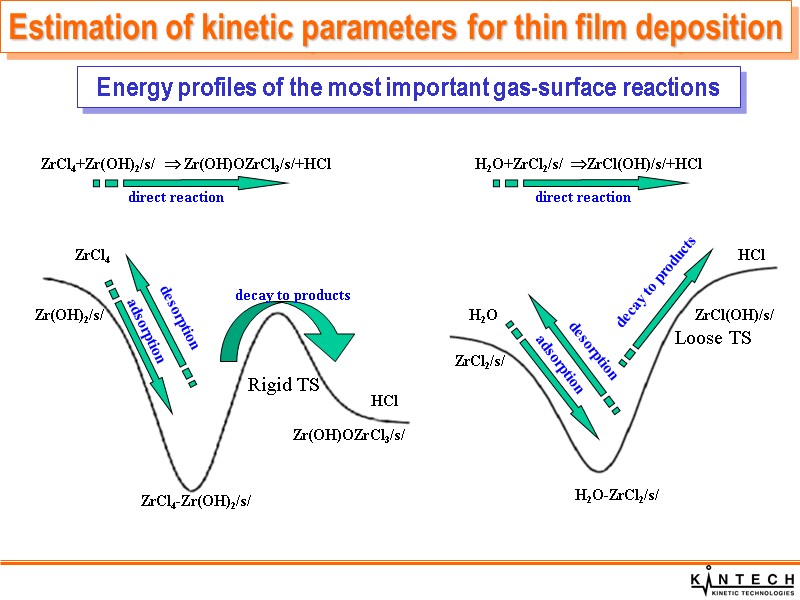
 ZrCl4+Zr(OH)2/s/ Zr(OH)OZrCl3/s/+HCl H2O+ZrCl2/s/ ZrCl(OH)/s/+HCl direct reaction direct reaction adsorption desorption H2O ZrCl2/s/ H2O-ZrCl2/s/ ZrCl(OH)/s/ HCl decay to products Energy profiles of the most important gas-surface reactions Estimation of kinetic parameters for thin film deposition Rigid TS Loose TS
ZrCl4+Zr(OH)2/s/ Zr(OH)OZrCl3/s/+HCl H2O+ZrCl2/s/ ZrCl(OH)/s/+HCl direct reaction direct reaction adsorption desorption H2O ZrCl2/s/ H2O-ZrCl2/s/ ZrCl(OH)/s/ HCl decay to products Energy profiles of the most important gas-surface reactions Estimation of kinetic parameters for thin film deposition Rigid TS Loose TS
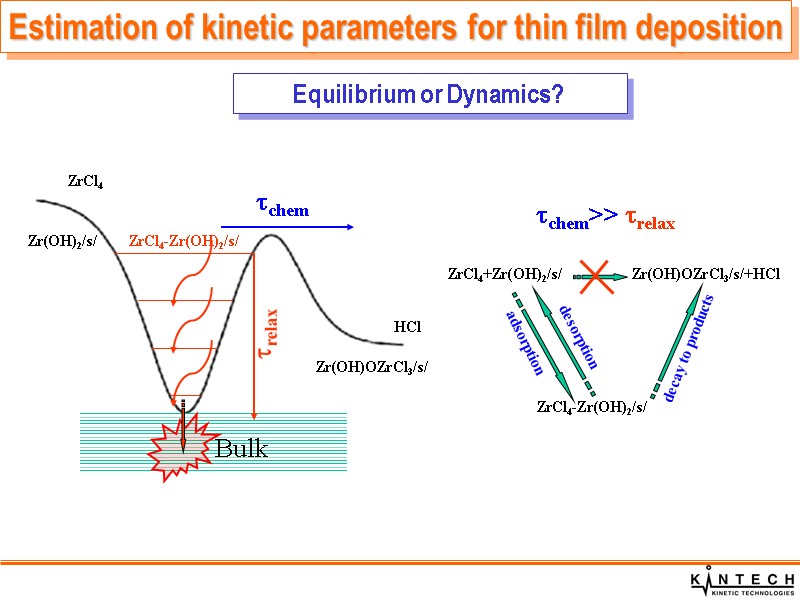
 ZrCl4 Zr(OH)2/s/ ZrCl4-Zr(OH)2/s/ Bulk ZrCl4 Zr(OH)2/s/ Zr(OH)OZrCl3/s/ HCl chem relax chem>> relax ZrCl4+Zr(OH)2/s/ Zr(OH)OZrCl3/s/+HCl ZrCl4-Zr(OH)2/s/ adsorption desorption decay to products Estimation of kinetic parameters for thin film deposition Equilibrium or Dynamics?
ZrCl4 Zr(OH)2/s/ ZrCl4-Zr(OH)2/s/ Bulk ZrCl4 Zr(OH)2/s/ Zr(OH)OZrCl3/s/ HCl chem relax chem>> relax ZrCl4+Zr(OH)2/s/ Zr(OH)OZrCl3/s/+HCl ZrCl4-Zr(OH)2/s/ adsorption desorption decay to products Estimation of kinetic parameters for thin film deposition Equilibrium or Dynamics?
