MRS USA-13 Safonov.pptx
- Количество слайдов: 27
 Chemical Department Laser-induced Copper Deposition from Solutions with the Addition of Surfactants and Oxidants Sergey Safonov Saint-Petersburg State University, Russian Federation Laser Chemistry and Laser Materials Science
Chemical Department Laser-induced Copper Deposition from Solutions with the Addition of Surfactants and Oxidants Sergey Safonov Saint-Petersburg State University, Russian Federation Laser Chemistry and Laser Materials Science
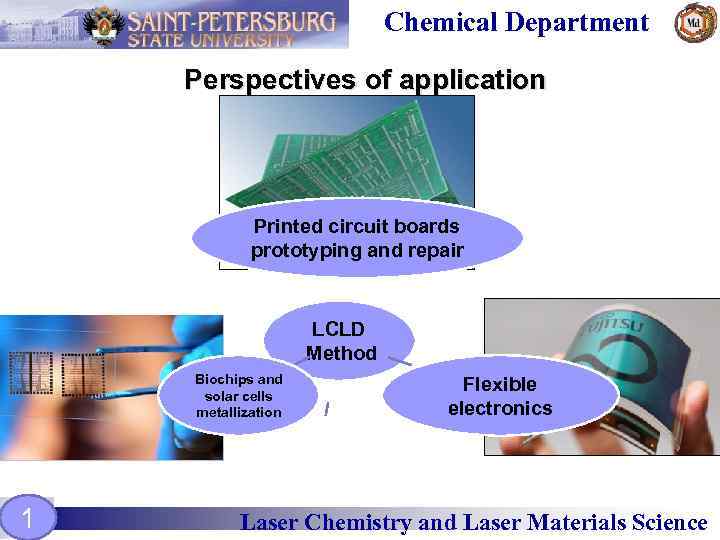 Chemical Department Perspectives of application Printed circuit boards prototyping and repair LCLD Method Biochips and solar cells metallization 1 Flexible electronics Laser Chemistry and Laser Materials Science
Chemical Department Perspectives of application Printed circuit boards prototyping and repair LCLD Method Biochips and solar cells metallization 1 Flexible electronics Laser Chemistry and Laser Materials Science
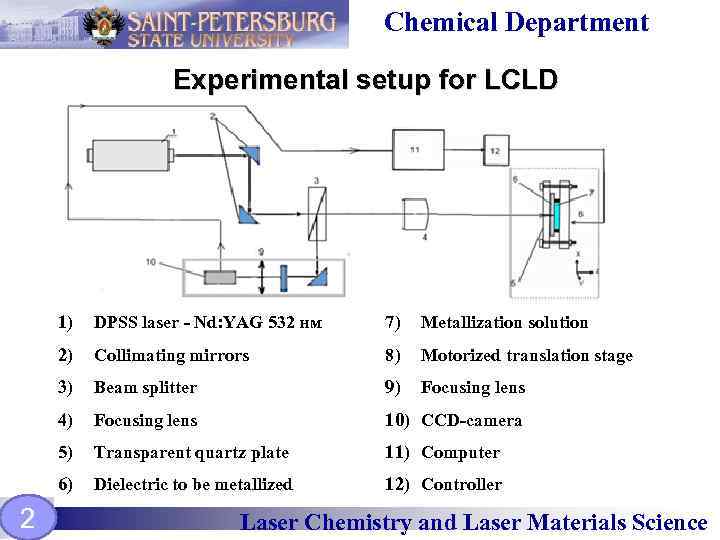 Chemical Department Experimental setup for LCLD 1) 7) Metallization solution 2) Collimating mirrors 8) Motorized translation stage 3) Beam splitter 9) Focusing lens 4) Focusing lens 10) CCD-camera 5) Transparent quartz plate 11) Computer 6) 2 DPSS laser - Nd: YAG 532 нм Dielectric to be metallized 12) Controller Laser Chemistry and Laser Materials Science
Chemical Department Experimental setup for LCLD 1) 7) Metallization solution 2) Collimating mirrors 8) Motorized translation stage 3) Beam splitter 9) Focusing lens 4) Focusing lens 10) CCD-camera 5) Transparent quartz plate 11) Computer 6) 2 DPSS laser - Nd: YAG 532 нм Dielectric to be metallized 12) Controller Laser Chemistry and Laser Materials Science
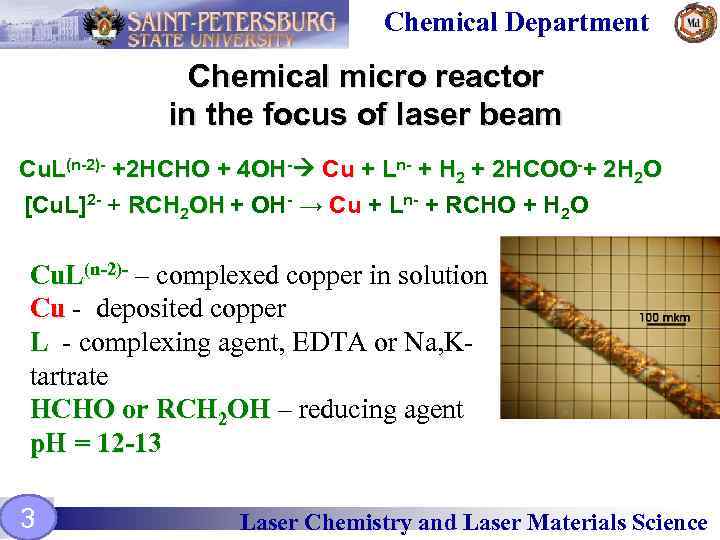 Chemical Department Chemical micro reactor in the focus of laser beam Cu. L(n-2)- +2 HCHO + 4 OH- Cu + Ln- + H 2 + 2 HCOO-+ 2 H 2 O [Cu. L]2 - + RCH 2 OH + OH- → Cu + Ln- + RCHO + H 2 O Cu. L(n-2)- – complexed copper in solution Cu - deposited copper L - complexing agent, EDTA or Na, Ktartrate НСНО or RCH 2 OH – reducing agent p. H = 12 -13 3 Laser Chemistry and Laser Materials Science
