ald_film.ppt
- Количество слайдов: 35
 Computational Materials Science: Multiscale Modeling of Atomic Layer Deposition of Thin Films Andrey Knizhnik
Computational Materials Science: Multiscale Modeling of Atomic Layer Deposition of Thin Films Andrey Knizhnik
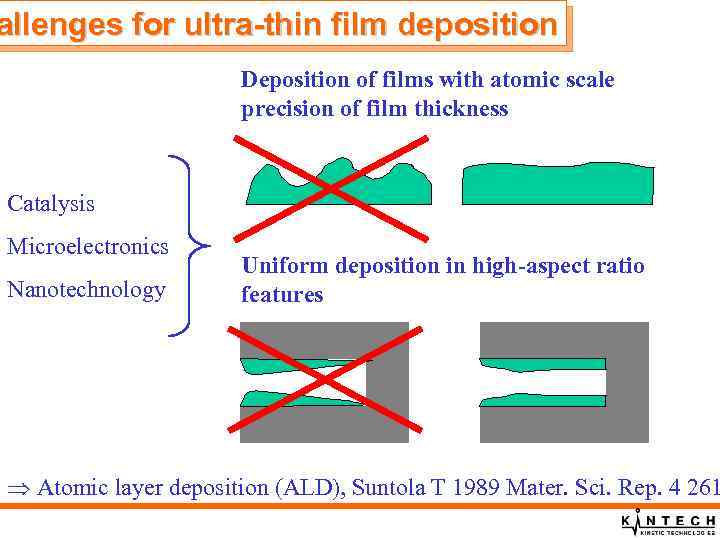 allenges for ultra-thin film deposition Deposition of films with atomic scale precision of film thickness Catalysis Microelectronics Nanotechnology Uniform deposition in high-aspect ratio features Atomic layer deposition (ALD), Suntola T 1989 Mater. Sci. Rep. 4 261
allenges for ultra-thin film deposition Deposition of films with atomic scale precision of film thickness Catalysis Microelectronics Nanotechnology Uniform deposition in high-aspect ratio features Atomic layer deposition (ALD), Suntola T 1989 Mater. Sci. Rep. 4 261
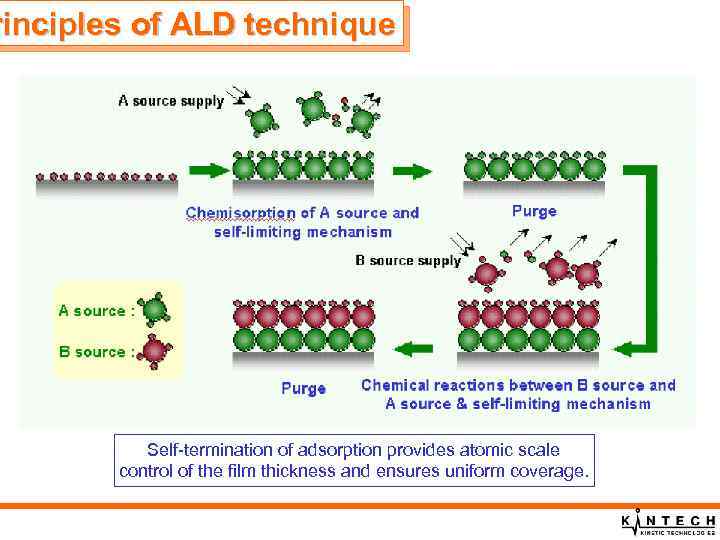 rinciples of ALD technique Self-termination of adsorption provides atomic scale control of the film thickness and ensures uniform coverage.
rinciples of ALD technique Self-termination of adsorption provides atomic scale control of the film thickness and ensures uniform coverage.
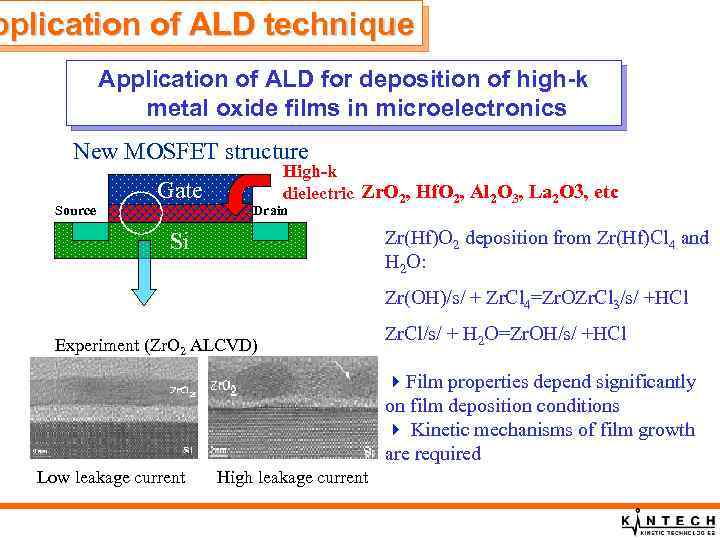 pplication of ALD technique Application of ALD for deposition of high-k metal oxide films in microelectronics New MOSFET structure Source Gate High-k dielectric Zr. O 2, Hf. O 2, Al 2 O 3, La 2 O 3, etc Drain Zr(Hf)O 2 deposition from Zr(Hf)Cl 4 and H 2 O: Si Zr(OH)/s/ + Zr. Cl 4=Zr. OZr. Cl 3/s/ +HCl Experiment (Zr. O 2 ALCVD) Zr. Cl/s/ + H 2 O=Zr. OH/s/ +HCl 4 Film properties depend significantly on film deposition conditions 4 Kinetic mechanisms of film growth are required Low leakage current High leakage current
pplication of ALD technique Application of ALD for deposition of high-k metal oxide films in microelectronics New MOSFET structure Source Gate High-k dielectric Zr. O 2, Hf. O 2, Al 2 O 3, La 2 O 3, etc Drain Zr(Hf)O 2 deposition from Zr(Hf)Cl 4 and H 2 O: Si Zr(OH)/s/ + Zr. Cl 4=Zr. OZr. Cl 3/s/ +HCl Experiment (Zr. O 2 ALCVD) Zr. Cl/s/ + H 2 O=Zr. OH/s/ +HCl 4 Film properties depend significantly on film deposition conditions 4 Kinetic mechanisms of film growth are required Low leakage current High leakage current
 eatures of ALD technique Main features of atomic layer deposition • • • Maximum film growth rate Temperature dependence of film growth rate Residual impurities in as-deposited films Selection of precursors Film roughness Influence with initial support state
eatures of ALD technique Main features of atomic layer deposition • • • Maximum film growth rate Temperature dependence of film growth rate Residual impurities in as-deposited films Selection of precursors Film roughness Influence with initial support state
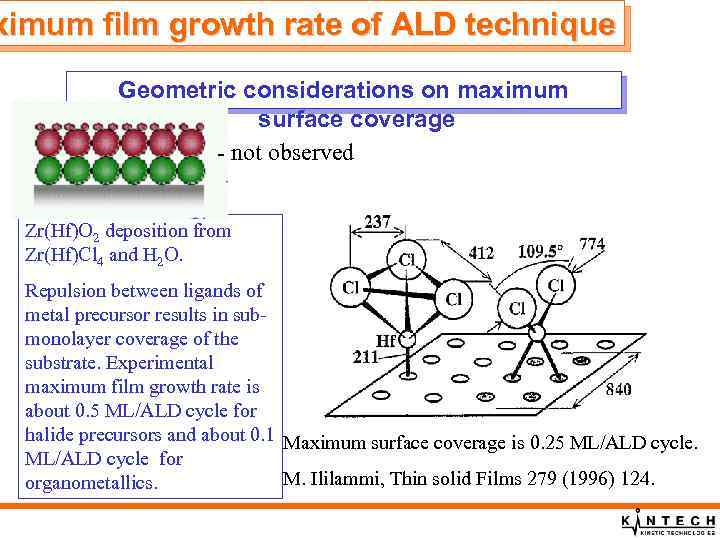 ximum film growth rate of ALD technique Geometric considerations on maximum surface coverage - not observed Zr(Hf)O 2 deposition from Zr(Hf)Cl 4 and H 2 O. Repulsion between ligands of metal precursor results in submonolayer coverage of the substrate. Experimental maximum film growth rate is about 0. 5 ML/ALD cycle for halide precursors and about 0. 1 Maximum surface coverage is 0. 25 ML/ALD cycle for M. Ililammi, Thin solid Films 279 (1996) 124. organometallics.
ximum film growth rate of ALD technique Geometric considerations on maximum surface coverage - not observed Zr(Hf)O 2 deposition from Zr(Hf)Cl 4 and H 2 O. Repulsion between ligands of metal precursor results in submonolayer coverage of the substrate. Experimental maximum film growth rate is about 0. 5 ML/ALD cycle for halide precursors and about 0. 1 Maximum surface coverage is 0. 25 ML/ALD cycle for M. Ililammi, Thin solid Films 279 (1996) 124. organometallics.
