1 GEN1030 Introduction to Environmental Studies Lecture 2









8734-gen1030_lecture2_su_2013.ppt
- Количество слайдов: 35
 1 GEN1030 Introduction to Environmental Studies Lecture 2 Chapter 2. Matter, Energy, and Environment Aliya Nurtaeva, Ph.D. KIMEP University Dept of General Education Office # 507/ Valikhanov [email protected]
1 GEN1030 Introduction to Environmental Studies Lecture 2 Chapter 2. Matter, Energy, and Environment Aliya Nurtaeva, Ph.D. KIMEP University Dept of General Education Office # 507/ Valikhanov [email protected]
 2 Matter, Energy and Environment
2 Matter, Energy and Environment
 3 Outline Principles of Matter & Energy Structure of Matter Energy Principles Energy Flows Energy, Matter & the Environment Biochemical Cycles
3 Outline Principles of Matter & Energy Structure of Matter Energy Principles Energy Flows Energy, Matter & the Environment Biochemical Cycles
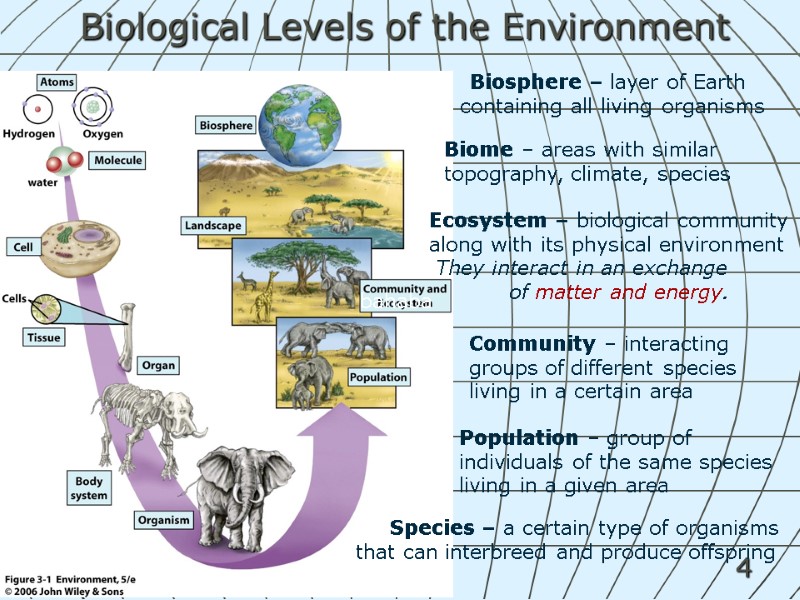
 4 Biological Levels of the Environment Ecosystem – biological community along with its physical environment They interact in an exchange of matter and energy. Species – a certain type of organisms that can interbreed and produce offspring Population – group of individuals of the same species living in a given area Community – interacting groups of different species living in a certain area Biosphere – layer of Earth containing all living organisms bahaba Biome – areas with similar topography, climate, species
