Chemistry and chemical elements. Chemistry is the science

chemistry.ppt
- Количество слайдов: 8
 Chemistry and chemical elements
Chemistry and chemical elements
 Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds. Chemistry is sometimes called ‘the central science’ because it connects physics with other natural sciences such as geology and biology. Chemistry is a branch of physical science but distinct from physics. Traditional chemistry starts with the study of elementary particles, atoms, molecules, substances, metals, crystals and other aggregates of matter in solid, liquid, and gas states, whether in isolation or combination. The interactions, reactions and transformations that are studied in chemistry are a result of interaction either between different chemical substances or between matter and energy. Such behaviors are studied in a chemistry laboratory using various forms of laboratory glassware.
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds. Chemistry is sometimes called ‘the central science’ because it connects physics with other natural sciences such as geology and biology. Chemistry is a branch of physical science but distinct from physics. Traditional chemistry starts with the study of elementary particles, atoms, molecules, substances, metals, crystals and other aggregates of matter in solid, liquid, and gas states, whether in isolation or combination. The interactions, reactions and transformations that are studied in chemistry are a result of interaction either between different chemical substances or between matter and energy. Such behaviors are studied in a chemistry laboratory using various forms of laboratory glassware.
 There are five major branches of chemistry: Organic chemistry focuses on the structure, properties, and preparation of chemical compounds that consist primarily of carbon and hydrogen. Inorganic chemistry deals with the properties and behavior of inorganic compounds. Analytical chemistry involves the qualitative and quantitative determination of the chemical components of substances. Physical chemistry studies the effect of chemical structure on the physical properties of a substance. Biochemistry is the study of chemical reactions that take place in living things. Thus, although there are five main branches of chemistry, there are many sub-branches such as quantum chemistry, surface chemistry, environmental chemistry, solid-state chemistry, organometallic chemistry, nuclear chemistry, polymer chemistry and many others. There is a huge overlap between chemistry and biology, medicine, physics, geology, and many other disciplines.
There are five major branches of chemistry: Organic chemistry focuses on the structure, properties, and preparation of chemical compounds that consist primarily of carbon and hydrogen. Inorganic chemistry deals with the properties and behavior of inorganic compounds. Analytical chemistry involves the qualitative and quantitative determination of the chemical components of substances. Physical chemistry studies the effect of chemical structure on the physical properties of a substance. Biochemistry is the study of chemical reactions that take place in living things. Thus, although there are five main branches of chemistry, there are many sub-branches such as quantum chemistry, surface chemistry, environmental chemistry, solid-state chemistry, organometallic chemistry, nuclear chemistry, polymer chemistry and many others. There is a huge overlap between chemistry and biology, medicine, physics, geology, and many other disciplines.
 An atom is the basic unit of chemistry. It consists of a positively charged core (the atomic nucleus) which contains protons and neutrons, and which maintains a number of electrons to balance the positive charge in the nucleus. The atom is also the smallest entity that can be envisaged to retain the chemical properties of the element, such as electronegativity, ionization potential, preferred oxidation state, coordination number, and preferred types of bonds to form (e.g., metallic, ionic, covalent). Molecules are typically a set of atoms bound together by covalent bonds, such that the structure is electrically neutral and all valence electrons are paired with other electrons either in bonds or in lone pairs. A chemical element is specifically a substance which is composed of a single type of atom. A chemical element is characterized by a particular number of protons in the nuclei of its atoms. This number is known as the atomic number of the element. For example, all atoms with 6 protons in their nuclei are atoms of the chemical element carbon, and all atoms with 92 protons in their nuclei are atoms of the element uranium.
An atom is the basic unit of chemistry. It consists of a positively charged core (the atomic nucleus) which contains protons and neutrons, and which maintains a number of electrons to balance the positive charge in the nucleus. The atom is also the smallest entity that can be envisaged to retain the chemical properties of the element, such as electronegativity, ionization potential, preferred oxidation state, coordination number, and preferred types of bonds to form (e.g., metallic, ionic, covalent). Molecules are typically a set of atoms bound together by covalent bonds, such that the structure is electrically neutral and all valence electrons are paired with other electrons either in bonds or in lone pairs. A chemical element is specifically a substance which is composed of a single type of atom. A chemical element is characterized by a particular number of protons in the nuclei of its atoms. This number is known as the atomic number of the element. For example, all atoms with 6 protons in their nuclei are atoms of the chemical element carbon, and all atoms with 92 protons in their nuclei are atoms of the element uranium.
 A compound is a substance with a particular ratio of atoms of particular chemical elements which determines its composition, and a particular organization which determines chemical properties. For example, water is a compound containing hydrogen and oxygen in the ratio of two to one, with the oxygen atom between the two hydrogen atoms, and an angle of 104.5° between them.
A compound is a substance with a particular ratio of atoms of particular chemical elements which determines its composition, and a particular organization which determines chemical properties. For example, water is a compound containing hydrogen and oxygen in the ratio of two to one, with the oxygen atom between the two hydrogen atoms, and an angle of 104.5° between them.