Chemical Department Chemical micro reactor in the focus of laser beam Cu. L(n-2)- +2 HCHO + 4 OH- Cu + Ln- + H 2 + 2 HCOO-+ 2 H 2 O [Cu. L]2 - + RCH 2 OH + OH- → Cu + Ln- + RCHO + H 2 O Cu. L(n-2)- – complexed copper in solution Cu - deposited copper L - complexing agent, EDTA or Na, Ktartrate НСНО or RCH 2 OH – reducing agent p. H = 12 -13 3 Laser Chemistry and Laser Materials Science
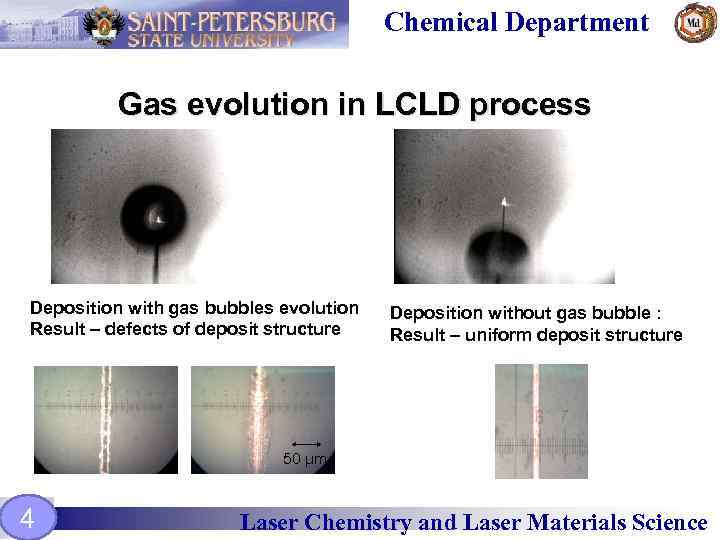 Chemical Department Gas evolution in LCLD process Deposition with gas bubbles evolution Result – defects of deposit structure Deposition without gas bubble : Result – uniform deposit structure 50 µm 4 Laser Chemistry and Laser Materials Science
Chemical Department Gas evolution in LCLD process Deposition with gas bubbles evolution Result – defects of deposit structure Deposition without gas bubble : Result – uniform deposit structure 50 µm 4 Laser Chemistry and Laser Materials Science
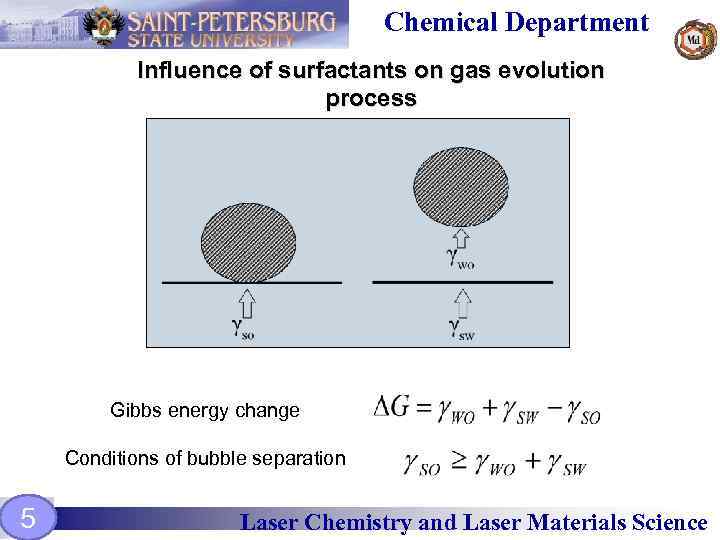 Chemical Department Influence of surfactants on gas evolution process Gibbs energy change Conditions of bubble separation 5 Laser Chemistry and Laser Materials Science
Chemical Department Influence of surfactants on gas evolution process Gibbs energy change Conditions of bubble separation 5 Laser Chemistry and Laser Materials Science
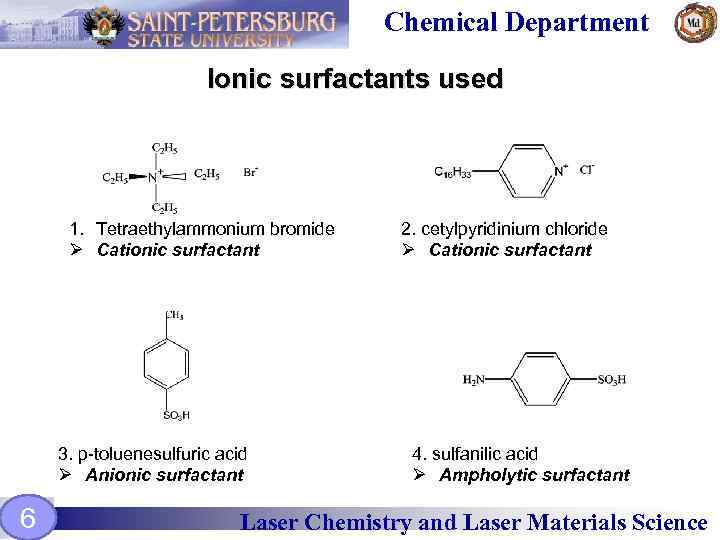 Chemical Department Ionic surfactants used 1. Tetraethylammonium bromide Ø Cationic surfactant 3. p-toluenesulfuric acid Ø Anionic surfactant 6 2. cetylpyridinium сhloride Ø Cationic surfactant 4. sulfanilic acid Ø Ampholytic surfactant Laser Chemistry and Laser Materials Science
Chemical Department Ionic surfactants used 1. Tetraethylammonium bromide Ø Cationic surfactant 3. p-toluenesulfuric acid Ø Anionic surfactant 6 2. cetylpyridinium сhloride Ø Cationic surfactant 4. sulfanilic acid Ø Ampholytic surfactant Laser Chemistry and Laser Materials Science
 Chemical Department Ionic surfactants: negative results Cationic surfactant 7 Anionic surfactant Ampholytic surfactant Laser Chemistry and Laser Materials Science