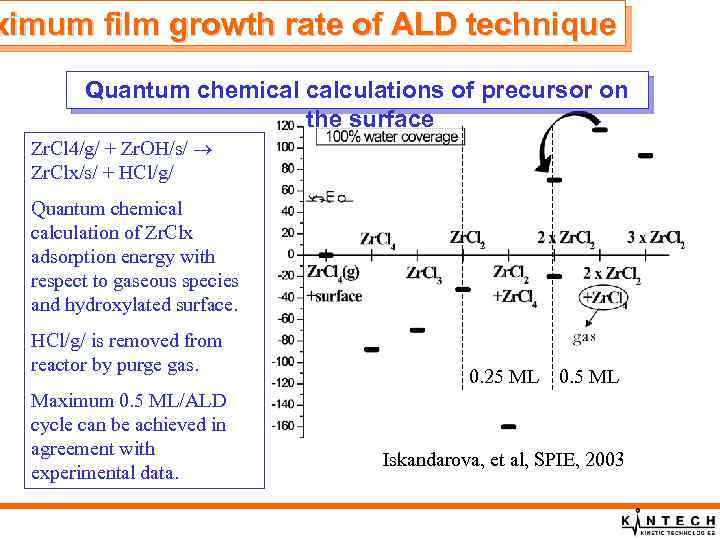 ximum film growth rate of ALD technique Quantum chemical calculations of precursor on the surface Zr. Cl 4/g/ + Zr. OH/s/ Zr. Clx/s/ + HCl/g/ Quantum chemical calculation of Zr. Clx adsorption energy with respect to gaseous species and hydroxylated surface. HCl/g/ is removed from reactor by purge gas. Maximum 0. 5 ML/ALD cycle can be achieved in agreement with experimental data. 0. 25 ML 0. 5 ML Iskandarova, et al, SPIE, 2003
ximum film growth rate of ALD technique Quantum chemical calculations of precursor on the surface Zr. Cl 4/g/ + Zr. OH/s/ Zr. Clx/s/ + HCl/g/ Quantum chemical calculation of Zr. Clx adsorption energy with respect to gaseous species and hydroxylated surface. HCl/g/ is removed from reactor by purge gas. Maximum 0. 5 ML/ALD cycle can be achieved in agreement with experimental data. 0. 25 ML 0. 5 ML Iskandarova, et al, SPIE, 2003
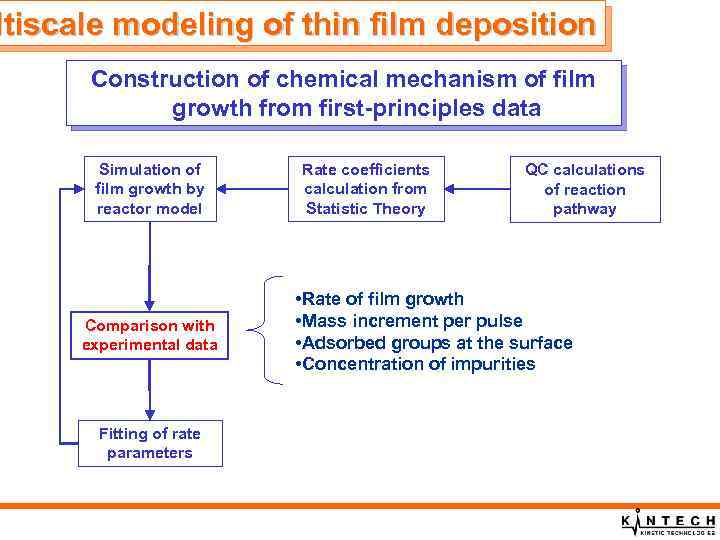 ltiscale modeling of thin film deposition Construction of chemical mechanism of film growth from first-principles data Simulation of film growth by reactor model Comparison with experimental data Fitting of rate parameters Rate coefficients calculation from Statistic Theory QC calculations of reaction pathway • Rate of film growth • Mass increment per pulse • Adsorbed groups at the surface • Concentration of impurities
ltiscale modeling of thin film deposition Construction of chemical mechanism of film growth from first-principles data Simulation of film growth by reactor model Comparison with experimental data Fitting of rate parameters Rate coefficients calculation from Statistic Theory QC calculations of reaction pathway • Rate of film growth • Mass increment per pulse • Adsorbed groups at the surface • Concentration of impurities
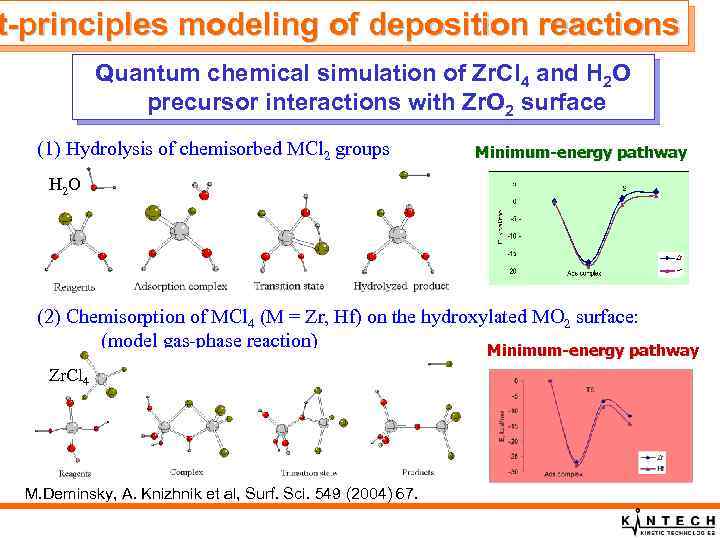 t-principles modeling of deposition reactions Quantum chemical simulation of Zr. Cl 4 and H 2 O precursor interactions with Zr. O 2 surface (1) Hydrolysis of chemisorbed MCl 2 groups Minimum-energy pathway H 2 O (2) Chemisorption of MCl 4 (M = Zr, Hf) on the hydroxylated MO 2 surface: (model gas-phase reaction) Minimum-energy pathway Zr. Cl 4 M. Deminsky, A. Knizhnik et al, Surf. Sci. 549 (2004) 67. .
t-principles modeling of deposition reactions Quantum chemical simulation of Zr. Cl 4 and H 2 O precursor interactions with Zr. O 2 surface (1) Hydrolysis of chemisorbed MCl 2 groups Minimum-energy pathway H 2 O (2) Chemisorption of MCl 4 (M = Zr, Hf) on the hydroxylated MO 2 surface: (model gas-phase reaction) Minimum-energy pathway Zr. Cl 4 M. Deminsky, A. Knizhnik et al, Surf. Sci. 549 (2004) 67. .