4 Biological Levels of the Environment Ecosystem – biological community along with its physical environment They interact in an exchange of matter and energy. Species – a certain type of organisms that can interbreed and produce offspring Population – group of individuals of the same species living in a given area Community – interacting groups of different species living in a certain area Biosphere – layer of Earth containing all living organisms bahaba Biome – areas with similar topography, climate, species
 5
5
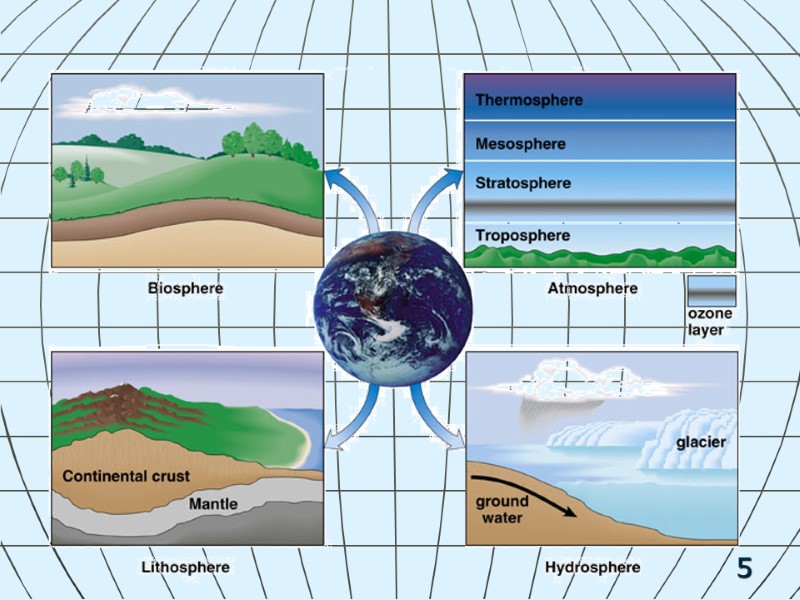
 6 Biotic Components Abiotic Components: Energy & Matter
6 Biotic Components Abiotic Components: Energy & Matter
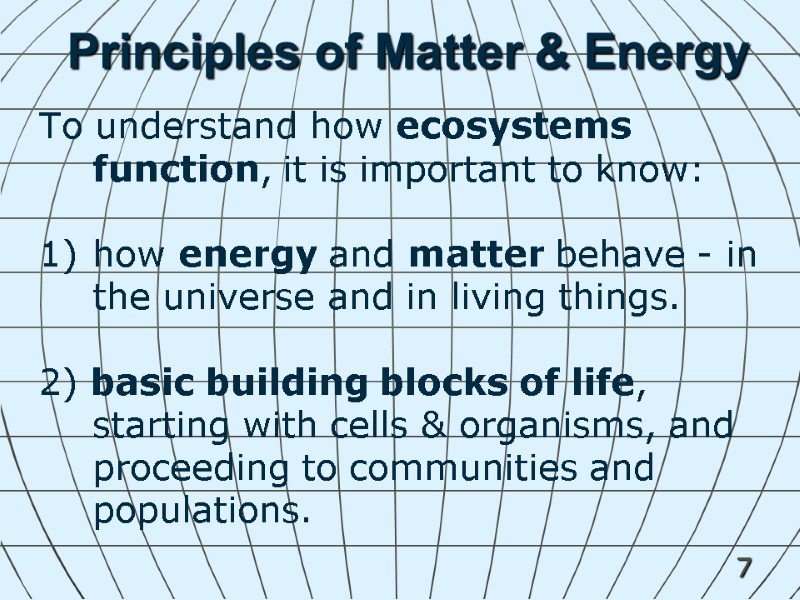
 7 Principles of Matter & Energy To understand how ecosystems function, it is important to know: how energy and matter behave - in the universe and in living things. 2) basic building blocks of life, starting with cells & organisms, and proceeding to communities and populations.
7 Principles of Matter & Energy To understand how ecosystems function, it is important to know: how energy and matter behave - in the universe and in living things. 2) basic building blocks of life, starting with cells & organisms, and proceeding to communities and populations.