Chemical Department Ionic surfactants: negative results Cationic surfactant 7 Anionic surfactant Ampholytic surfactant Laser Chemistry and Laser Materials Science
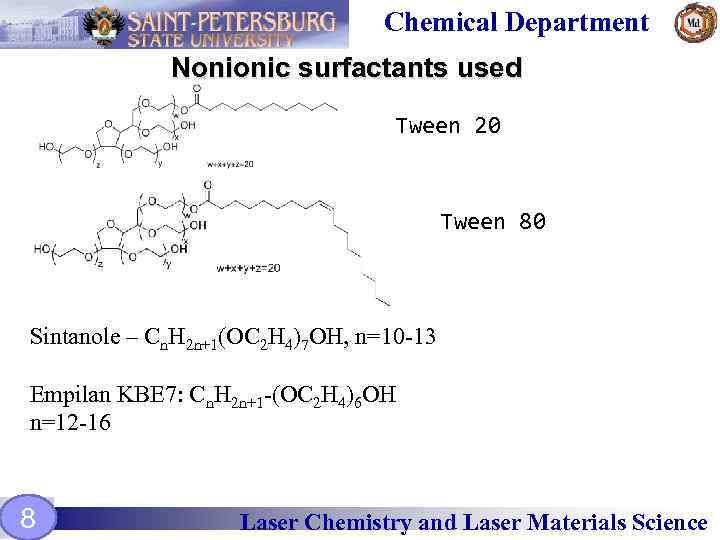 Chemical Department Nonionic surfactants used Tween 20 Tween 80 Sintanole – Сn. H 2 n+1(OC 2 H 4)7 OH, n=10 -13 Empilan KBE 7: Cn. H 2 n+1 -(OC 2 H 4)6 OH n=12 -16 8 Laser Chemistry and Laser Materials Science
Chemical Department Nonionic surfactants used Tween 20 Tween 80 Sintanole – Сn. H 2 n+1(OC 2 H 4)7 OH, n=10 -13 Empilan KBE 7: Cn. H 2 n+1 -(OC 2 H 4)6 OH n=12 -16 8 Laser Chemistry and Laser Materials Science
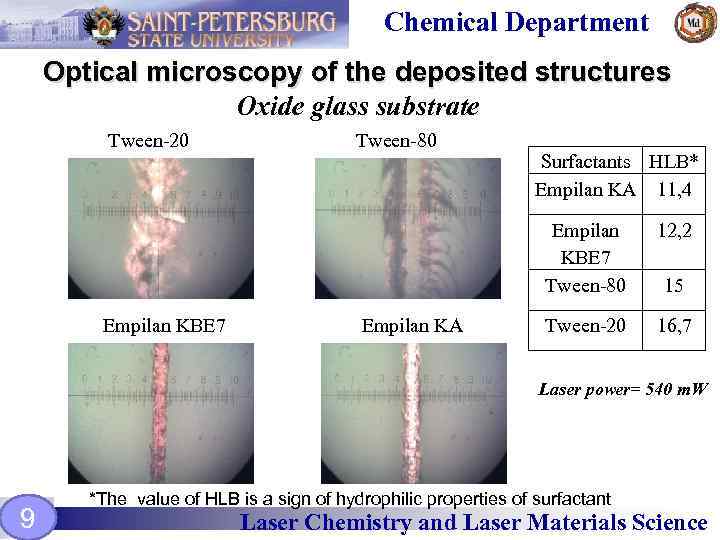 Chemical Department Optical microscopy of the deposited structures Oxide glass substrate Tween-20 Tween-80 Surfactants HLB* Empilan KA 11, 4 Empilan KBE 7 Tween-80 Empilan KBE 7 Empilan KA 12, 2 Tween-20 16, 7 15 Laser power= 540 m. W 9 *The value of HLB is a sign of hydrophilic properties of surfactant Laser Chemistry and Laser Materials Science
Chemical Department Optical microscopy of the deposited structures Oxide glass substrate Tween-20 Tween-80 Surfactants HLB* Empilan KA 11, 4 Empilan KBE 7 Tween-80 Empilan KBE 7 Empilan KA 12, 2 Tween-20 16, 7 15 Laser power= 540 m. W 9 *The value of HLB is a sign of hydrophilic properties of surfactant Laser Chemistry and Laser Materials Science
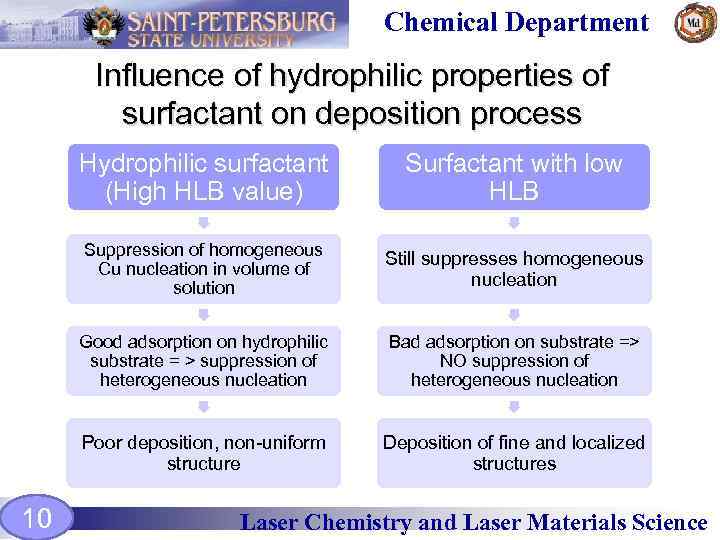 Chemical Department Influence of hydrophilic properties of surfactant on deposition process Hydrophilic surfactant (High HLB value) Suppression of homogeneous Cu nucleation in volume of solution Still suppresses homogeneous nucleation Good adsorption on hydrophilic substrate = > suppression of heterogeneous nucleation Bad adsorption on substrate => NO suppression of heterogeneous nucleation Poor deposition, non-uniform structure 10 Surfactant with low HLB Deposition of fine and localized structures Laser Chemistry and Laser Materials Science