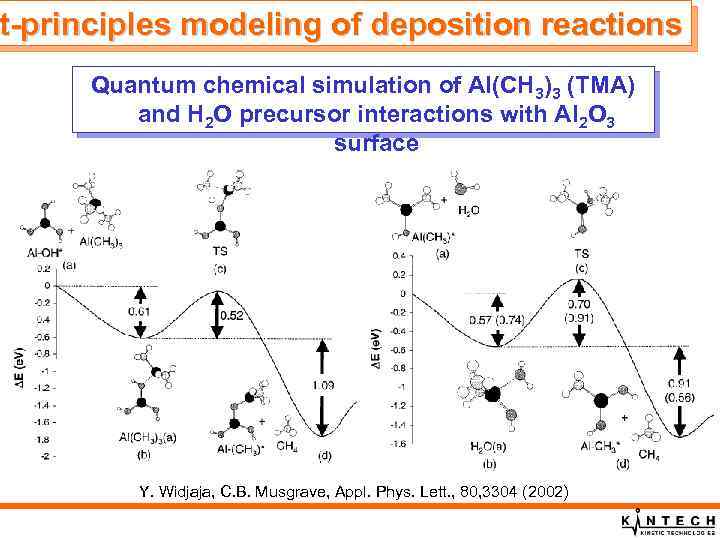 t-principles st-principles modeling of deposition reactions Quantum chemical simulation of Al(CH 3)3 (TMA) and H 2 O precursor interactions with Al 2 O 3 surface Y. Widjaja, C. B. Musgrave, Appl. Phys. Lett. , 80, 3304 (2002)
t-principles st-principles modeling of deposition reactions Quantum chemical simulation of Al(CH 3)3 (TMA) and H 2 O precursor interactions with Al 2 O 3 surface Y. Widjaja, C. B. Musgrave, Appl. Phys. Lett. , 80, 3304 (2002)
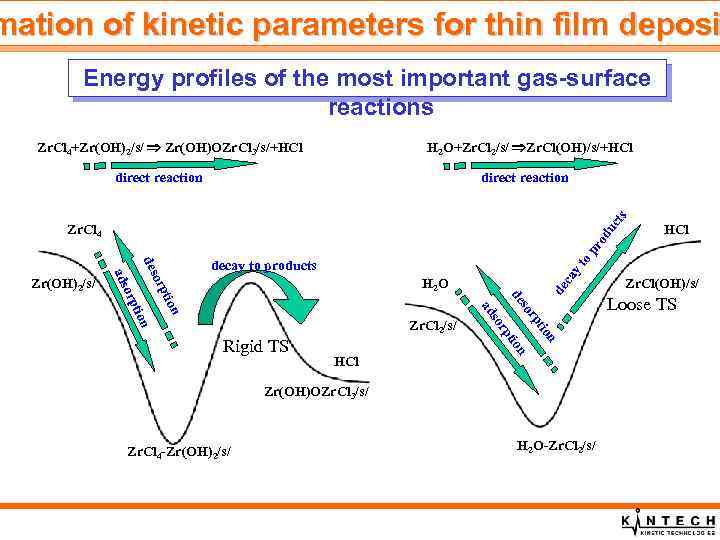 mation of kinetic parameters for thin film deposi Energy profiles of the most important gas-surface reactions Zr. Cl 4+Zr(OH)2/s/ Zr(OH)OZr. Cl 3/s/+HCl H 2 O+Zr. Cl 2/s/ Zr. Cl(OH)/s/+HCl direct reaction du cts direct reaction HCl n tio rp so n tio rp Rigid TS so ad n Zr. Cl 2/s/ n tio de orp H 2 O de ca yt decay to products des orp ads Zr(OH)2/s/ Zr(OH)OZr. Cl 3/s/ Zr. Cl 4 -Zr(OH)2/s/ HCl op ro Zr. Cl 4 H 2 O-Zr. Cl 2/s/ Zr. Cl(OH)/s/ Loose TS
mation of kinetic parameters for thin film deposi Energy profiles of the most important gas-surface reactions Zr. Cl 4+Zr(OH)2/s/ Zr(OH)OZr. Cl 3/s/+HCl H 2 O+Zr. Cl 2/s/ Zr. Cl(OH)/s/+HCl direct reaction du cts direct reaction HCl n tio rp so n tio rp Rigid TS so ad n Zr. Cl 2/s/ n tio de orp H 2 O de ca yt decay to products des orp ads Zr(OH)2/s/ Zr(OH)OZr. Cl 3/s/ Zr. Cl 4 -Zr(OH)2/s/ HCl op ro Zr. Cl 4 H 2 O-Zr. Cl 2/s/ Zr. Cl(OH)/s/ Loose TS
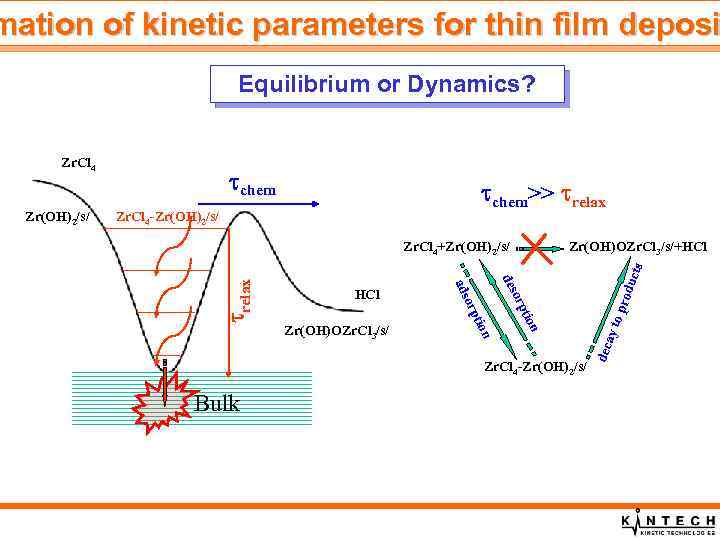 mation of kinetic parameters for thin film deposi Equilibrium or Dynamics? chem>> relax n Bulk o pr tio orp Zr. Cl 4 -Zr(OH)2/s/ odu des n tio orp Zr(OH)OZr. Cl 3/s/ ads HCl Zr(OH)OZr. Cl 3/s/+HCl cts Zr. Cl 4+Zr(OH)2/s/ ay t Zr. Cl 4 -Zr(OH)2/s/ relax Zr(OH)2/s/ Zr(OH)2 chem dec Zr. Cl 4
mation of kinetic parameters for thin film deposi Equilibrium or Dynamics? chem>> relax n Bulk o pr tio orp Zr. Cl 4 -Zr(OH)2/s/ odu des n tio orp Zr(OH)OZr. Cl 3/s/ ads HCl Zr(OH)OZr. Cl 3/s/+HCl cts Zr. Cl 4+Zr(OH)2/s/ ay t Zr. Cl 4 -Zr(OH)2/s/ relax Zr(OH)2/s/ Zr(OH)2 chem dec Zr. Cl 4
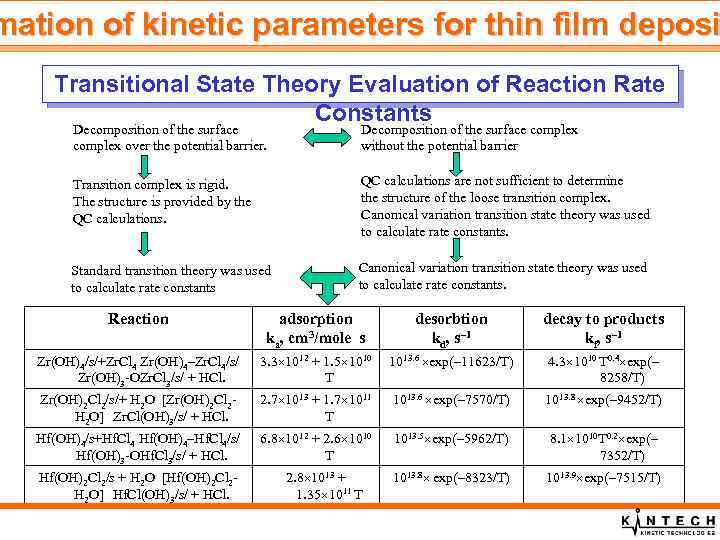 mation of kinetic parameters for thin film deposi Transitional State Theory Evaluation of Reaction Rate Constants Decomposition of the surface complex over the potential barrier. Decomposition of the surface complex without the potential barrier Transition complex is rigid. The structure is provided by the QC calculations are not sufficient to determine the structure of the loose transition complex. Canonical variation transition state theory was used to calculate rate constants. Standard transition theory was used to calculate rate constants Canonical variation transition state theory was used to calculate rate constants. Reaction adsorption ka, cm 3/mole s desorbtion kd, s– 1 decay to products kf, s– 1 Zr(OH)4/s/+Zr. Cl 4 Zr(OH)4–Zr. Cl 4/s/ 3. 3 1012 + 1. 5 1010 1013. 6 exp(– 11623/T) Zr(OH)3 -OZr. Cl 3/s/ + HCl. T 4. 3 1010 T 0. 4 exp(– 8258/T) 2. 7 1013 + 1. 7 1011 T 1013. 6 exp(– 7570/T) 1013. 8 exp(– 9452/T) Hf(OH)4/s+Hf. Cl 4 Hf(OH)4–Hf. Cl 4/s/ 6. 8 1012 + 2. 6 1010 Hf(OH)3 -OHf. Cl 3/s/ + HCl. T 1013. 5 exp(– 5962/T) 8. 1 1010 T 0. 2 exp(– 7352/T) Hf(OH)2 Cl 2/s + H 2 O [Hf(OH)2 Cl 2 - H 2 O] Hf. Cl(OH)3/s/ + HCl. 1013. 8 exp(– 8323/T) 1013. 9 exp(– 7515/T) Zr(OH)2 Cl 2/s/+ H 2 O [Zr(OH)2 Cl 2 - H 2 O] Zr. Cl(OH)3/s/ + HCl. 2. 8 1013 + 1. 35 1011 T