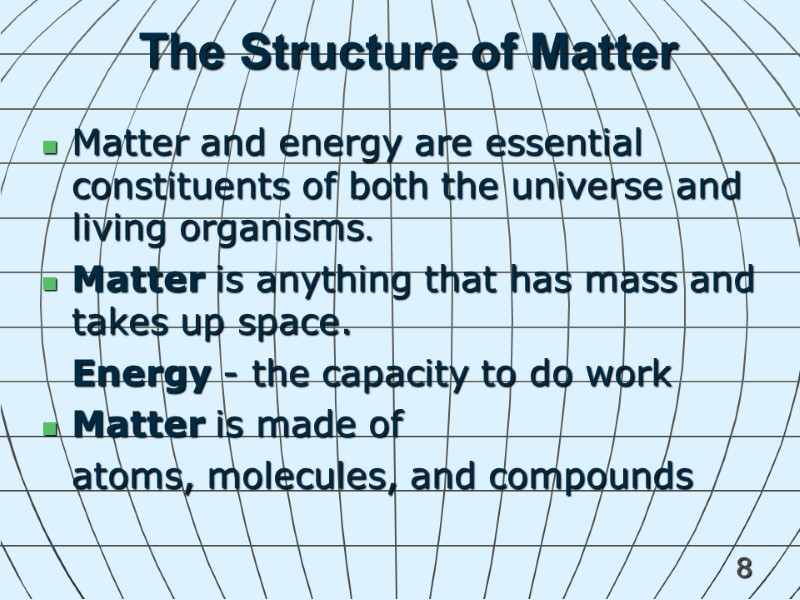
 8 The Structure of Matter Matter and energy are essential constituents of both the universe and living organisms. Matter is anything that has mass and takes up space. Energy - the capacity to do work Matter is made of atoms, molecules, and compounds
8 The Structure of Matter Matter and energy are essential constituents of both the universe and living organisms. Matter is anything that has mass and takes up space. Energy - the capacity to do work Matter is made of atoms, molecules, and compounds
 9 Atomic Structure Atom - fundamental unit of matter. 92 types of atoms found in nature, with each being composed of: Protons (‘+’ charge) Neutrons (neutral: ‘0’ charge ) Electrons (‘-’ charge) Each kind of atom forms a specific type of matter known as an element.
9 Atomic Structure Atom - fundamental unit of matter. 92 types of atoms found in nature, with each being composed of: Protons (‘+’ charge) Neutrons (neutral: ‘0’ charge ) Electrons (‘-’ charge) Each kind of atom forms a specific type of matter known as an element.
 10 Atoms, Molecules, & Compounds Atom - the smallest particle of matter still remaining the elemental properties of matter Molecule – a combination of two or more atoms Compound – a molecule made up of 2 or more kinds of atoms (elements) held together by chemical bonds Isotopes - atoms of the same element that differ in the number of neutrons they contain. Isotopes have the same number of protons & electrons, but different number of neutrons.