Chemical Department Influence of hydrophilic properties of surfactant on deposition process Hydrophilic surfactant (High HLB value) Suppression of homogeneous Cu nucleation in volume of solution Still suppresses homogeneous nucleation Good adsorption on hydrophilic substrate = > suppression of heterogeneous nucleation Bad adsorption on substrate => NO suppression of heterogeneous nucleation Poor deposition, non-uniform structure 10 Surfactant with low HLB Deposition of fine and localized structures Laser Chemistry and Laser Materials Science
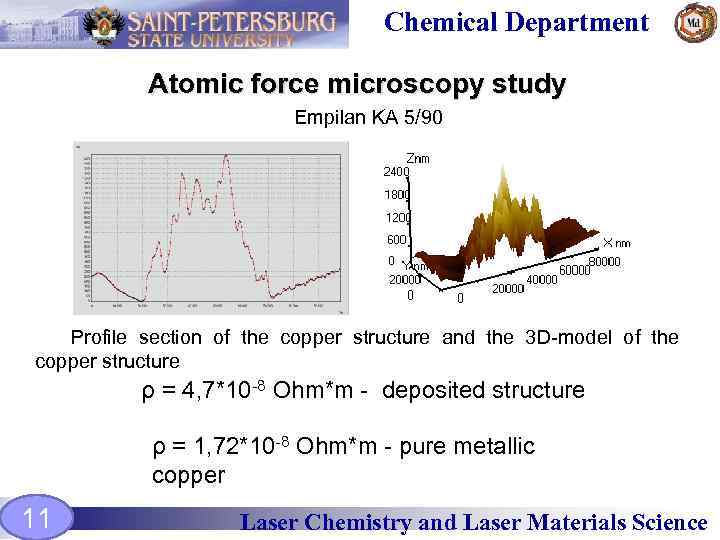 Chemical Department Atomic force microscopy study Empilan KA 5/90 Profile section of the copper structure and the 3 D-model of the copper structure ρ = 4, 7*10 -8 Ohm*m - deposited structure ρ = 1, 72*10 -8 Ohm*m - pure metallic copper 11 Laser Chemistry and Laser Materials Science
Chemical Department Atomic force microscopy study Empilan KA 5/90 Profile section of the copper structure and the 3 D-model of the copper structure ρ = 4, 7*10 -8 Ohm*m - deposited structure ρ = 1, 72*10 -8 Ohm*m - pure metallic copper 11 Laser Chemistry and Laser Materials Science
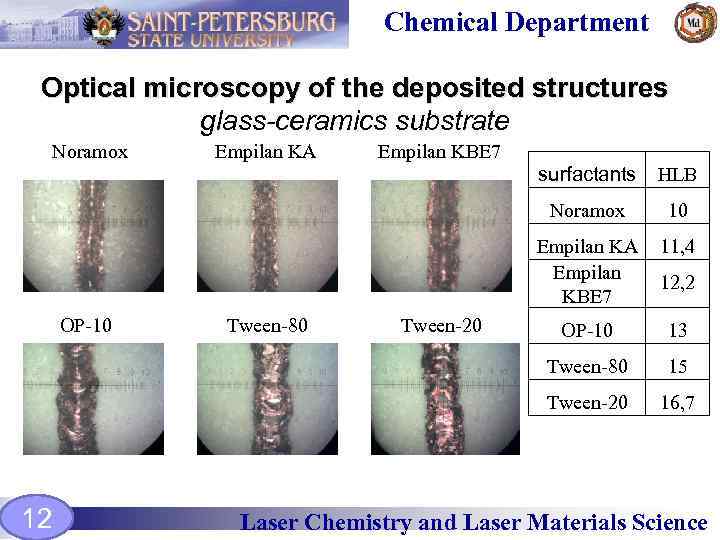 Chemical Department Optical microscopy of the deposited structures glass-ceramics substrate Noramox Empilan KA Empilan KBE 7 surfactants Noramox 12 OP-10 13 15 Tween-20 Тween-20 11, 4 Tween-80 Тween-80 10 Empilan KA Empilan KBE 7 ОP-10 HLB 16, 7 12, 2 Laser Chemistry and Laser Materials Science
Chemical Department Optical microscopy of the deposited structures glass-ceramics substrate Noramox Empilan KA Empilan KBE 7 surfactants Noramox 12 OP-10 13 15 Tween-20 Тween-20 11, 4 Tween-80 Тween-80 10 Empilan KA Empilan KBE 7 ОP-10 HLB 16, 7 12, 2 Laser Chemistry and Laser Materials Science
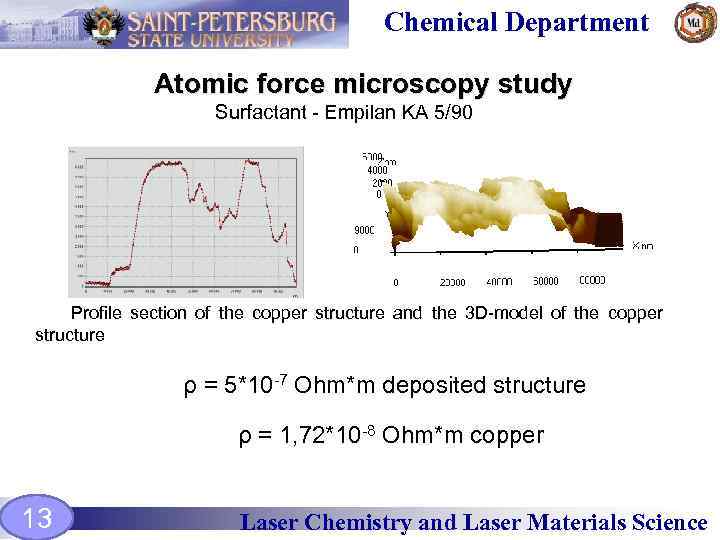 Chemical Department Atomic force microscopy study Surfactant - Empilan KA 5/90 Profile section of the copper structure and the 3 D-model of the copper structure ρ = 5*10 -7 Ohm*m deposited structure ρ = 1, 72*10 -8 Ohm*m copper 13 Laser Chemistry and Laser Materials Science