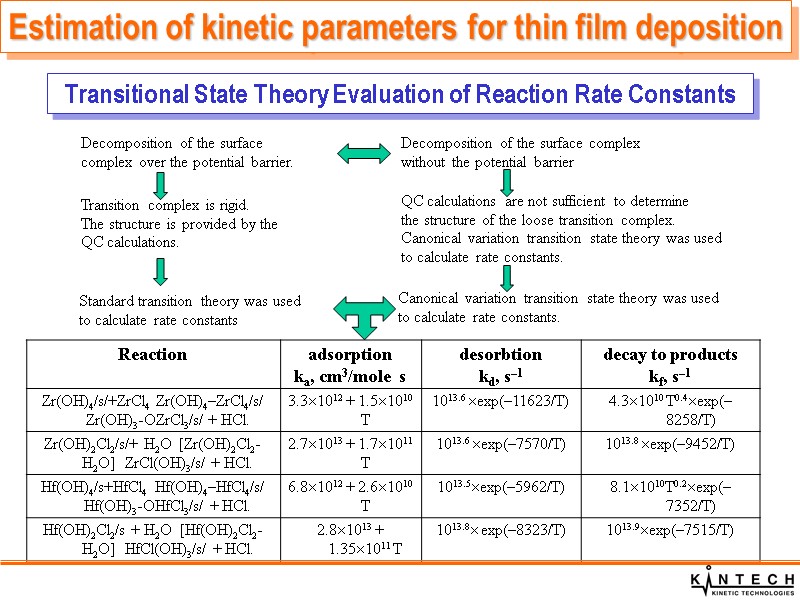
 Decomposition of the surface complex over the potential barrier. Transition complex is rigid. The structure is provided by the QC calculations. Decomposition of the surface complex without the potential barrier QC calculations are not sufficient to determine the structure of the loose transition complex. Canonical variation transition state theory was used to calculate rate constants. Standard transition theory was used to calculate rate constants Canonical variation transition state theory was used to calculate rate constants. Transitional State Theory Evaluation of Reaction Rate Constants Estimation of kinetic parameters for thin film deposition
Decomposition of the surface complex over the potential barrier. Transition complex is rigid. The structure is provided by the QC calculations. Decomposition of the surface complex without the potential barrier QC calculations are not sufficient to determine the structure of the loose transition complex. Canonical variation transition state theory was used to calculate rate constants. Standard transition theory was used to calculate rate constants Canonical variation transition state theory was used to calculate rate constants. Transitional State Theory Evaluation of Reaction Rate Constants Estimation of kinetic parameters for thin film deposition
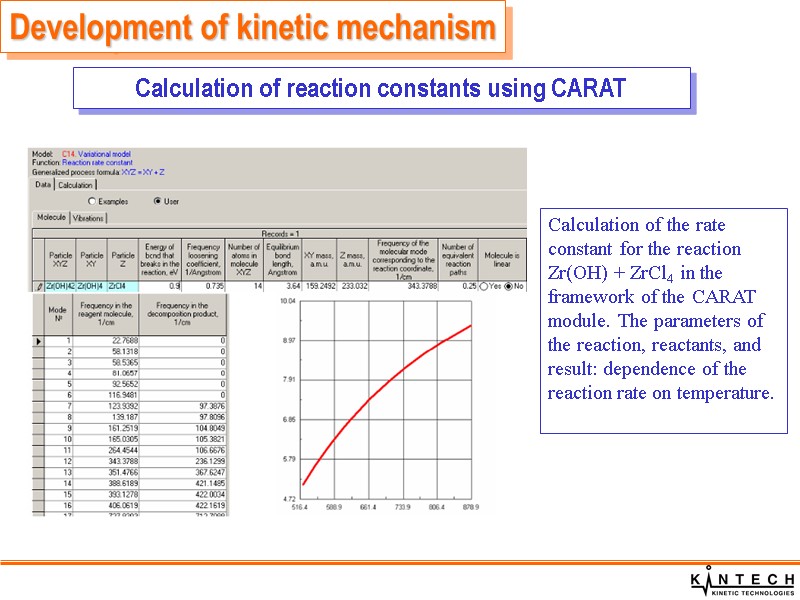
 Development of kinetic mechanism Calculation of reaction constants using CARAT Calculation of the rate constant for the reaction Zr(OH) + ZrCl4 in the framework of the CARAT module. The parameters of the reaction, reactants, and result: dependence of the reaction rate on temperature.
Development of kinetic mechanism Calculation of reaction constants using CARAT Calculation of the rate constant for the reaction Zr(OH) + ZrCl4 in the framework of the CARAT module. The parameters of the reaction, reactants, and result: dependence of the reaction rate on temperature.
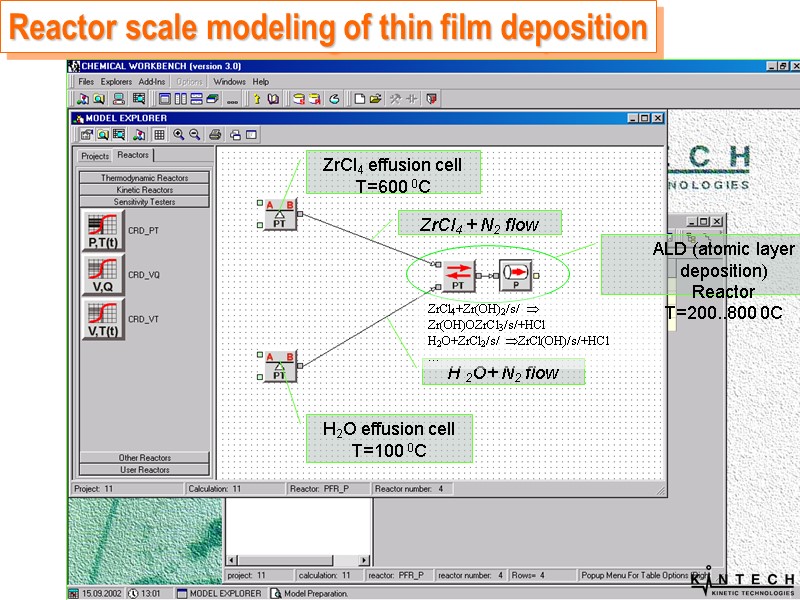
 Reactor scale modeling of thin film deposition
Reactor scale modeling of thin film deposition
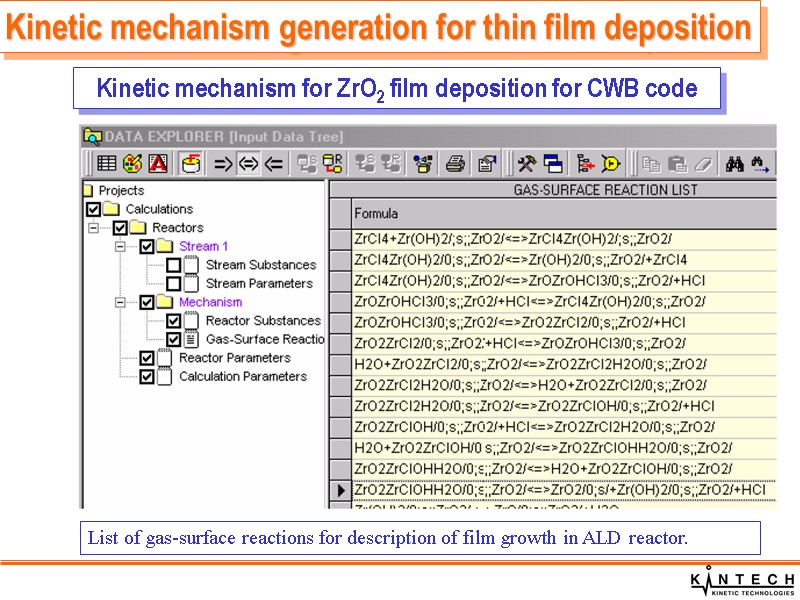
 Kinetic mechanism for ZrO2 film deposition for CWB code Kinetic mechanism generation for thin film deposition List of gas-surface reactions for description of film growth in ALD reactor.