10 Atoms, Molecules, & Compounds Atom - the smallest particle of matter still remaining the elemental properties of matter Molecule – a combination of two or more atoms Compound – a molecule made up of 2 or more kinds of atoms (elements) held together by chemical bonds Isotopes - atoms of the same element that differ in the number of neutrons they contain. Isotopes have the same number of protons & electrons, but different number of neutrons.
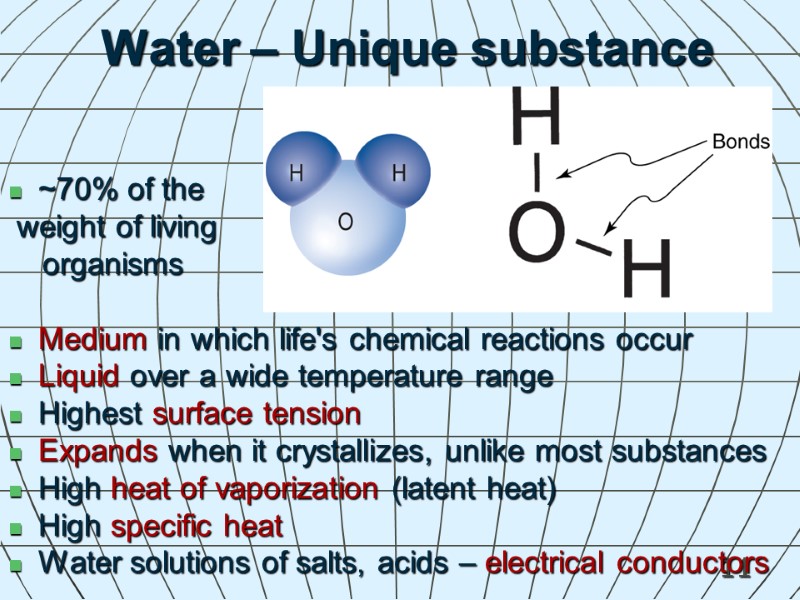
 11 Water – Unique substance ~70% of the weight of living organisms Medium in which life's chemical reactions occur Liquid over a wide temperature range Highest surface tension Expands when it crystallizes, unlike most substances High heat of vaporization (latent heat) High specific heat Water solutions of salts, acids – electrical conductors
11 Water – Unique substance ~70% of the weight of living organisms Medium in which life's chemical reactions occur Liquid over a wide temperature range Highest surface tension Expands when it crystallizes, unlike most substances High heat of vaporization (latent heat) High specific heat Water solutions of salts, acids – electrical conductors
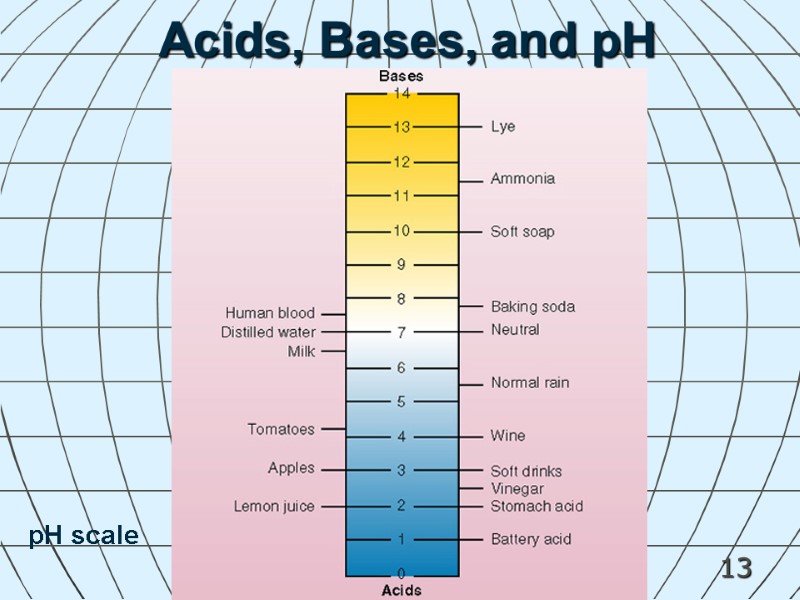
 12 Acids, Bases, and pH Acid - a compound that releases hydrogen ions in water solution. Base - a compound that accepts hydrogen ions in water solution. pH indicator shows acidity or hydrogen ion concentration. The scale is inverse and logarithmic pH = 7: neutral 0 < pH < 7: acidic (more H+ than OH-) 7 < pH < 14 : basic (less H+ than OH-)
12 Acids, Bases, and pH Acid - a compound that releases hydrogen ions in water solution. Base - a compound that accepts hydrogen ions in water solution. pH indicator shows acidity or hydrogen ion concentration. The scale is inverse and logarithmic pH = 7: neutral 0 < pH < 7: acidic (more H+ than OH-) 7 < pH < 14 : basic (less H+ than OH-)
 13 Acids, Bases, and pH pH scale
13 Acids, Bases, and pH pH scale