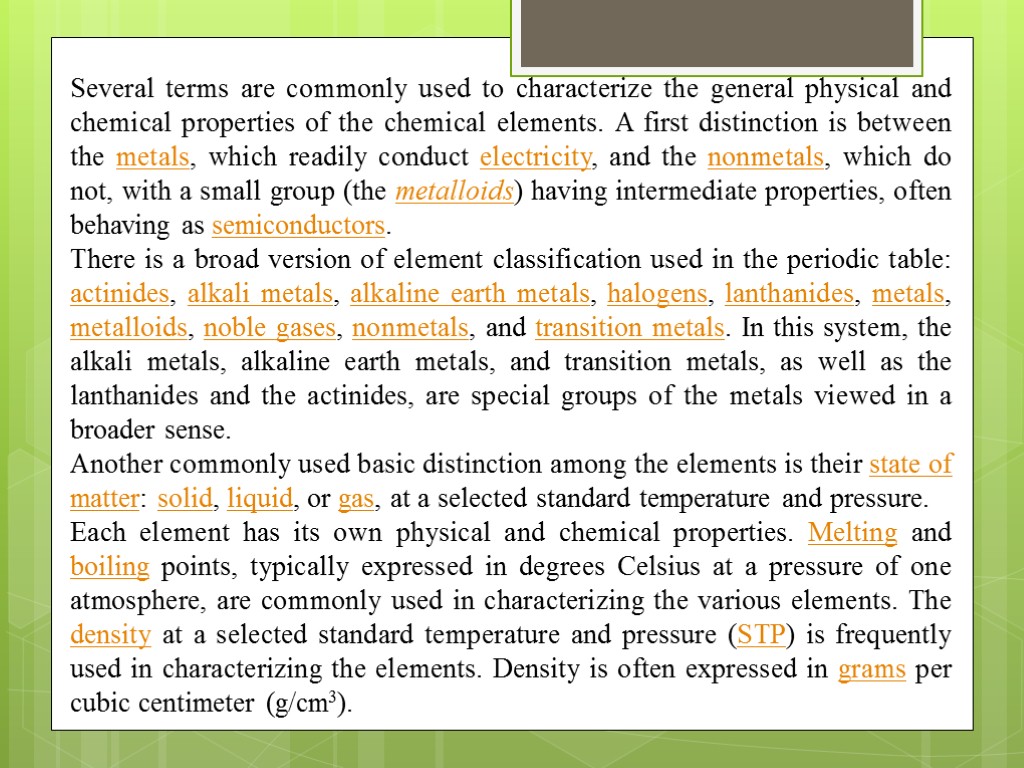
 Several terms are commonly used to characterize the general physical and chemical properties of the chemical elements. A first distinction is between the metals, which readily conduct electricity, and the nonmetals, which do not, with a small group (the metalloids) having intermediate properties, often behaving as semiconductors. There is a broad version of element classification used in the periodic table: actinides, alkali metals, alkaline earth metals, halogens, lanthanides, metals, metalloids, noble gases, nonmetals, and transition metals. In this system, the alkali metals, alkaline earth metals, and transition metals, as well as the lanthanides and the actinides, are special groups of the metals viewed in a broader sense. Another commonly used basic distinction among the elements is their state of matter: solid, liquid, or gas, at a selected standard temperature and pressure. Each element has its own physical and chemical properties. Melting and boiling points, typically expressed in degrees Celsius at a pressure of one atmosphere, are commonly used in characterizing the various elements. The density at a selected standard temperature and pressure (STP) is frequently used in characterizing the elements. Density is often expressed in grams per cubic centimeter (g/cm3).
Several terms are commonly used to characterize the general physical and chemical properties of the chemical elements. A first distinction is between the metals, which readily conduct electricity, and the nonmetals, which do not, with a small group (the metalloids) having intermediate properties, often behaving as semiconductors. There is a broad version of element classification used in the periodic table: actinides, alkali metals, alkaline earth metals, halogens, lanthanides, metals, metalloids, noble gases, nonmetals, and transition metals. In this system, the alkali metals, alkaline earth metals, and transition metals, as well as the lanthanides and the actinides, are special groups of the metals viewed in a broader sense. Another commonly used basic distinction among the elements is their state of matter: solid, liquid, or gas, at a selected standard temperature and pressure. Each element has its own physical and chemical properties. Melting and boiling points, typically expressed in degrees Celsius at a pressure of one atmosphere, are commonly used in characterizing the various elements. The density at a selected standard temperature and pressure (STP) is frequently used in characterizing the elements. Density is often expressed in grams per cubic centimeter (g/cm3).