Chemical Department Atomic force microscopy study Surfactant - Empilan KA 5/90 Profile section of the copper structure and the 3 D-model of the copper structure ρ = 5*10 -7 Ohm*m deposited structure ρ = 1, 72*10 -8 Ohm*m copper 13 Laser Chemistry and Laser Materials Science
 Chemical Department Electron microscopy study Deposition without surfuctants glass-ceramic substrate oxide glasses substrate Deposition with surfuctants Empilan KA 5/90 14 Laser Chemistry and Laser Materials Science
Chemical Department Electron microscopy study Deposition without surfuctants glass-ceramic substrate oxide glasses substrate Deposition with surfuctants Empilan KA 5/90 14 Laser Chemistry and Laser Materials Science
 Chemical Department LCLD problem: Slow scanning speed Currently used scanning speeds: 2. 5 µm/sec , 10 µm/sec Desirable scanning speed: 100 µm/sec and more Catalyzing agent proposed in previous works for HCHO solutions: p-benzoquinone. CH 2 O + O=C 6 H 4=O + OH- -> HCOO- + HO-C 6 H 4 -OH +H 2 O HO-C 6 H 4 -OH + Cu 2+ -> Cu 0 + O=C 6 H 4=O Oxidizing potential of p-benzoquinone is not enough for polyol -containing solutions: new catalyzing additives have to be proposed. 15 Laser Chemistry and Laser Materials Science
Chemical Department LCLD problem: Slow scanning speed Currently used scanning speeds: 2. 5 µm/sec , 10 µm/sec Desirable scanning speed: 100 µm/sec and more Catalyzing agent proposed in previous works for HCHO solutions: p-benzoquinone. CH 2 O + O=C 6 H 4=O + OH- -> HCOO- + HO-C 6 H 4 -OH +H 2 O HO-C 6 H 4 -OH + Cu 2+ -> Cu 0 + O=C 6 H 4=O Oxidizing potential of p-benzoquinone is not enough for polyol -containing solutions: new catalyzing additives have to be proposed. 15 Laser Chemistry and Laser Materials Science
 Chemical Department Oxidizing catalysis of laser-induced chemical deposition of copper Reducing agent: C 2 H 5 OH No Additive speed: 0. 01 mm/s (4 times increased) Reducing agent: C 2 H 5 OH Additive: Fe. Cl 3 speed: 0. 01 mm/s (4 times increased) Reducing agent: glycerol No Additive speed: 0. 01 mm/s (4 times increased) Reducing agent: glycerol Additive: KMn. O 4 speed: 0. 01 mm/s (4 times increased) 16 Laser Chemistry and Laser Materials Science