 14 Inorganic and Organic Matter Organic matter consists of molecules containing carbon atoms that are bonded to form chains or rings. All living things contain molecules of organic compounds. Chemical bonds in organic molecules contain a large amount of chemical energy that can be released when the bonds are broken. Essential Elements of Life: • carbon, hydrogen, oxygen, and nitrogen • make up 96% of living matter
14 Inorganic and Organic Matter Organic matter consists of molecules containing carbon atoms that are bonded to form chains or rings. All living things contain molecules of organic compounds. Chemical bonds in organic molecules contain a large amount of chemical energy that can be released when the bonds are broken. Essential Elements of Life: • carbon, hydrogen, oxygen, and nitrogen • make up 96% of living matter
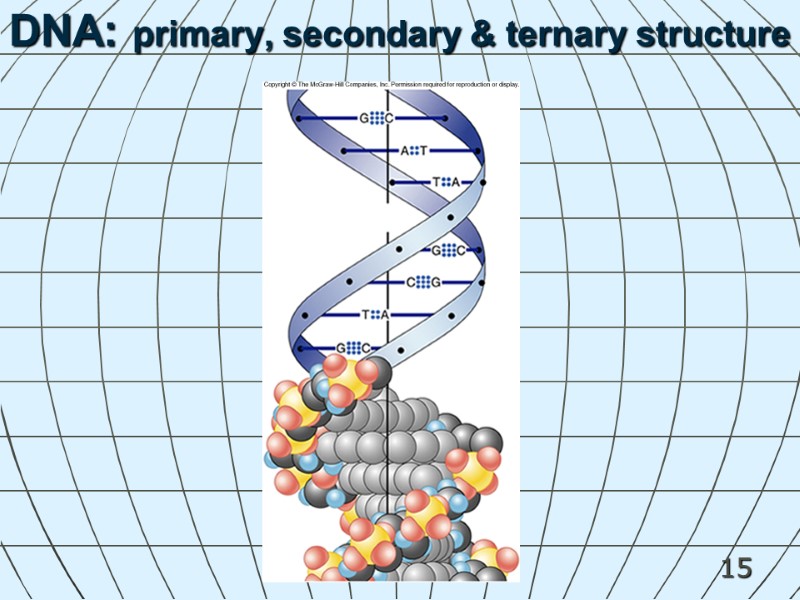
 15 DNA: primary, secondary & ternary structure
15 DNA: primary, secondary & ternary structure
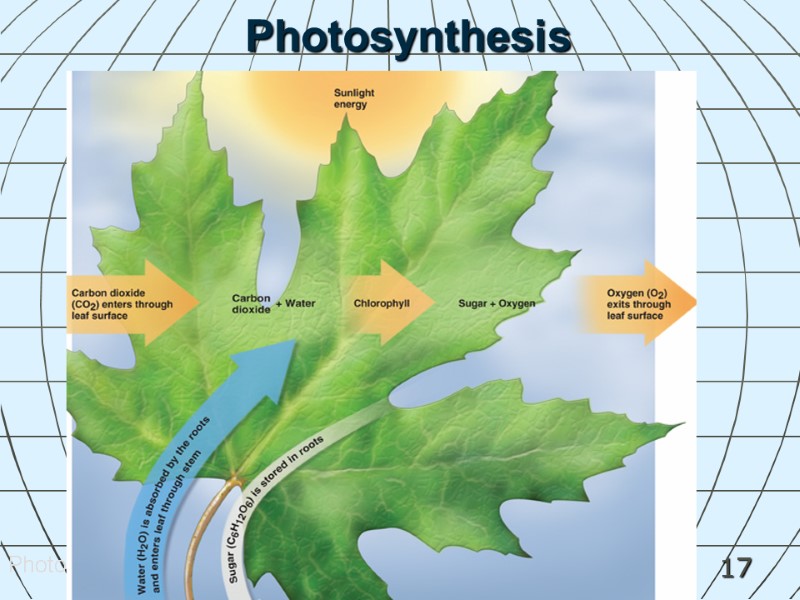
 16 Chemical Reactions in Living Things Photosynthesis is a process used by plants to convert inorganic material into organic material using light. Carbon dioxide + water (in the presence of sunlight) produces glucose + oxygen. 6CO2 + 6H2O + E (sunlight) C6H12O6 + 6O2 Enzymes contained in living organisms act as biocatalysts speeding up chemical reactions.
16 Chemical Reactions in Living Things Photosynthesis is a process used by plants to convert inorganic material into organic material using light. Carbon dioxide + water (in the presence of sunlight) produces glucose + oxygen. 6CO2 + 6H2O + E (sunlight) C6H12O6 + 6O2 Enzymes contained in living organisms act as biocatalysts speeding up chemical reactions.