mation of kinetic parameters for thin film deposi Transitional State Theory Evaluation of Reaction Rate Constants Decomposition of the surface complex over the potential barrier. Decomposition of the surface complex without the potential barrier Transition complex is rigid. The structure is provided by the QC calculations are not sufficient to determine the structure of the loose transition complex. Canonical variation transition state theory was used to calculate rate constants. Standard transition theory was used to calculate rate constants Canonical variation transition state theory was used to calculate rate constants. Reaction adsorption ka, cm 3/mole s desorbtion kd, s– 1 decay to products kf, s– 1 Zr(OH)4/s/+Zr. Cl 4 Zr(OH)4–Zr. Cl 4/s/ 3. 3 1012 + 1. 5 1010 1013. 6 exp(– 11623/T) Zr(OH)3 -OZr. Cl 3/s/ + HCl. T 4. 3 1010 T 0. 4 exp(– 8258/T) 2. 7 1013 + 1. 7 1011 T 1013. 6 exp(– 7570/T) 1013. 8 exp(– 9452/T) Hf(OH)4/s+Hf. Cl 4 Hf(OH)4–Hf. Cl 4/s/ 6. 8 1012 + 2. 6 1010 Hf(OH)3 -OHf. Cl 3/s/ + HCl. T 1013. 5 exp(– 5962/T) 8. 1 1010 T 0. 2 exp(– 7352/T) Hf(OH)2 Cl 2/s + H 2 O [Hf(OH)2 Cl 2 - H 2 O] Hf. Cl(OH)3/s/ + HCl. 1013. 8 exp(– 8323/T) 1013. 9 exp(– 7515/T) Zr(OH)2 Cl 2/s/+ H 2 O [Zr(OH)2 Cl 2 - H 2 O] Zr. Cl(OH)3/s/ + HCl. 2. 8 1013 + 1. 35 1011 T
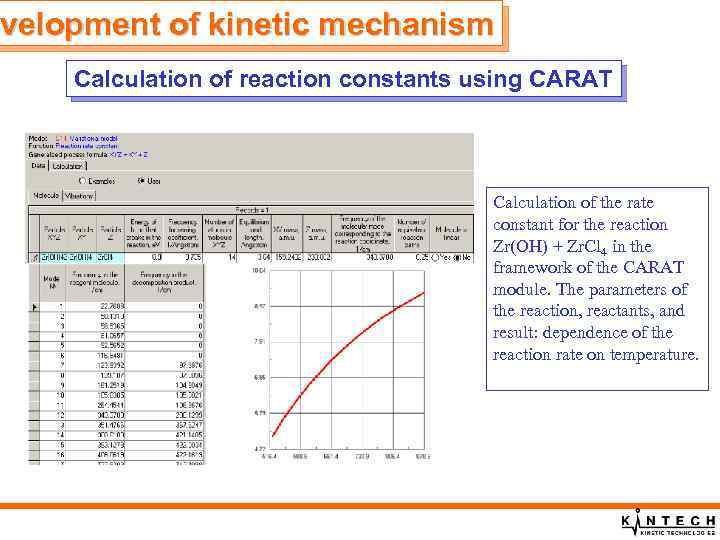 evelopment of kinetic mechanism Calculation of reaction constants using CARAT Calculation of the rate constant for the reaction Zr(OH) + Zr. Cl 4 in the framework of the CARAT module. The parameters of the reaction, reactants, and result: dependence of the reaction rate on temperature.
evelopment of kinetic mechanism Calculation of reaction constants using CARAT Calculation of the rate constant for the reaction Zr(OH) + Zr. Cl 4 in the framework of the CARAT module. The parameters of the reaction, reactants, and result: dependence of the reaction rate on temperature.
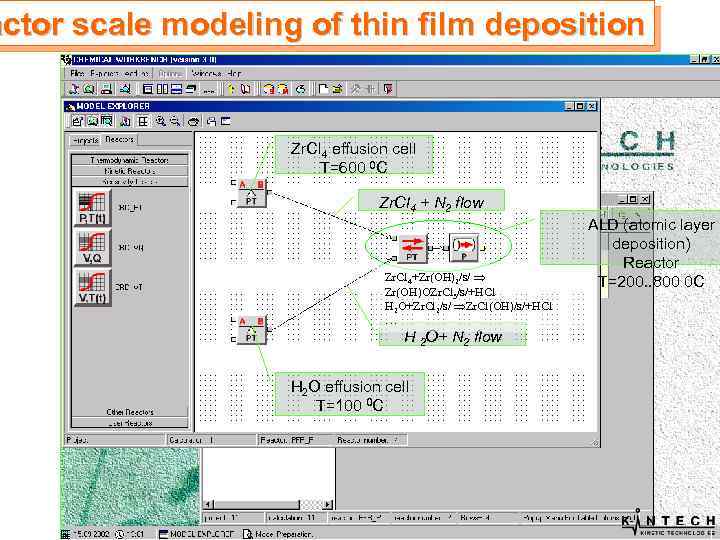 actor scale modeling of thin film deposition Zr. Cl 4 effusion cell T=600 0 C Zr. Cl 4 + N 2 flow Zr. Cl 4+Zr(OH)2/s/ Zr(OH)OZr. Cl 3/s/+HCl H 2 O+Zr. Cl 2/s/ Zr. Cl(OH)/s/+HCl … H 2 O+ N 2 flow H 2 O effusion cell T=100 0 C ALD (atomic layer deposition) Reactor T=200. . 800 0 C
actor scale modeling of thin film deposition Zr. Cl 4 effusion cell T=600 0 C Zr. Cl 4 + N 2 flow Zr. Cl 4+Zr(OH)2/s/ Zr(OH)OZr. Cl 3/s/+HCl H 2 O+Zr. Cl 2/s/ Zr. Cl(OH)/s/+HCl … H 2 O+ N 2 flow H 2 O effusion cell T=100 0 C ALD (atomic layer deposition) Reactor T=200. . 800 0 C
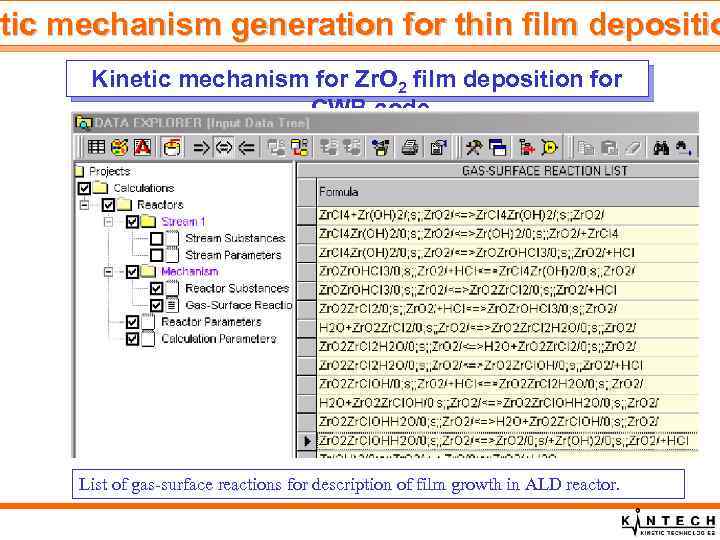 tic etic mechanism generation for thin film depositio Kinetic mechanism for Zr. O 2 film deposition for CWB code List of gas-surface reactions for description of film growth in ALD reactor.
tic etic mechanism generation for thin film depositio Kinetic mechanism for Zr. O 2 film deposition for CWB code List of gas-surface reactions for description of film growth in ALD reactor.