Chemical Department Oxidizing catalysis of laser-induced chemical deposition of copper Reducing agent: C 2 H 5 OH No Additive speed: 0. 01 mm/s (4 times increased) Reducing agent: C 2 H 5 OH Additive: Fe. Cl 3 speed: 0. 01 mm/s (4 times increased) Reducing agent: glycerol No Additive speed: 0. 01 mm/s (4 times increased) Reducing agent: glycerol Additive: KMn. O 4 speed: 0. 01 mm/s (4 times increased) 16 Laser Chemistry and Laser Materials Science
 Chemical Department n n n 17 Conclusions Laser-induced copper deposition process is highly affected by type of added surfactant Ionic surfactants addition decrease quality of copper deposits; Non-ionic surfactants may increase quality of copper deposits. The main criteria of high quality deposit: low surface tension of solution and low HLB value Addition of oxidizing agents to plating solution with polyols as reducing agents may increase deposition speed. Mechanisms should be disclosed in further research Laser Chemistry and Laser Materials Science
Chemical Department n n n 17 Conclusions Laser-induced copper deposition process is highly affected by type of added surfactant Ionic surfactants addition decrease quality of copper deposits; Non-ionic surfactants may increase quality of copper deposits. The main criteria of high quality deposit: low surface tension of solution and low HLB value Addition of oxidizing agents to plating solution with polyols as reducing agents may increase deposition speed. Mechanisms should be disclosed in further research Laser Chemistry and Laser Materials Science
 Chemical Department Thank you for attention! Sergey Safonov Saint-Petersburg State University, Department of Chemistry Russian Federation sergey. saphonov@gmail. com Skype: scitourn Laser Chemistry and Laser Materials Science
Chemical Department Thank you for attention! Sergey Safonov Saint-Petersburg State University, Department of Chemistry Russian Federation sergey. saphonov@gmail. com Skype: scitourn Laser Chemistry and Laser Materials Science