 17 Photosynthesis Photosynthesis
17 Photosynthesis Photosynthesis
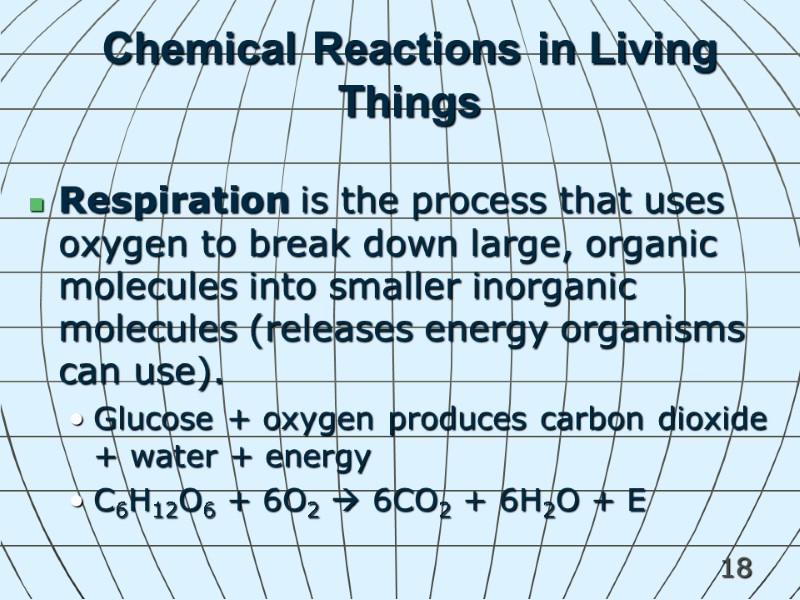
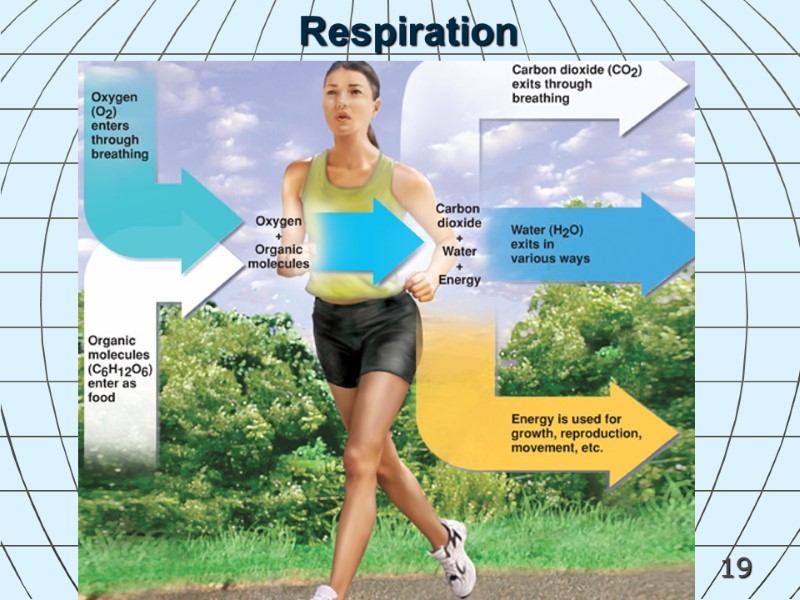
 18 Chemical Reactions in Living Things Respiration is the process that uses oxygen to break down large, organic molecules into smaller inorganic molecules (releases energy organisms can use). Glucose + oxygen produces carbon dioxide + water + energy C6H12O6 + 6O2 6CO2 + 6H2O + E
18 Chemical Reactions in Living Things Respiration is the process that uses oxygen to break down large, organic molecules into smaller inorganic molecules (releases energy organisms can use). Glucose + oxygen produces carbon dioxide + water + energy C6H12O6 + 6O2 6CO2 + 6H2O + E
 19 Respiration
19 Respiration
 20 Energy Principles Energy is the ability to perform work. Kinetic energy is the energy contained by moving objects. Potential energy is energy due to relative position (latent, available for use).
20 Energy Principles Energy is the ability to perform work. Kinetic energy is the energy contained by moving objects. Potential energy is energy due to relative position (latent, available for use).