Kinetic mechanism for ZrO2 film deposition for CWB code Kinetic mechanism generation for thin film deposition List of gas-surface reactions for description of film growth in ALD reactor.
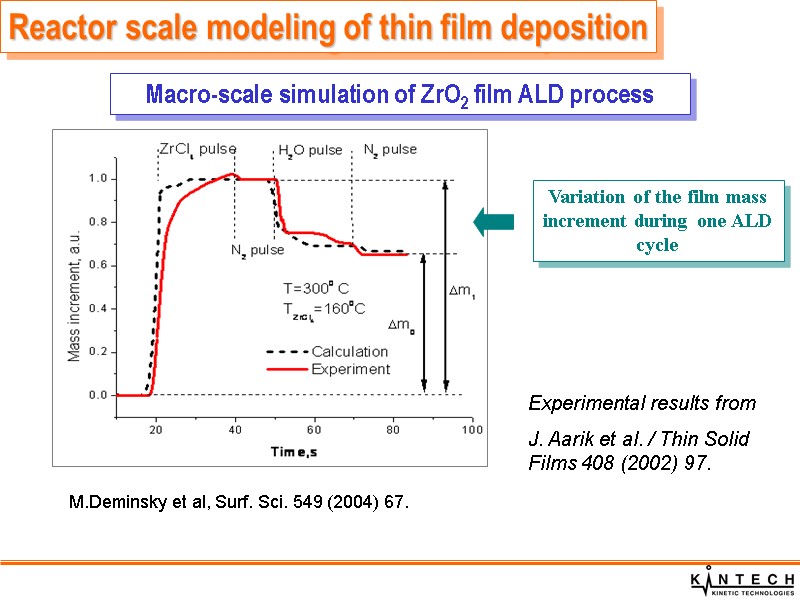
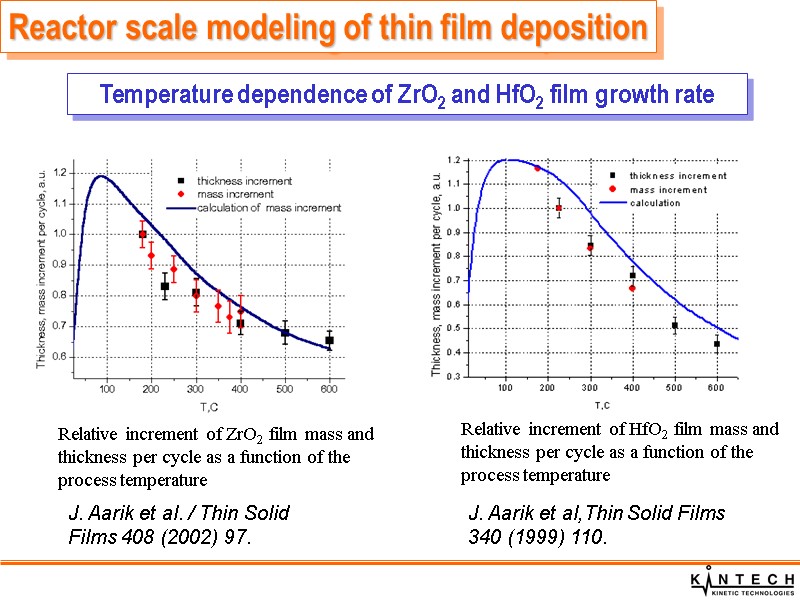
 Macro-scale simulation of ZrO2 film ALD process Variation of the film mass increment during one ALD cycle Reactor scale modeling of thin film deposition Experimental results from J. Aarik et al. / Thin Solid Films 408 (2002) 97. M.Deminsky et al, Surf. Sci. 549 (2004) 67.
Macro-scale simulation of ZrO2 film ALD process Variation of the film mass increment during one ALD cycle Reactor scale modeling of thin film deposition Experimental results from J. Aarik et al. / Thin Solid Films 408 (2002) 97. M.Deminsky et al, Surf. Sci. 549 (2004) 67.
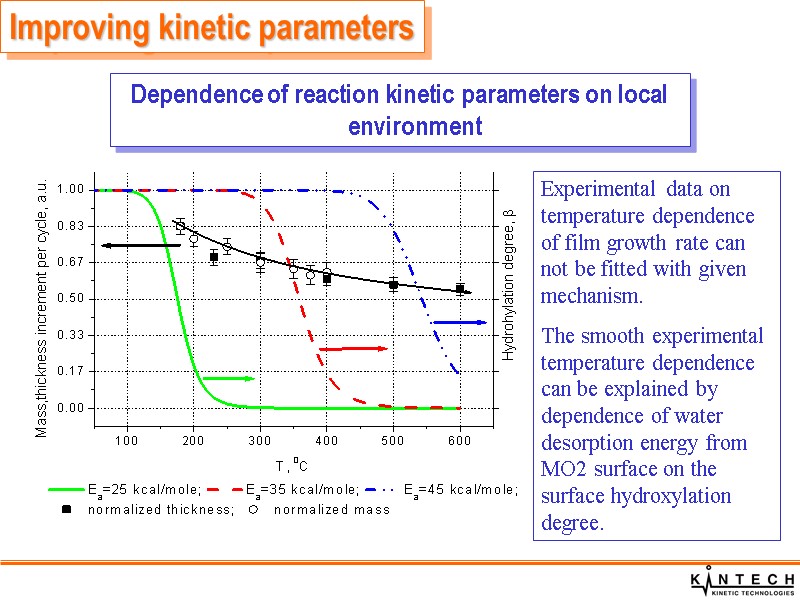
 Improving kinetic parameters Dependence of reaction kinetic parameters on local environment Experimental data on temperature dependence of film growth rate can not be fitted with given mechanism. The smooth experimental temperature dependence can be explained by dependence of water desorption energy from MO2 surface on the surface hydroxylation degree.
Improving kinetic parameters Dependence of reaction kinetic parameters on local environment Experimental data on temperature dependence of film growth rate can not be fitted with given mechanism. The smooth experimental temperature dependence can be explained by dependence of water desorption energy from MO2 surface on the surface hydroxylation degree.