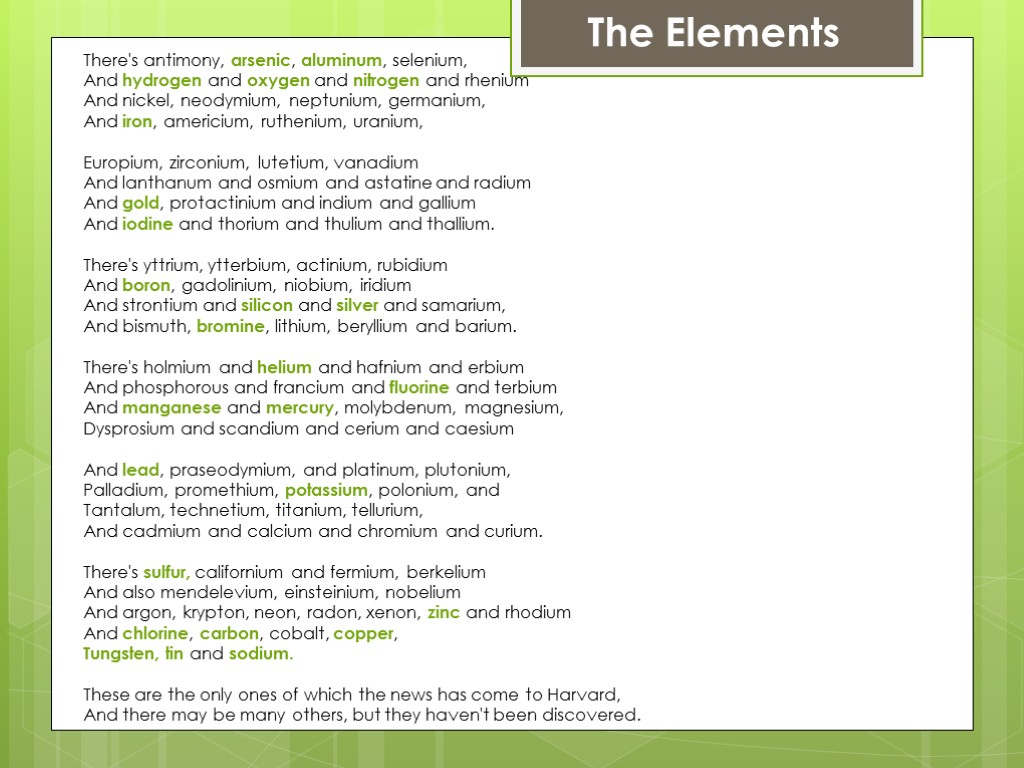
 There's antimony, arsenic, aluminum, selenium, And hydrogen and oxygen and nitrogen and rhenium And nickel, neodymium, neptunium, germanium, And iron, americium, ruthenium, uranium, Europium, zirconium, lutetium, vanadium And lanthanum and osmium and astatine and radium And gold, protactinium and indium and gallium And iodine and thorium and thulium and thallium. There's yttrium, ytterbium, actinium, rubidium And boron, gadolinium, niobium, iridium And strontium and silicon and silver and samarium, And bismuth, bromine, lithium, beryllium and barium. There's holmium and helium and hafnium and erbium And phosphorous and francium and fluorine and terbium And manganese and mercury, molybdenum, magnesium, Dysprosium and scandium and cerium and caesium And lead, praseodymium, and platinum, plutonium, Palladium, promethium, potassium, polonium, and Tantalum, technetium, titanium, tellurium, And cadmium and calcium and chromium and curium. There's sulfur, californium and fermium, berkelium And also mendelevium, einsteinium, nobelium And argon, krypton, neon, radon, xenon, zinc and rhodium And chlorine, carbon, cobalt, copper, Tungsten, tin and sodium. These are the only ones of which the news has come to Harvard, And there may be many others, but they haven't been discovered. The Elements
There's antimony, arsenic, aluminum, selenium, And hydrogen and oxygen and nitrogen and rhenium And nickel, neodymium, neptunium, germanium, And iron, americium, ruthenium, uranium, Europium, zirconium, lutetium, vanadium And lanthanum and osmium and astatine and radium And gold, protactinium and indium and gallium And iodine and thorium and thulium and thallium. There's yttrium, ytterbium, actinium, rubidium And boron, gadolinium, niobium, iridium And strontium and silicon and silver and samarium, And bismuth, bromine, lithium, beryllium and barium. There's holmium and helium and hafnium and erbium And phosphorous and francium and fluorine and terbium And manganese and mercury, molybdenum, magnesium, Dysprosium and scandium and cerium and caesium And lead, praseodymium, and platinum, plutonium, Palladium, promethium, potassium, polonium, and Tantalum, technetium, titanium, tellurium, And cadmium and calcium and chromium and curium. There's sulfur, californium and fermium, berkelium And also mendelevium, einsteinium, nobelium And argon, krypton, neon, radon, xenon, zinc and rhodium And chlorine, carbon, cobalt, copper, Tungsten, tin and sodium. These are the only ones of which the news has come to Harvard, And there may be many others, but they haven't been discovered. The Elements