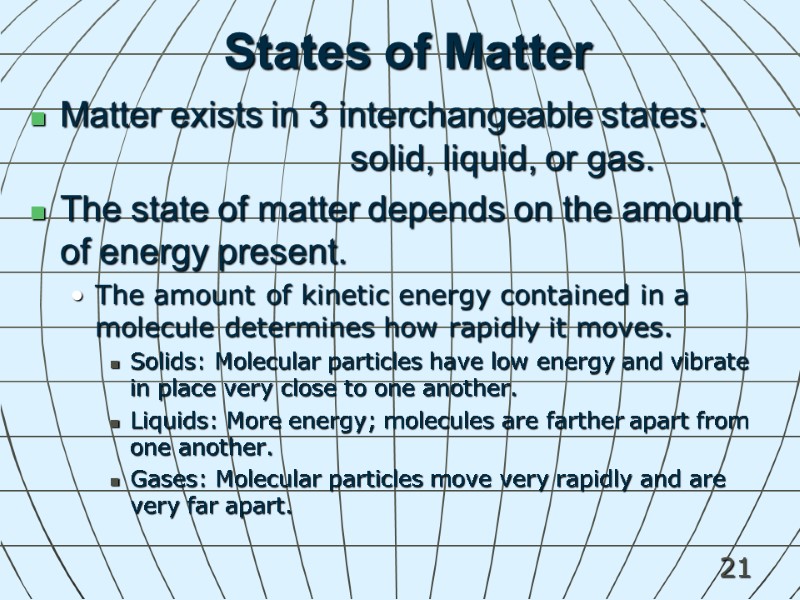
 21 States of Matter Matter exists in 3 interchangeable states: solid, liquid, or gas. The state of matter depends on the amount of energy present. The amount of kinetic energy contained in a molecule determines how rapidly it moves. Solids: Molecular particles have low energy and vibrate in place very close to one another. Liquids: More energy; molecules are farther apart from one another. Gases: Molecular particles move very rapidly and are very far apart.
21 States of Matter Matter exists in 3 interchangeable states: solid, liquid, or gas. The state of matter depends on the amount of energy present. The amount of kinetic energy contained in a molecule determines how rapidly it moves. Solids: Molecular particles have low energy and vibrate in place very close to one another. Liquids: More energy; molecules are farther apart from one another. Gases: Molecular particles move very rapidly and are very far apart.
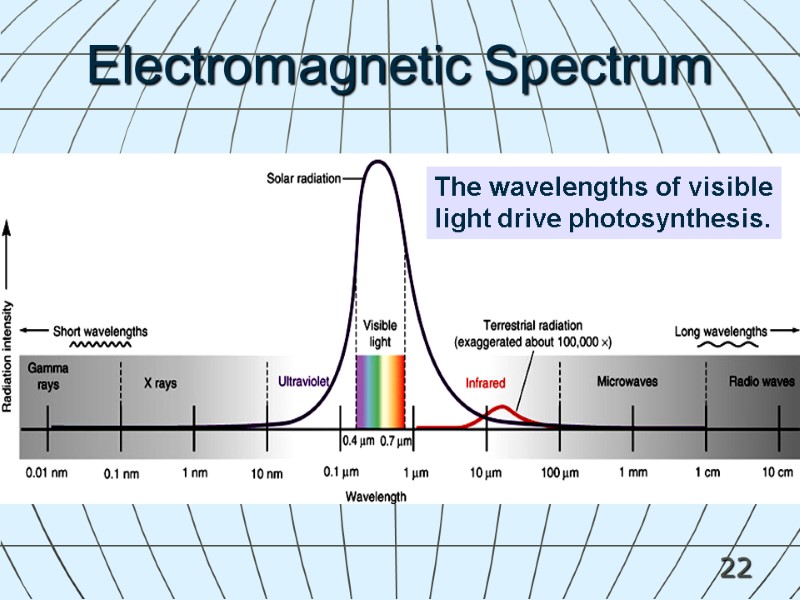
 22 The wavelengths of visible light drive photosynthesis. Electromagnetic Spectrum
22 The wavelengths of visible light drive photosynthesis. Electromagnetic Spectrum
 23 Properties of Energy Most energy used in ecosystems originates as solar energy. Green plants convert energy to chemical energy, which is then converted to heat or kinetic energy by the animal that eats the plant. Energy cannot be recycled. Energy is reused, but it is constantly degraded or lost from the system.
23 Properties of Energy Most energy used in ecosystems originates as solar energy. Green plants convert energy to chemical energy, which is then converted to heat or kinetic energy by the animal that eats the plant. Energy cannot be recycled. Energy is reused, but it is constantly degraded or lost from the system.