 Chemical Department ADDITIONAL SLIDES Laser Chemistry and Laser Materials Science
Chemical Department ADDITIONAL SLIDES Laser Chemistry and Laser Materials Science
 Chemical Department Study of surfactants effect (glass substrate) Experimental Deposition was carried out on oxide glass substrate by using Ar + laser (λ = 488 nm). Composition of water solution of copper plating component Cu. Cl 2 EDTA Na. OH surfactant 0, 01 concentration M 21 0, 011 M 0, 05 M 10 -5 M formaldehyde P-Benzochinon 0, 075 M 0, 003 M Laser Chemistry and Laser Materials Science
Chemical Department Study of surfactants effect (glass substrate) Experimental Deposition was carried out on oxide glass substrate by using Ar + laser (λ = 488 nm). Composition of water solution of copper plating component Cu. Cl 2 EDTA Na. OH surfactant 0, 01 concentration M 21 0, 011 M 0, 05 M 10 -5 M formaldehyde P-Benzochinon 0, 075 M 0, 003 M Laser Chemistry and Laser Materials Science
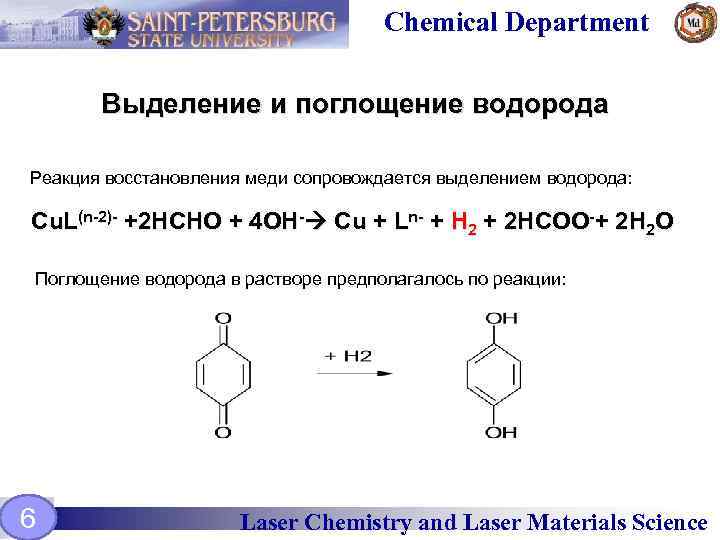 Chemical Department Выделение и поглощение водорода Реакция восстановления меди сопровождается выделением водорода: Cu. L(n-2)- +2 HCHO + 4 OH- Cu + Ln- + H 2 + 2 HCOO-+ 2 H 2 O Поглощение водорода в растворе предполагалось по реакции: 6 Laser Chemistry and Laser Materials Science
Chemical Department Выделение и поглощение водорода Реакция восстановления меди сопровождается выделением водорода: Cu. L(n-2)- +2 HCHO + 4 OH- Cu + Ln- + H 2 + 2 HCOO-+ 2 H 2 O Поглощение водорода в растворе предполагалось по реакции: 6 Laser Chemistry and Laser Materials Science
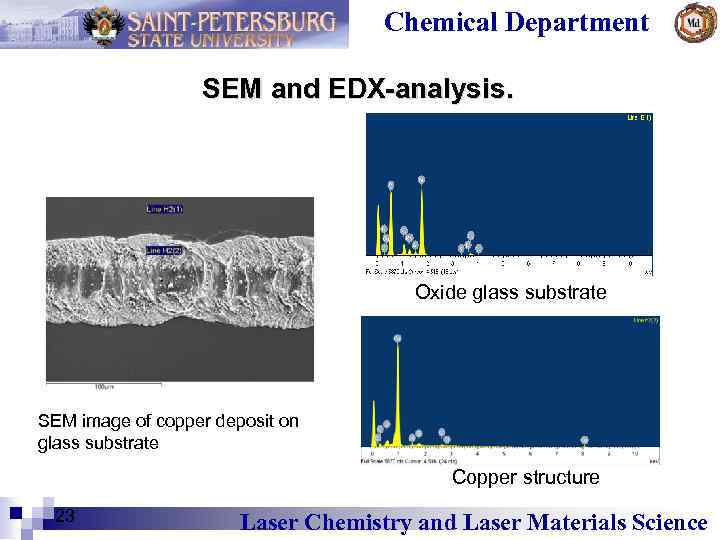 Chemical Department SEM and EDX-analysis. Oxide glass substrate SEM image of copper deposit on glass substrate Copper structure 23 Laser Chemistry and Laser Materials Science
Chemical Department SEM and EDX-analysis. Oxide glass substrate SEM image of copper deposit on glass substrate Copper structure 23 Laser Chemistry and Laser Materials Science