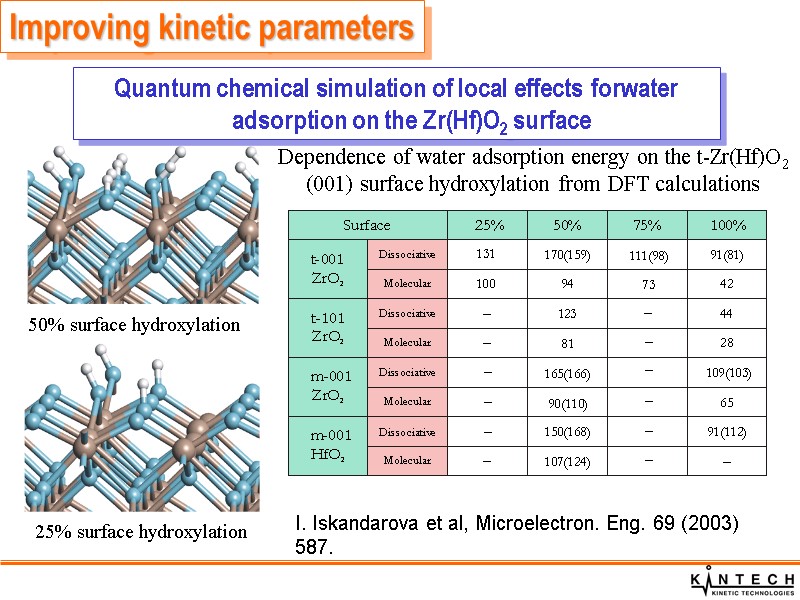
 25% surface hydroxylation 50% surface hydroxylation Dependence of water adsorption energy on the t-Zr(Hf)O2 (001) surface hydroxylation from DFT calculations Quantum chemical simulation of local effects forwater adsorption on the Zr(Hf)O2 surface I. Iskandarova et al, Microelectron. Eng. 69 (2003) 587. Improving kinetic parameters
25% surface hydroxylation 50% surface hydroxylation Dependence of water adsorption energy on the t-Zr(Hf)O2 (001) surface hydroxylation from DFT calculations Quantum chemical simulation of local effects forwater adsorption on the Zr(Hf)O2 surface I. Iskandarova et al, Microelectron. Eng. 69 (2003) 587. Improving kinetic parameters
 Relative increment of ZrO2 film mass and thickness per cycle as a function of the process temperature Relative increment of HfO2 film mass and thickness per cycle as a function of the process temperature Reactor scale modeling of thin film deposition Temperature dependence of ZrO2 and HfO2 film growth rate J. Aarik et al. / Thin Solid Films 408 (2002) 97. J. Aarik et al,Thin Solid Films 340 (1999) 110.
Relative increment of ZrO2 film mass and thickness per cycle as a function of the process temperature Relative increment of HfO2 film mass and thickness per cycle as a function of the process temperature Reactor scale modeling of thin film deposition Temperature dependence of ZrO2 and HfO2 film growth rate J. Aarik et al. / Thin Solid Films 408 (2002) 97. J. Aarik et al,Thin Solid Films 340 (1999) 110.
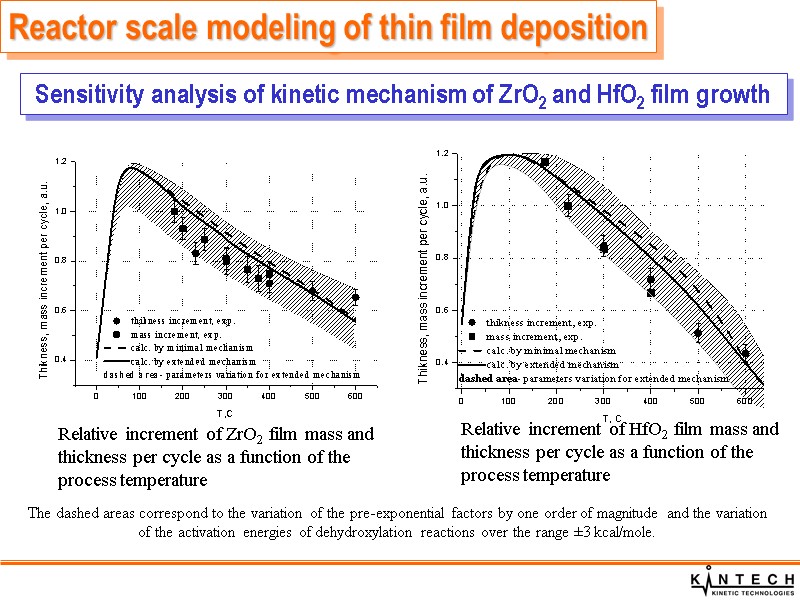
 Relative increment of ZrO2 film mass and thickness per cycle as a function of the process temperature Relative increment of HfO2 film mass and thickness per cycle as a function of the process temperature The dashed areas correspond to the variation of the pre-exponential factors by one order of magnitude and the variation of the activation energies of dehydroxylation reactions over the range ±3 kcal/mole. Sensitivity analysis of kinetic mechanism of ZrO2 and HfO2 film growth Reactor scale modeling of thin film deposition
Relative increment of ZrO2 film mass and thickness per cycle as a function of the process temperature Relative increment of HfO2 film mass and thickness per cycle as a function of the process temperature The dashed areas correspond to the variation of the pre-exponential factors by one order of magnitude and the variation of the activation energies of dehydroxylation reactions over the range ±3 kcal/mole. Sensitivity analysis of kinetic mechanism of ZrO2 and HfO2 film growth Reactor scale modeling of thin film deposition
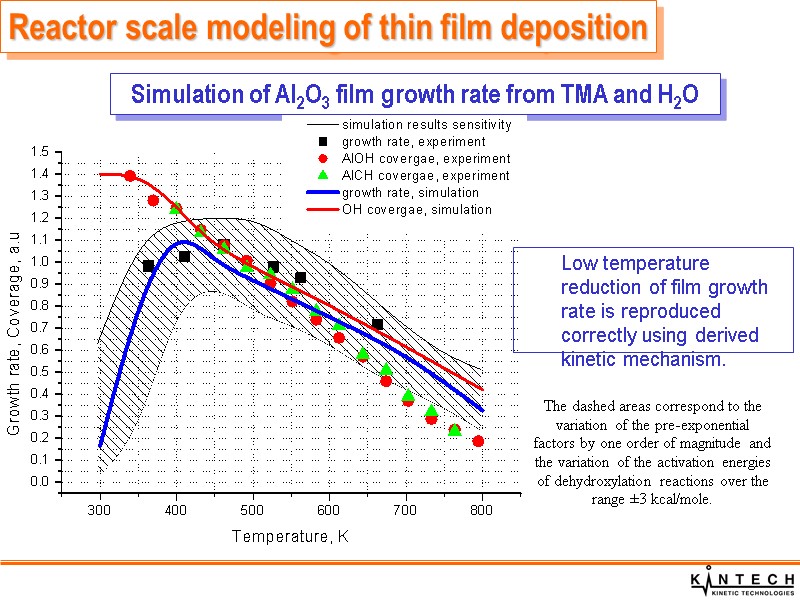
 Simulation of Al2O3 film growth rate from TMA and H2O Reactor scale modeling of thin film deposition Low temperature reduction of film growth rate is reproduced correctly using derived kinetic mechanism. The dashed areas correspond to the variation of the pre-exponential factors by one order of magnitude and the variation of the activation energies of dehydroxylation reactions over the range ±3 kcal/mole.