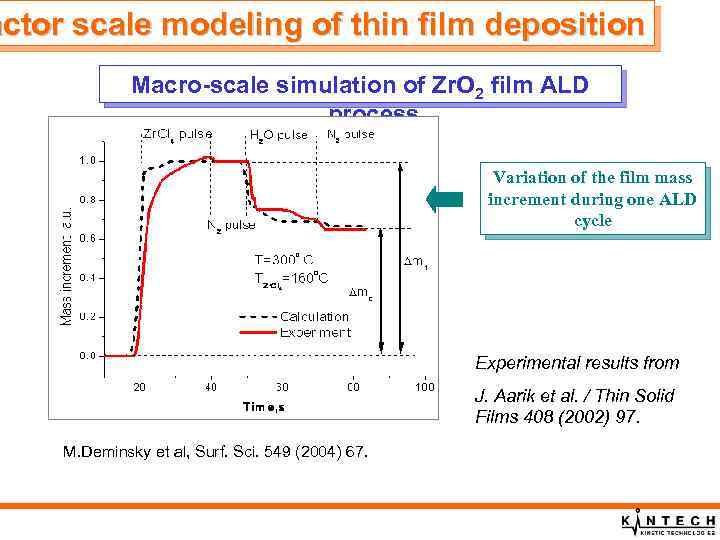 actor scale modeling of thin film deposition Macro-scale simulation of Zr. O 2 film ALD process Variation of the film mass increment during one ALD cycle Experimental results from J. Aarik et al. / Thin Solid Films 408 (2002) 97. M. Deminsky et al, Surf. Sci. 549 (2004) 67.
actor scale modeling of thin film deposition Macro-scale simulation of Zr. O 2 film ALD process Variation of the film mass increment during one ALD cycle Experimental results from J. Aarik et al. / Thin Solid Films 408 (2002) 97. M. Deminsky et al, Surf. Sci. 549 (2004) 67.
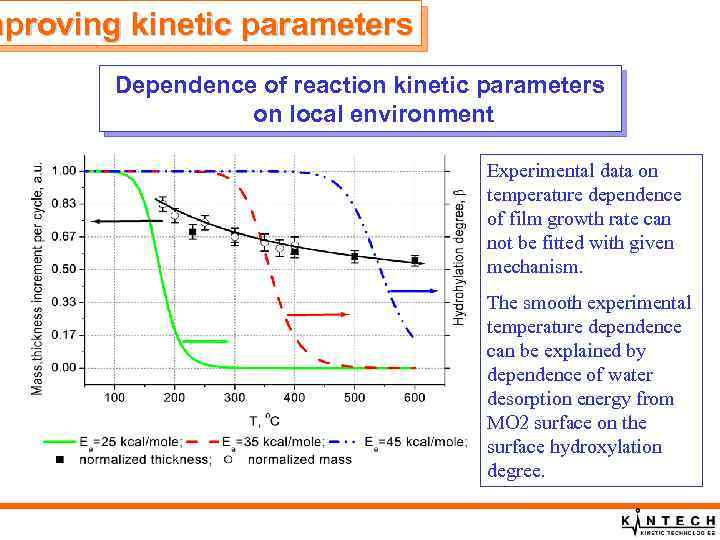 mproving kinetic parameters Dependence of reaction kinetic parameters on local environment Experimental data on temperature dependence of film growth rate can not be fitted with given mechanism. The smooth experimental temperature dependence can be explained by dependence of water desorption energy from MO 2 surface on the surface hydroxylation degree.
mproving kinetic parameters Dependence of reaction kinetic parameters on local environment Experimental data on temperature dependence of film growth rate can not be fitted with given mechanism. The smooth experimental temperature dependence can be explained by dependence of water desorption energy from MO 2 surface on the surface hydroxylation degree.
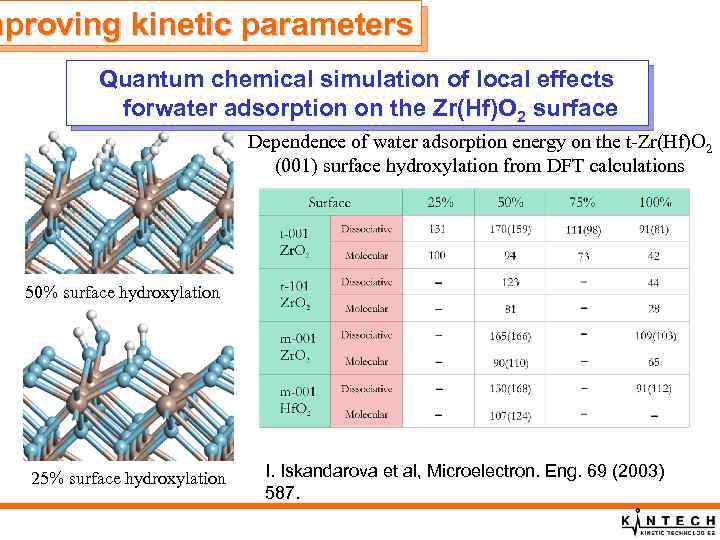 mproving kinetic parameters Quantum chemical simulation of local effects forwater adsorption on the Zr(Hf)O 2 surface Dependence of water adsorption energy on the t-Zr(Hf)O 2 (001) surface hydroxylation from DFT calculations 50% surface hydroxylation 25% surface hydroxylation I. Iskandarova et al, Microelectron. Eng. 69 (2003) 587.
mproving kinetic parameters Quantum chemical simulation of local effects forwater adsorption on the Zr(Hf)O 2 surface Dependence of water adsorption energy on the t-Zr(Hf)O 2 (001) surface hydroxylation from DFT calculations 50% surface hydroxylation 25% surface hydroxylation I. Iskandarova et al, Microelectron. Eng. 69 (2003) 587.
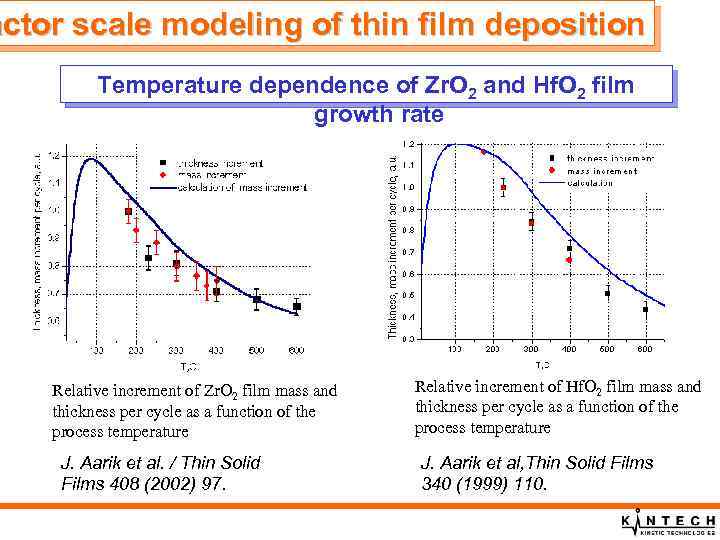 actor scale modeling of thin film deposition Temperature dependence of Zr. O 2 and Hf. O 2 film growth rate Relative increment of Zr. O 2 film mass and thickness per cycle as a function of the process temperature J. Aarik et al. / Thin Solid Films 408 (2002) 97. Relative increment of Hf. O 2 film mass and thickness per cycle as a function of the process temperature J. Aarik et al, Thin Solid Films 340 (1999) 110.
actor scale modeling of thin film deposition Temperature dependence of Zr. O 2 and Hf. O 2 film growth rate Relative increment of Zr. O 2 film mass and thickness per cycle as a function of the process temperature J. Aarik et al. / Thin Solid Films 408 (2002) 97. Relative increment of Hf. O 2 film mass and thickness per cycle as a function of the process temperature J. Aarik et al, Thin Solid Films 340 (1999) 110.
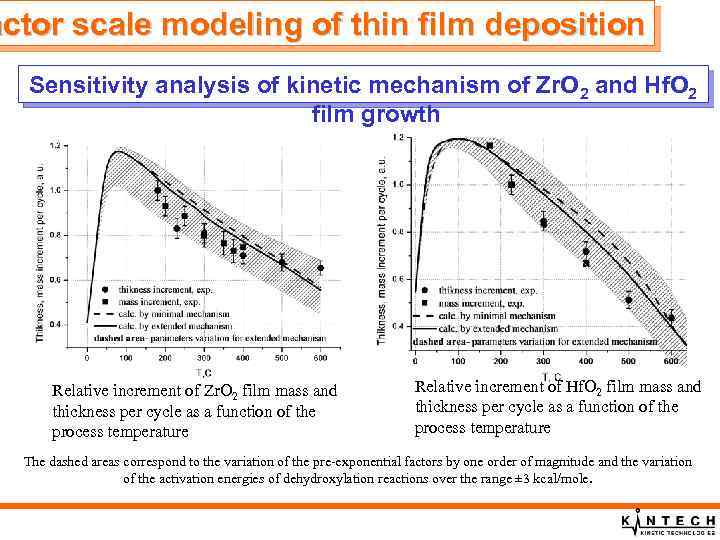 actor scale modeling of thin film deposition Sensitivity analysis of kinetic mechanism of Zr. O 2 and Hf. O 2 film growth Relative increment of Zr. O 2 film mass and thickness per cycle as a function of the process temperature Relative increment of Hf. O 2 film mass and thickness per cycle as a function of the process temperature The dashed areas correspond to the variation of the pre-exponential factors by one order of magnitude and the variation of the activation energies of dehydroxylation reactions over the range ± 3 kcal/mole.
actor scale modeling of thin film deposition Sensitivity analysis of kinetic mechanism of Zr. O 2 and Hf. O 2 film growth Relative increment of Zr. O 2 film mass and thickness per cycle as a function of the process temperature Relative increment of Hf. O 2 film mass and thickness per cycle as a function of the process temperature The dashed areas correspond to the variation of the pre-exponential factors by one order of magnitude and the variation of the activation energies of dehydroxylation reactions over the range ± 3 kcal/mole.