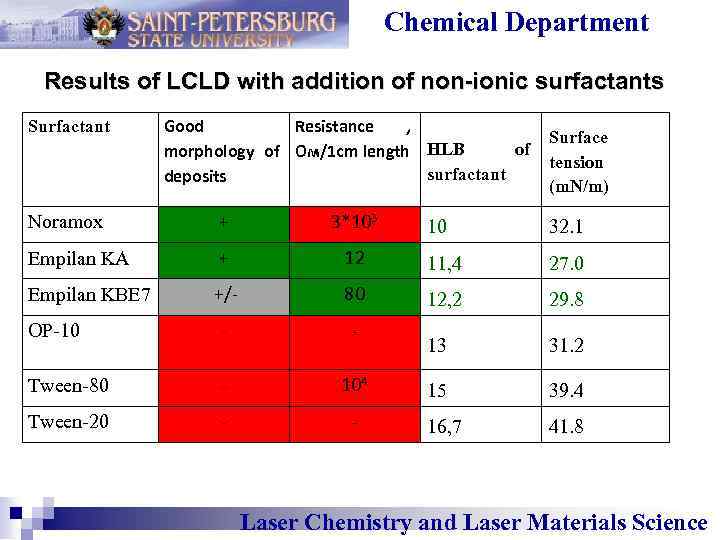 Chemical Department Results of LCLD with addition of non-ionic surfactants Surfactant Good Resistance , of morphology of Ом/1 cm length HLB surfactant deposits Noramox + 3*103 Empilan KA + Empilan KBE 7 Surface tension (m. N/m) 10 32. 1 12 11, 4 27. 0 +/- 80 12, 2 29. 8 ОP-10 - - 13 31. 2 Tween-80 - 104 15 39. 4 Tween-20 - - 16, 7 41. 8 Laser Chemistry and Laser Materials Science
Chemical Department Results of LCLD with addition of non-ionic surfactants Surfactant Good Resistance , of morphology of Ом/1 cm length HLB surfactant deposits Noramox + 3*103 Empilan KA + Empilan KBE 7 Surface tension (m. N/m) 10 32. 1 12 11, 4 27. 0 +/- 80 12, 2 29. 8 ОP-10 - - 13 31. 2 Tween-80 - 104 15 39. 4 Tween-20 - - 16, 7 41. 8 Laser Chemistry and Laser Materials Science
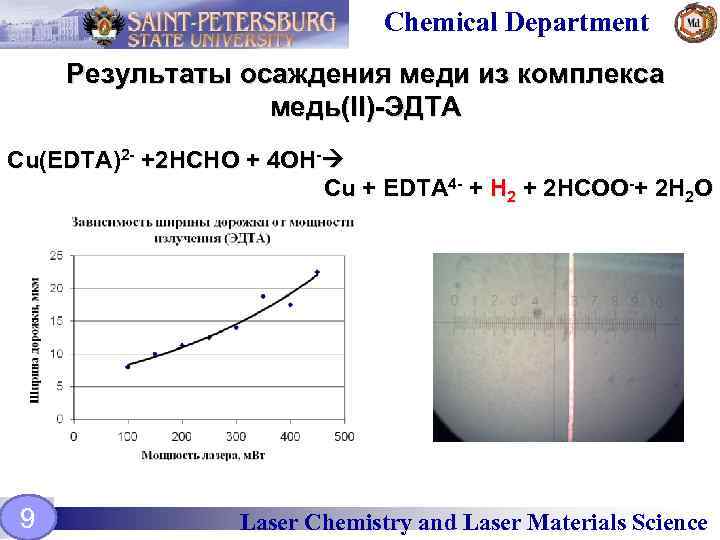 Chemical Department Результаты осаждения меди из комплекса медь(II)-ЭДТА Cu(EDTA)2 - +2 HCHO + 4 OH- Cu + EDTA 4 - + H 2 + 2 HCOO-+ 2 H 2 O 9 Laser Chemistry and Laser Materials Science
Chemical Department Результаты осаждения меди из комплекса медь(II)-ЭДТА Cu(EDTA)2 - +2 HCHO + 4 OH- Cu + EDTA 4 - + H 2 + 2 HCOO-+ 2 H 2 O 9 Laser Chemistry and Laser Materials Science
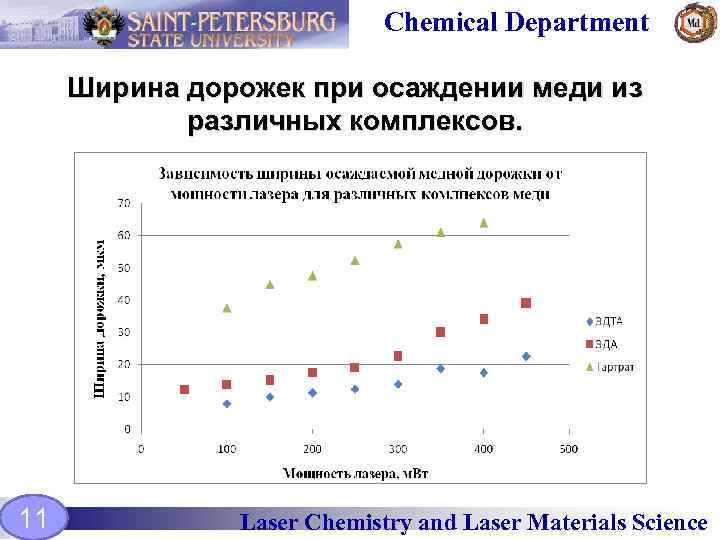 Chemical Department Ширина дорожек при осаждении меди из различных комплексов. 11 Laser Chemistry and Laser Materials Science
Chemical Department Ширина дорожек при осаждении меди из различных комплексов. 11 Laser Chemistry and Laser Materials Science
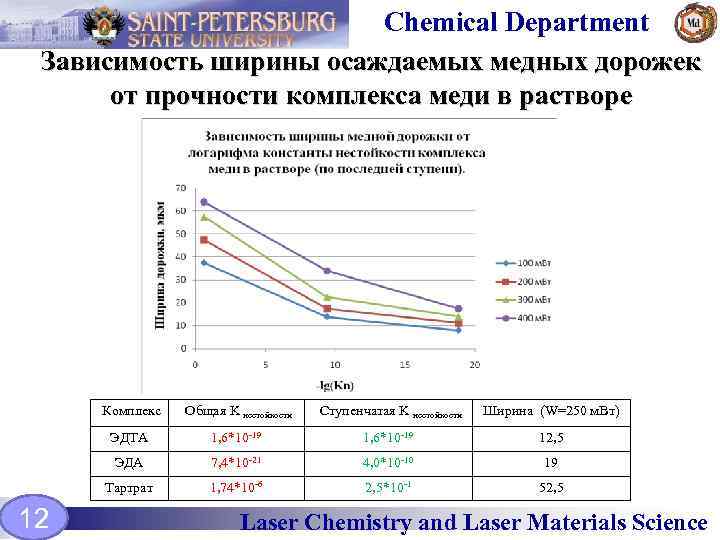 Chemical Department Зависимость ширины осаждаемых медных дорожек от прочности комплекса меди в растворе Комплекс Ступенчатая K нестойкости Ширина (W=250 м. Вт) ЭДТА 1, 6*10 -19 12, 5 ЭДА 7, 4*10 -21 4, 0*10 -10 19 Тартрат 12 Общая K нестойкости 1, 74*10 -6 2, 5*10 -1 52, 5 Laser Chemistry and Laser Materials Science
Chemical Department Зависимость ширины осаждаемых медных дорожек от прочности комплекса меди в растворе Комплекс Ступенчатая K нестойкости Ширина (W=250 м. Вт) ЭДТА 1, 6*10 -19 12, 5 ЭДА 7, 4*10 -21 4, 0*10 -10 19 Тартрат 12 Общая K нестойкости 1, 74*10 -6 2, 5*10 -1 52, 5 Laser Chemistry and Laser Materials Science