Simulation of Al2O3 film growth rate from TMA and H2O Reactor scale modeling of thin film deposition Low temperature reduction of film growth rate is reproduced correctly using derived kinetic mechanism. The dashed areas correspond to the variation of the pre-exponential factors by one order of magnitude and the variation of the activation energies of dehydroxylation reactions over the range ±3 kcal/mole.
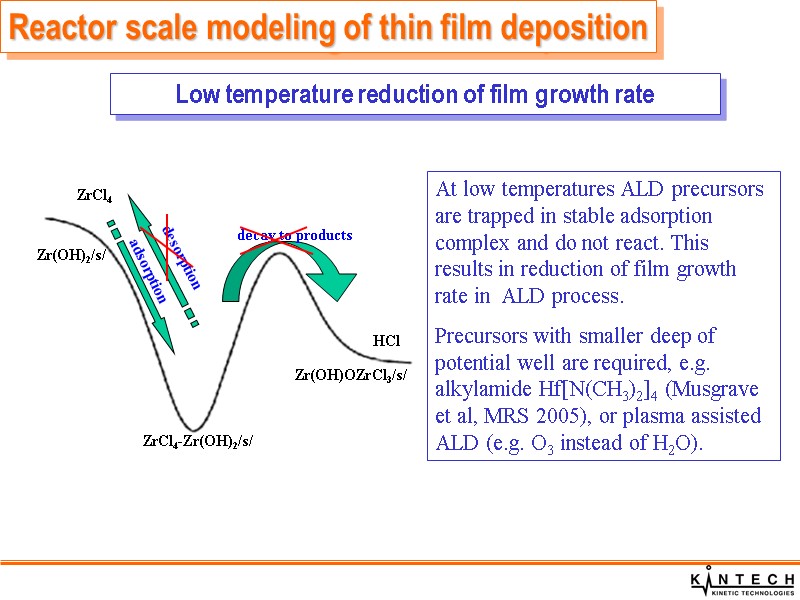
 Reactor scale modeling of thin film deposition Low temperature reduction of film growth rate At low temperatures ALD precursors are trapped in stable adsorption complex and do not react. This results in reduction of film growth rate in ALD process. Precursors with smaller deep of potential well are required, e.g. alkylamide Hf[N(CH3)2]4 (Musgrave et al, MRS 2005), or plasma assisted ALD (e.g. O3 instead of H2O).
Reactor scale modeling of thin film deposition Low temperature reduction of film growth rate At low temperatures ALD precursors are trapped in stable adsorption complex and do not react. This results in reduction of film growth rate in ALD process. Precursors with smaller deep of potential well are required, e.g. alkylamide Hf[N(CH3)2]4 (Musgrave et al, MRS 2005), or plasma assisted ALD (e.g. O3 instead of H2O).
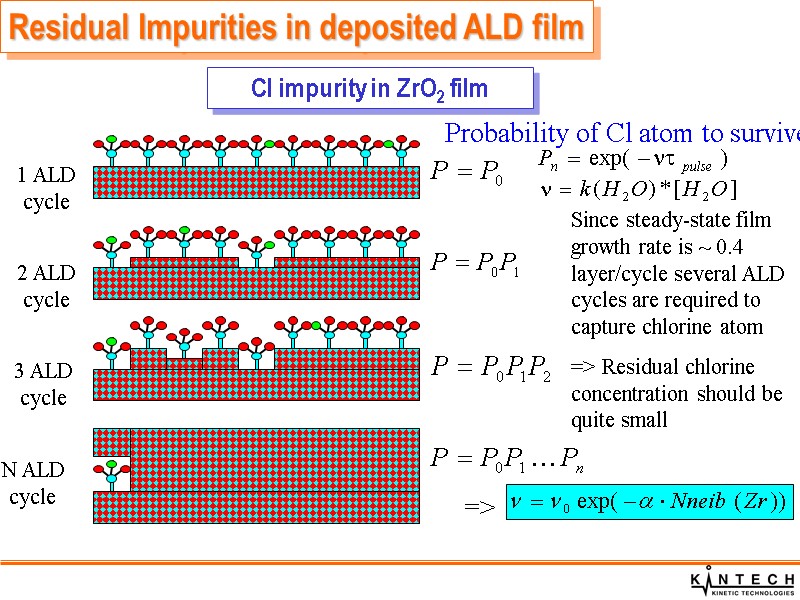
 1 ALD cycle 2 ALD cycle 3 ALD cycle N ALD cycle Since steady-state film growth rate is ~ 0.4 layer/cycle several ALD cycles are required to capture chlorine atom => Residual chlorine concentration should be quite small => Probability of Cl atom to survive: Cl impurity in ZrO2 film Residual Impurities in deposited ALD film
1 ALD cycle 2 ALD cycle 3 ALD cycle N ALD cycle Since steady-state film growth rate is ~ 0.4 layer/cycle several ALD cycles are required to capture chlorine atom => Residual chlorine concentration should be quite small => Probability of Cl atom to survive: Cl impurity in ZrO2 film Residual Impurities in deposited ALD film
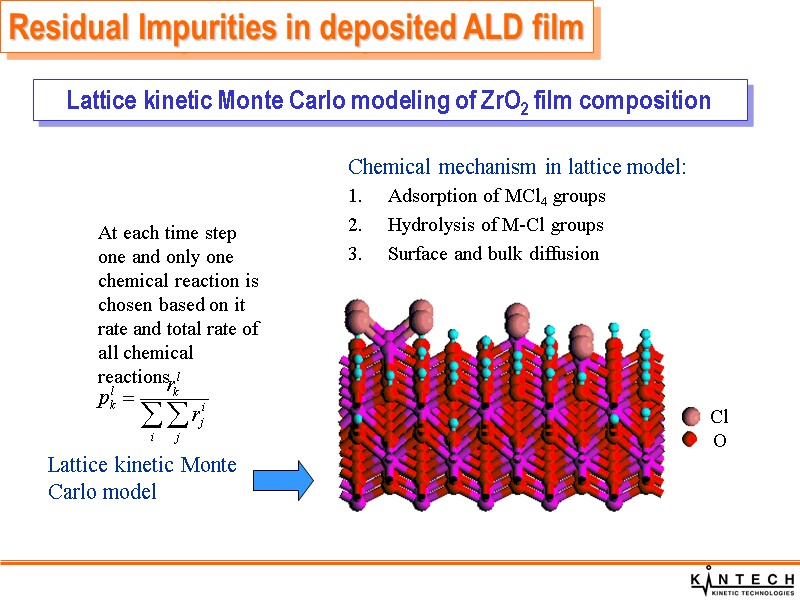