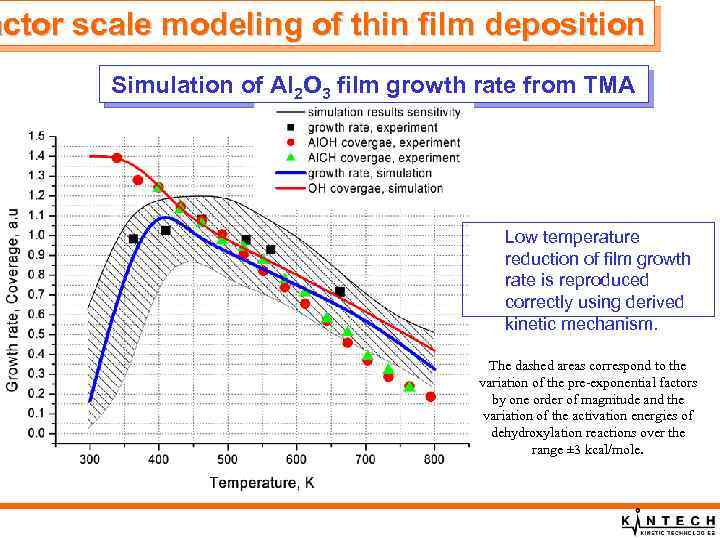 actor scale modeling of thin film deposition Simulation of Al 2 O 3 film growth rate from TMA and H 2 O Low temperature reduction of film growth rate is reproduced correctly using derived kinetic mechanism. The dashed areas correspond to the variation of the pre-exponential factors by one order of magnitude and the variation of the activation energies of dehydroxylation reactions over the range ± 3 kcal/mole.
actor scale modeling of thin film deposition Simulation of Al 2 O 3 film growth rate from TMA and H 2 O Low temperature reduction of film growth rate is reproduced correctly using derived kinetic mechanism. The dashed areas correspond to the variation of the pre-exponential factors by one order of magnitude and the variation of the activation energies of dehydroxylation reactions over the range ± 3 kcal/mole.
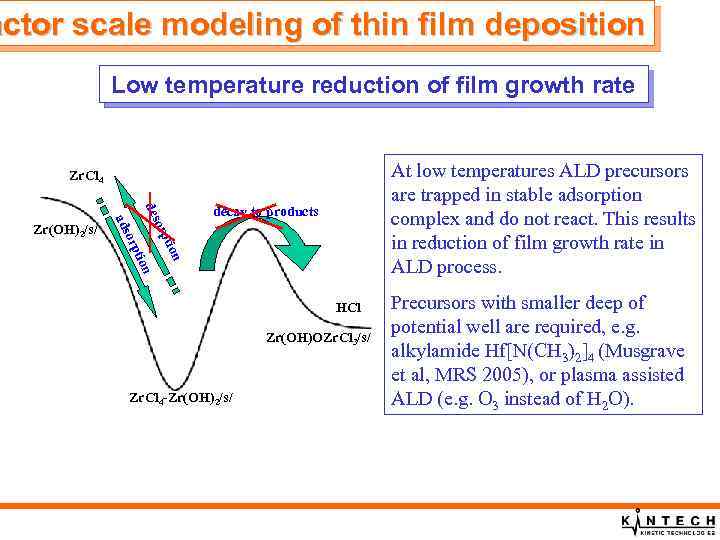 actor scale modeling of thin film deposition Low temperature reduction of film growth rate At low temperatures ALD precursors are trapped in stable adsorption complex and do not react. This results in reduction of film growth rate in ALD process. Zr. Cl 4 n n tio orp des orp ads Zr(OH)2/s/ decay to products HCl Zr(OH)OZr. Cl 3/s/ Zr. Cl 4 -Zr(OH)2/s/ Precursors with smaller deep of potential well are required, e. g. alkylamide Hf[N(CH 3)2]4 (Musgrave et al, MRS 2005), or plasma assisted ALD (e. g. O 3 instead of H 2 O).
actor scale modeling of thin film deposition Low temperature reduction of film growth rate At low temperatures ALD precursors are trapped in stable adsorption complex and do not react. This results in reduction of film growth rate in ALD process. Zr. Cl 4 n n tio orp des orp ads Zr(OH)2/s/ decay to products HCl Zr(OH)OZr. Cl 3/s/ Zr. Cl 4 -Zr(OH)2/s/ Precursors with smaller deep of potential well are required, e. g. alkylamide Hf[N(CH 3)2]4 (Musgrave et al, MRS 2005), or plasma assisted ALD (e. g. O 3 instead of H 2 O).
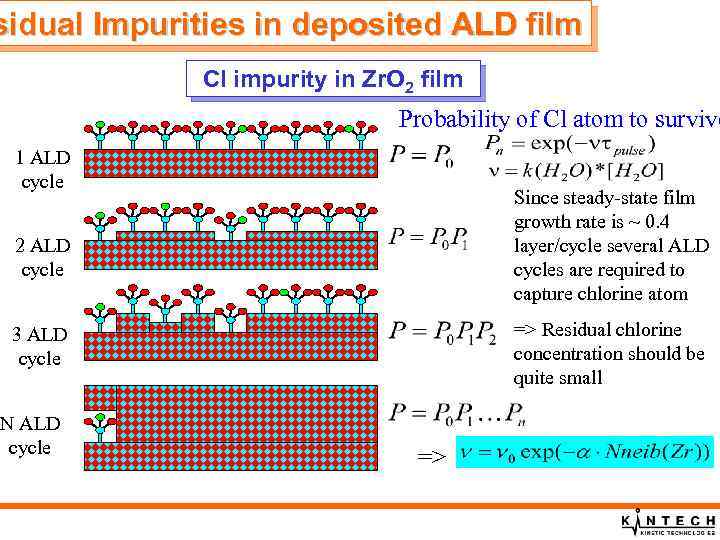 sidual Impurities in deposited ALD film Cl impurity in Zr. O 2 film Probability of Cl atom to survive 1 ALD cycle Since steady-state film growth rate is ~ 0. 4 layer/cycle several ALD cycles are required to capture chlorine atom 2 ALD cycle => Residual chlorine concentration should be quite small 3 ALD cycle N ALD cycle =>
sidual Impurities in deposited ALD film Cl impurity in Zr. O 2 film Probability of Cl atom to survive 1 ALD cycle Since steady-state film growth rate is ~ 0. 4 layer/cycle several ALD cycles are required to capture chlorine atom 2 ALD cycle => Residual chlorine concentration should be quite small 3 ALD cycle N ALD cycle =>
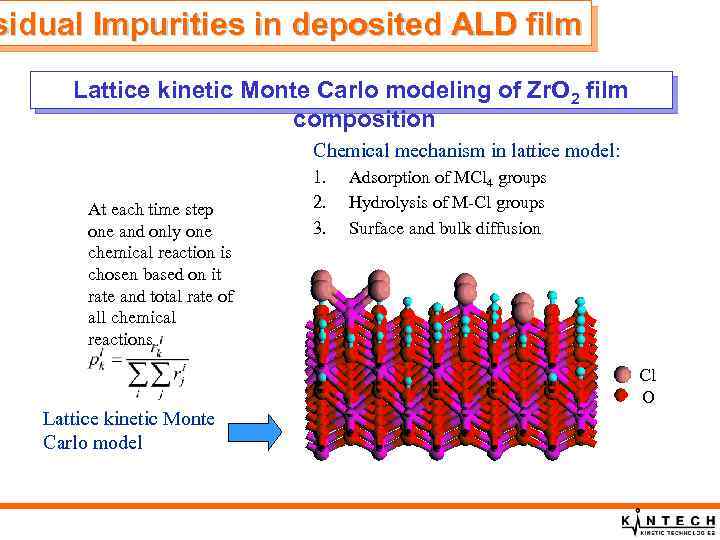 sidual Impurities in deposited ALD film Lattice kinetic Monte Carlo modeling of Zr. O 2 film composition Chemical mechanism in lattice model: At each time step one and only one chemical reaction is chosen based on it rate and total rate of all chemical reactions 1. 2. 3. Adsorption of MCl 4 groups Hydrolysis of M-Cl groups Surface and bulk diffusion Cl O Lattice kinetic Monte Carlo model