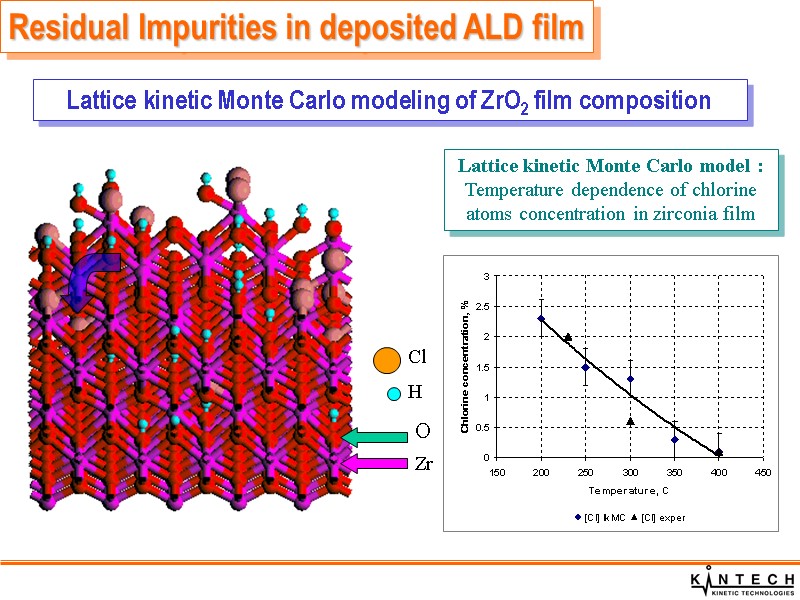
 At each time step one and only one chemical reaction is chosen based on it rate and total rate of all chemical reactions Chemical mechanism in lattice model: Adsorption of MCl4 groups Hydrolysis of M-Cl groups Surface and bulk diffusion Lattice kinetic Monte Carlo model Cl O Lattice kinetic Monte Carlo modeling of ZrO2 film composition Residual Impurities in deposited ALD film
At each time step one and only one chemical reaction is chosen based on it rate and total rate of all chemical reactions Chemical mechanism in lattice model: Adsorption of MCl4 groups Hydrolysis of M-Cl groups Surface and bulk diffusion Lattice kinetic Monte Carlo model Cl O Lattice kinetic Monte Carlo modeling of ZrO2 film composition Residual Impurities in deposited ALD film
 Lattice kinetic Monte Carlo modeling of ZrO2 film composition Lattice kinetic Monte Carlo model : Temperature dependence of chlorine atoms concentration in zirconia film Residual Impurities in deposited ALD film
Lattice kinetic Monte Carlo modeling of ZrO2 film composition Lattice kinetic Monte Carlo model : Temperature dependence of chlorine atoms concentration in zirconia film Residual Impurities in deposited ALD film
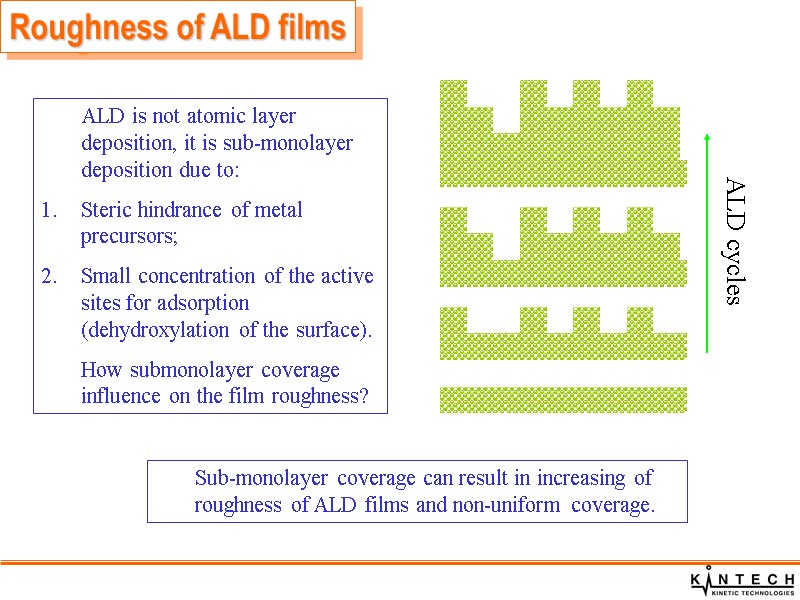
 Roughness of ALD films ALD is not atomic layer deposition, it is sub-monolayer deposition due to: Steric hindrance of metal precursors; Small concentration of the active sites for adsorption (dehydroxylation of the surface). How submonolayer coverage influence on the film roughness? Sub-monolayer coverage can result in increasing of roughness of ALD films and non-uniform coverage.
Roughness of ALD films ALD is not atomic layer deposition, it is sub-monolayer deposition due to: Steric hindrance of metal precursors; Small concentration of the active sites for adsorption (dehydroxylation of the surface). How submonolayer coverage influence on the film roughness? Sub-monolayer coverage can result in increasing of roughness of ALD films and non-uniform coverage.