sidual Impurities in deposited ALD film Lattice kinetic Monte Carlo modeling of Zr. O 2 film composition Chemical mechanism in lattice model: At each time step one and only one chemical reaction is chosen based on it rate and total rate of all chemical reactions 1. 2. 3. Adsorption of MCl 4 groups Hydrolysis of M-Cl groups Surface and bulk diffusion Cl O Lattice kinetic Monte Carlo model
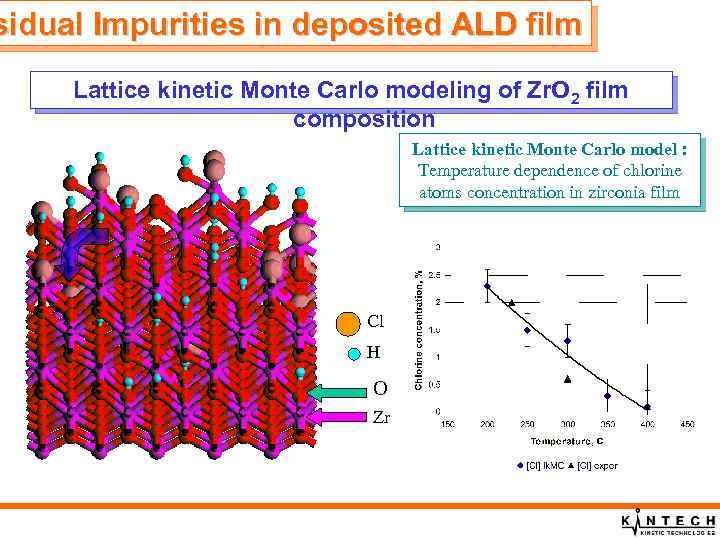 sidual Impurities in deposited ALD film Lattice kinetic Monte Carlo modeling of Zr. O 2 film composition Lattice kinetic Monte Carlo model : Temperature dependence of chlorine atoms concentration in zirconia film Cl H O Zr
sidual Impurities in deposited ALD film Lattice kinetic Monte Carlo modeling of Zr. O 2 film composition Lattice kinetic Monte Carlo model : Temperature dependence of chlorine atoms concentration in zirconia film Cl H O Zr
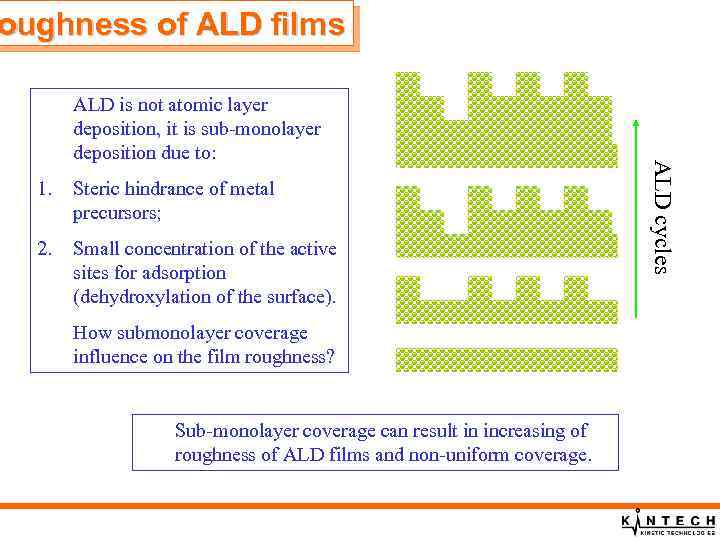 oughness of ALD films 1. Steric hindrance of metal precursors; 2. Small concentration of the active sites for adsorption (dehydroxylation of the surface). How submonolayer coverage influence on the film roughness? Sub-monolayer coverage can result in increasing of roughness of ALD films and non-uniform coverage. ALD cycles ALD is not atomic layer deposition, it is sub-monolayer deposition due to:
oughness of ALD films 1. Steric hindrance of metal precursors; 2. Small concentration of the active sites for adsorption (dehydroxylation of the surface). How submonolayer coverage influence on the film roughness? Sub-monolayer coverage can result in increasing of roughness of ALD films and non-uniform coverage. ALD cycles ALD is not atomic layer deposition, it is sub-monolayer deposition due to:
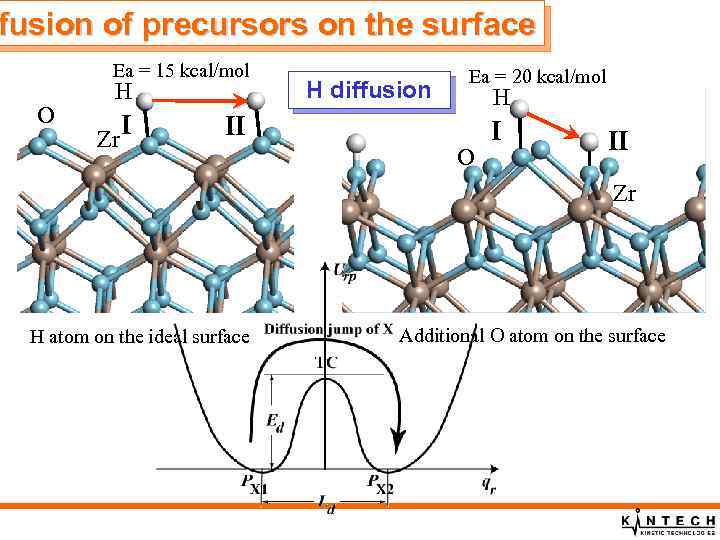 fusion of precursors on the surface Ea = 15 kcal/mol O H Zr I H diffusion Ea = 20 kcal/mol H II O I II Zr H atom on the ideal surface Additional O atom on the surface
fusion of precursors on the surface Ea = 15 kcal/mol O H Zr I H diffusion Ea = 20 kcal/mol H II O I II Zr H atom on the ideal surface Additional O atom on the surface
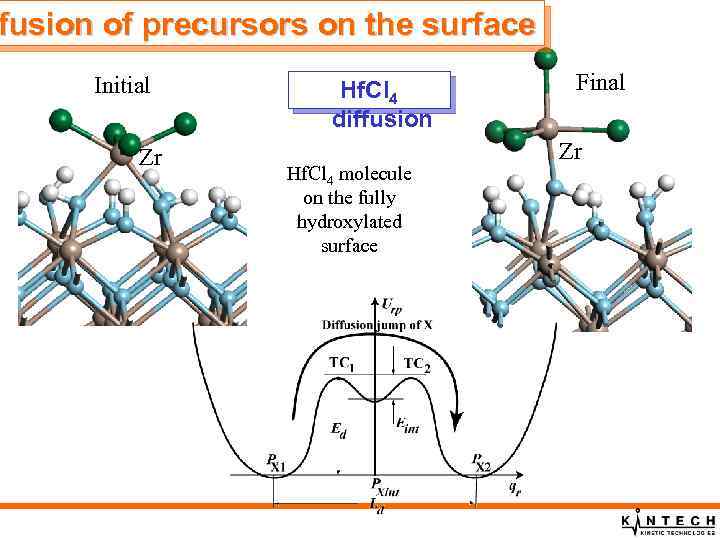 fusion of precursors on the surface Initial Zr Hf. Cl 4 diffusion Hf. Cl 4 molecule on the fully hydroxylated surface Final Zr
fusion of precursors on the surface Initial Zr Hf. Cl 4 diffusion Hf. Cl 4 molecule on the fully hydroxylated surface Final Zr
 fusion of precursors on the surface Summary of precursor diffusion properties q. Diffusion of H atoms is rather rapid q. Diffusion of OH groups over t- and m-MO 2(001) surfaces is very slow q. Diffusion of Hf. Cl 4 molecules over the fully hydroxylated t- Hf. O 2(001) surfaces is rapid q. Diffusion of Hf. Cl 4 molecules over the bare surface is slow q. Diffusion of chemically adsorbed Hf. Cl 3 molecules over the bare surface is slow, only local relaxation of Hf. Cl 3 molecules can take place.
fusion of precursors on the surface Summary of precursor diffusion properties q. Diffusion of H atoms is rather rapid q. Diffusion of OH groups over t- and m-MO 2(001) surfaces is very slow q. Diffusion of Hf. Cl 4 molecules over the fully hydroxylated t- Hf. O 2(001) surfaces is rapid q. Diffusion of Hf. Cl 4 molecules over the bare surface is slow q. Diffusion of chemically adsorbed Hf. Cl 3 molecules over the bare surface is slow, only local relaxation of Hf. Cl 3 molecules can take place.