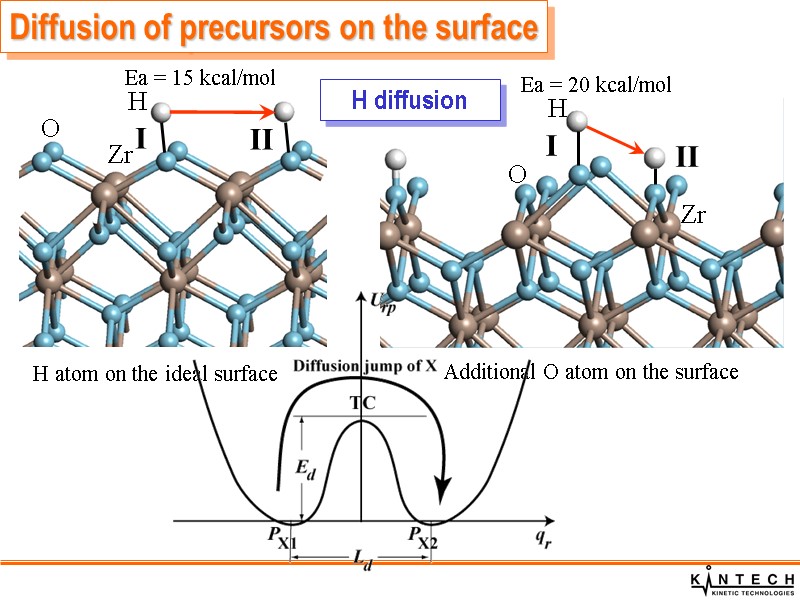
 Diffusion of precursors on the surface H diffusion O Zr O Zr H H
Diffusion of precursors on the surface H diffusion O Zr O Zr H H
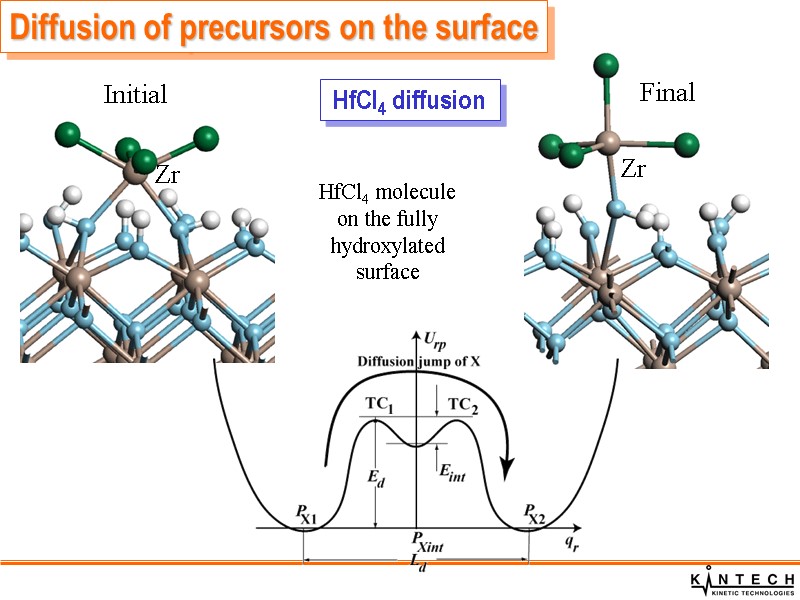
 HfCl4 diffusion HfCl4 molecule on the fully hydroxylated surface Initial Final Diffusion of precursors on the surface Zr Zr
HfCl4 diffusion HfCl4 molecule on the fully hydroxylated surface Initial Final Diffusion of precursors on the surface Zr Zr
 Diffusion of H atoms is rather rapid Diffusion of OH groups over t- and m-MO2(001) surfaces is very slow Diffusion of HfCl4 molecules over the fully hydroxylated t-HfO2(001) surfaces is rapid Diffusion of HfCl4 molecules over the bare surface is slow Diffusion of chemically adsorbed HfCl3 molecules over the bare surface is slow, only local relaxation of HfCl3 molecules can take place. Diffusion of precursors on the surface Summary of precursor diffusion properties
Diffusion of H atoms is rather rapid Diffusion of OH groups over t- and m-MO2(001) surfaces is very slow Diffusion of HfCl4 molecules over the fully hydroxylated t-HfO2(001) surfaces is rapid Diffusion of HfCl4 molecules over the bare surface is slow Diffusion of chemically adsorbed HfCl3 molecules over the bare surface is slow, only local relaxation of HfCl3 molecules can take place. Diffusion of precursors on the surface Summary of precursor diffusion properties
![>[1] Ref. [5] gives an unreasonable value of the threshold (more than 90 kcal/mol), >[1] Ref. [5] gives an unreasonable value of the threshold (more than 90 kcal/mol),](https://present5.com/presentacii-2/20171213\35794-ald_film.ppt\35794-ald_film_31.jpg) [1] Ref. [5] gives an unreasonable value of the threshold (more than 90 kcal/mol), which we think is in error. Instead, we use the difference between initial and final state (17 – 26 kcal/mol), like our lowest estimation. [2] Formally this reaction describes the formation of a bulk phase. But the formula AlO(OH)2(S) presumes that the surface complex still consists of AlO and 2(OH), so that this “virtual reaction” was introduced in order to have formation of a bulk phase in the model. To avoid any effect on the overall mechanism rate, we assumed the largest possible rate coefficient for this reaction. [i] HIKE deliverable D3.1 (Oct 2002). Steric hindrance of precursors does not in increasing of film roughness. Only dehyroxylation of the surface results in growth of film roughness with film thickness. Lattice kinetic Monte Carlo modeling of HfO2 film roughness Roughness of ALD films Surface profile with local relaxation at T=100 C
[1] Ref. [5] gives an unreasonable value of the threshold (more than 90 kcal/mol), which we think is in error. Instead, we use the difference between initial and final state (17 – 26 kcal/mol), like our lowest estimation. [2] Formally this reaction describes the formation of a bulk phase. But the formula AlO(OH)2(S) presumes that the surface complex still consists of AlO and 2(OH), so that this “virtual reaction” was introduced in order to have formation of a bulk phase in the model. To avoid any effect on the overall mechanism rate, we assumed the largest possible rate coefficient for this reaction. [i] HIKE deliverable D3.1 (Oct 2002). Steric hindrance of precursors does not in increasing of film roughness. Only dehyroxylation of the surface results in growth of film roughness with film thickness. Lattice kinetic Monte Carlo modeling of HfO2 film roughness Roughness of ALD films Surface profile with local relaxation at T=100 C
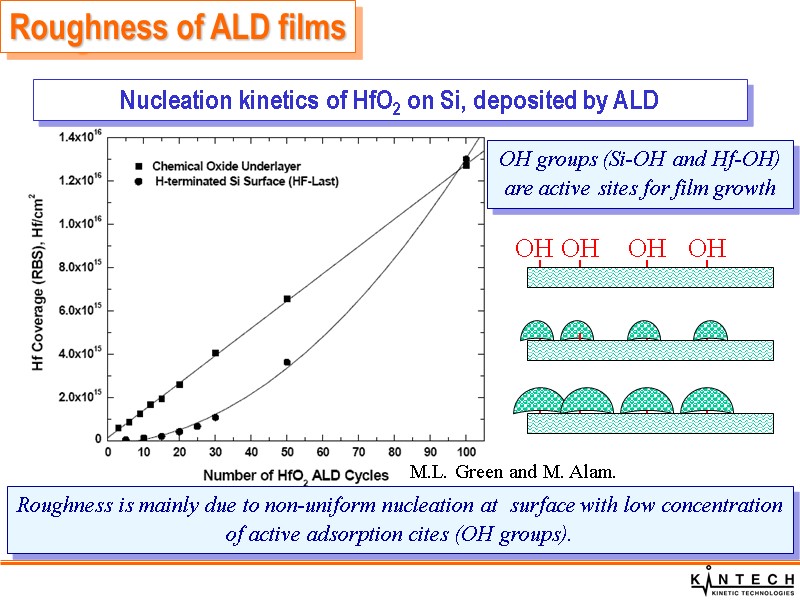
 Roughness of ALD films Roughness is mainly due to non-uniform nucleation at surface with low concentration of active adsorption cites (OH groups). Nucleation kinetics of HfO2 on Si, deposited by ALD M.L. Green and M. Alam.