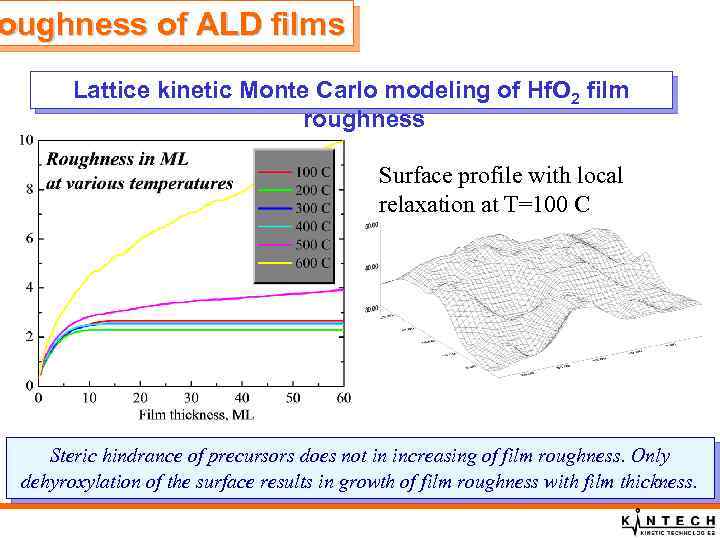 oughness of ALD films Lattice kinetic Monte Carlo modeling of Hf. O 2 film roughness Surface profile with local relaxation at T=100 C Steric hindrance of precursors does not in increasing of film roughness. Only dehyroxylation of the surface results in growth of film roughness with film thickness.
oughness of ALD films Lattice kinetic Monte Carlo modeling of Hf. O 2 film roughness Surface profile with local relaxation at T=100 C Steric hindrance of precursors does not in increasing of film roughness. Only dehyroxylation of the surface results in growth of film roughness with film thickness.
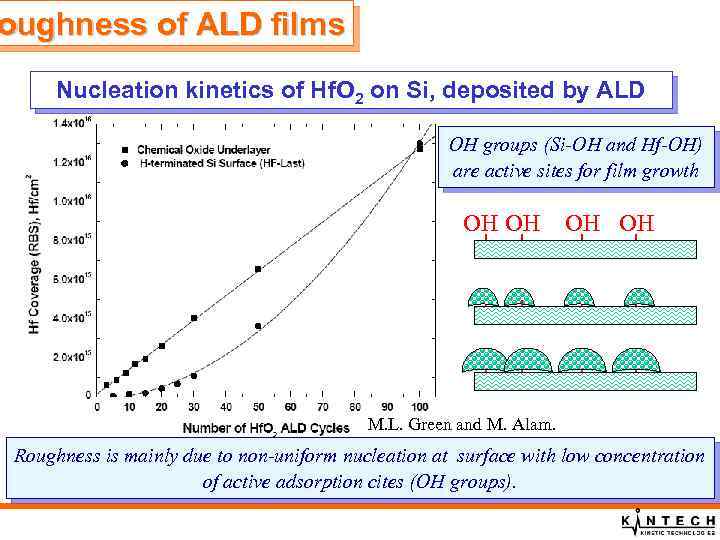 oughness of ALD films Nucleation kinetics of Hf. O 2 on Si, deposited by ALD OH groups (Si-OH and Hf-OH) are active sites for film growth OH OH M. L. Green and M. Alam. Roughness is mainly due to non-uniform nucleation at surface with low concentration of active adsorption cites (OH groups).
oughness of ALD films Nucleation kinetics of Hf. O 2 on Si, deposited by ALD OH groups (Si-OH and Hf-OH) are active sites for film growth OH OH M. L. Green and M. Alam. Roughness is mainly due to non-uniform nucleation at surface with low concentration of active adsorption cites (OH groups).
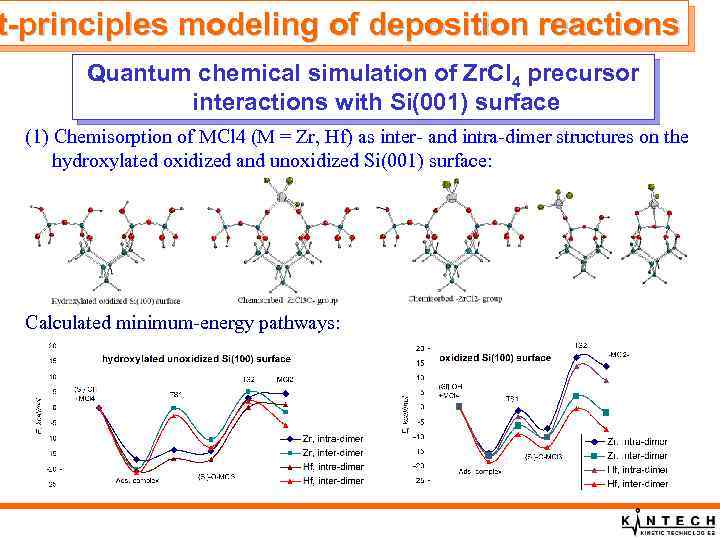 t-principles modeling of deposition reactions Quantum chemical simulation of Zr. Cl 4 precursor interactions with Si(001) surface (1) Chemisorption of MCl 4 (M = Zr, Hf) as inter- and intra-dimer structures on the hydroxylated oxidized and unoxidized Si(001) surface: Calculated minimum-energy pathways:
t-principles modeling of deposition reactions Quantum chemical simulation of Zr. Cl 4 precursor interactions with Si(001) surface (1) Chemisorption of MCl 4 (M = Zr, Hf) as inter- and intra-dimer structures on the hydroxylated oxidized and unoxidized Si(001) surface: Calculated minimum-energy pathways:
 Conclusions q ALD is a promising tool for deposition of uniform ultra thin films with atomic scale precision. q Steric hindrance of precursors in a ALD process reduces film growth rate, but not increase significantly film roughness. q Temperature dependences are generally smooth due to dependence of rate constants on local chemical environment. q. Low temperature growth is restricted by formation of stable intermediate complex. q More reactive precursors are needed to reduce temperature of an ALD process – plasma enhanced ALD can be used. q Nucleation of the film determines mainly film roughness.
Conclusions q ALD is a promising tool for deposition of uniform ultra thin films with atomic scale precision. q Steric hindrance of precursors in a ALD process reduces film growth rate, but not increase significantly film roughness. q Temperature dependences are generally smooth due to dependence of rate constants on local chemical environment. q. Low temperature growth is restricted by formation of stable intermediate complex. q More reactive precursors are needed to reduce temperature of an ALD process – plasma enhanced ALD can be used. q Nucleation of the film determines mainly film roughness.
 Acknowledgements • • • Boris Potapkin Alexander Bagatur’yants Elena Rykova Alexey Gavrikov Andrey Knizhnik Maxim Deminsky Ilya Polishchuk Mikhail Nechaev Inna Iskandarova Elena Shulakova • Vladimir Brodskii • Stanislav Umanskii • Andrey Safonov • Dima Bazhanov • Ivan Belov • Ilya Mutigullin • Anton Arkhipov • Evgeni Burovski • Maxim Miterev • • • Anatoli Korkin Ed Hall Marius Orlovski Matthew Stoker Leonardo Fonseca Jamie Schaeffer • Bill Johnson • Phil Tobin
Acknowledgements • • • Boris Potapkin Alexander Bagatur’yants Elena Rykova Alexey Gavrikov Andrey Knizhnik Maxim Deminsky Ilya Polishchuk Mikhail Nechaev Inna Iskandarova Elena Shulakova • Vladimir Brodskii • Stanislav Umanskii • Andrey Safonov • Dima Bazhanov • Ivan Belov • Ilya Mutigullin • Anton Arkhipov • Evgeni Burovski • Maxim Miterev • • • Anatoli Korkin Ed Hall Marius Orlovski Matthew Stoker Leonardo Fonseca Jamie Schaeffer • Bill Johnson • Phil Tobin