Roughness of ALD films Roughness is mainly due to non-uniform nucleation at surface with low concentration of active adsorption cites (OH groups). Nucleation kinetics of HfO2 on Si, deposited by ALD M.L. Green and M. Alam.
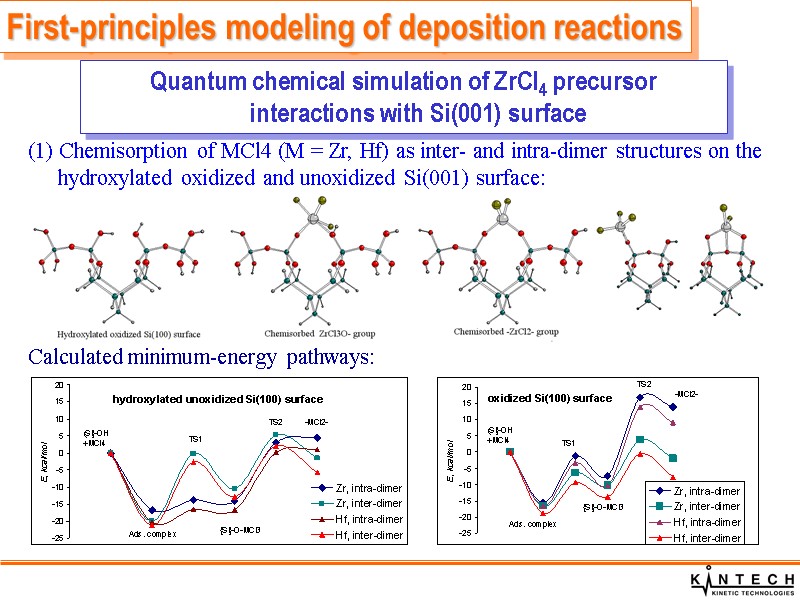
 (1) Chemisorption of MCl4 (M = Zr, Hf) as inter- and intra-dimer structures on the hydroxylated oxidized and unoxidized Si(001) surface: Calculated minimum-energy pathways: First-principles modeling of deposition reactions Quantum chemical simulation of ZrCl4 precursor interactions with Si(001) surface
(1) Chemisorption of MCl4 (M = Zr, Hf) as inter- and intra-dimer structures on the hydroxylated oxidized and unoxidized Si(001) surface: Calculated minimum-energy pathways: First-principles modeling of deposition reactions Quantum chemical simulation of ZrCl4 precursor interactions with Si(001) surface
 Conclusions ALD is a promising tool for deposition of uniform ultra thin films with atomic scale precision. Steric hindrance of precursors in a ALD process reduces film growth rate, but not increase significantly film roughness. Temperature dependences are generally smooth due to dependence of rate constants on local chemical environment. Low temperature growth is restricted by formation of stable intermediate complex. More reactive precursors are needed to reduce temperature of an ALD process – plasma enhanced ALD can be used. Nucleation of the film determines mainly film roughness.
Conclusions ALD is a promising tool for deposition of uniform ultra thin films with atomic scale precision. Steric hindrance of precursors in a ALD process reduces film growth rate, but not increase significantly film roughness. Temperature dependences are generally smooth due to dependence of rate constants on local chemical environment. Low temperature growth is restricted by formation of stable intermediate complex. More reactive precursors are needed to reduce temperature of an ALD process – plasma enhanced ALD can be used. Nucleation of the film determines mainly film roughness.
 Acknowledgements Boris Potapkin Alexander Bagatur’yants Elena Rykova Alexey Gavrikov Andrey Knizhnik Maxim Deminsky Ilya Polishchuk Mikhail Nechaev Inna Iskandarova Elena Shulakova Vladimir Brodskii Stanislav Umanskii Andrey Safonov Dima Bazhanov Ivan Belov Ilya Mutigullin Anton Arkhipov Evgeni Burovski Maxim Miterev Anatoli Korkin Ed Hall Marius Orlovski Matthew Stoker Leonardo Fonseca Jamie Schaeffer Bill Johnson Phil Tobin
Acknowledgements Boris Potapkin Alexander Bagatur’yants Elena Rykova Alexey Gavrikov Andrey Knizhnik Maxim Deminsky Ilya Polishchuk Mikhail Nechaev Inna Iskandarova Elena Shulakova Vladimir Brodskii Stanislav Umanskii Andrey Safonov Dima Bazhanov Ivan Belov Ilya Mutigullin Anton Arkhipov Evgeni Burovski Maxim Miterev Anatoli Korkin Ed Hall Marius Orlovski Matthew Stoker Leonardo Fonseca Jamie Schaeffer Bill Johnson Phil Tobin