 24 Organism (species) Population Biological Community Ecosystem Biosphere See also slide #4 Energy & Matter in the Environment
24 Organism (species) Population Biological Community Ecosystem Biosphere See also slide #4 Energy & Matter in the Environment
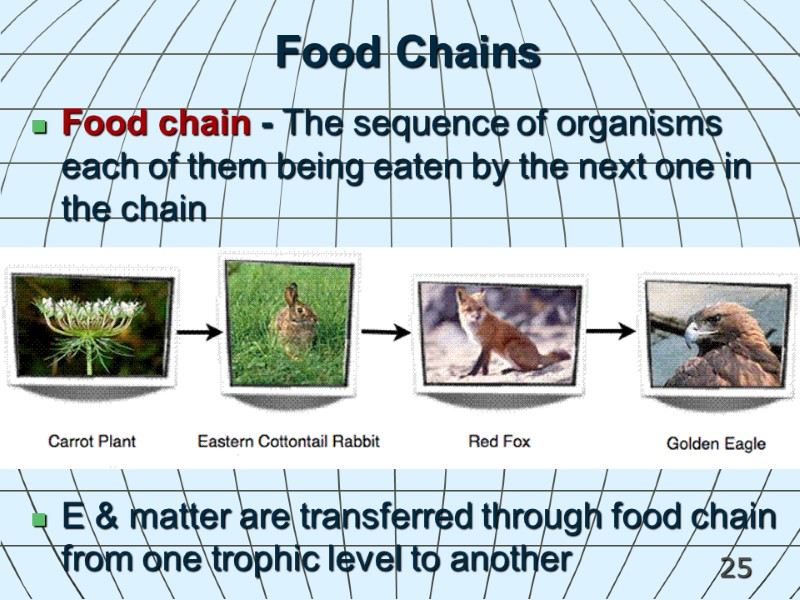
 25 Food Chains Food chain - The sequence of organisms each of them being eaten by the next one in the chain E & matter are transferred through food chain from one trophic level to another
25 Food Chains Food chain - The sequence of organisms each of them being eaten by the next one in the chain E & matter are transferred through food chain from one trophic level to another
 26 Cross-connected Food Chains Food web A complex, interlocking series of individual food chains in an ecosystem.
26 Cross-connected Food Chains Food web A complex, interlocking series of individual food chains in an ecosystem.
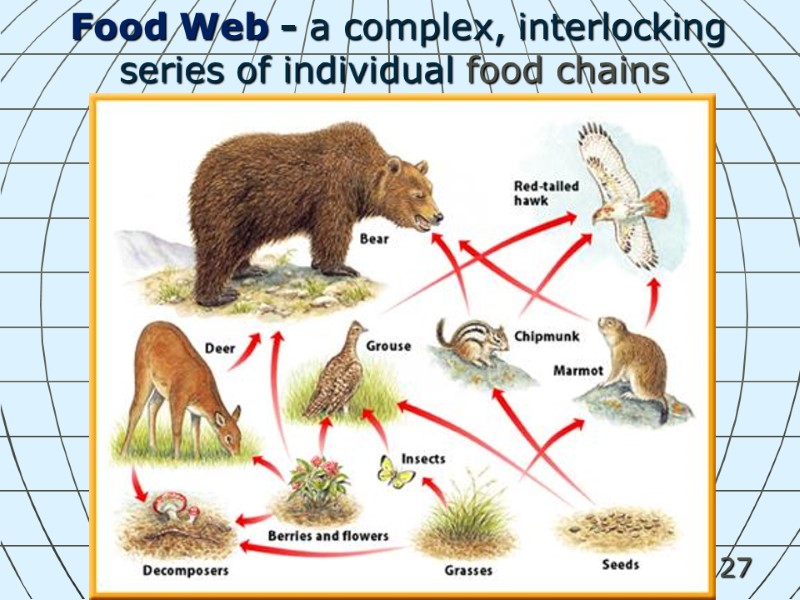
 27 Food Web - a complex, interlocking series of individual food chains
27 Food Web - a complex, interlocking series of individual food chains
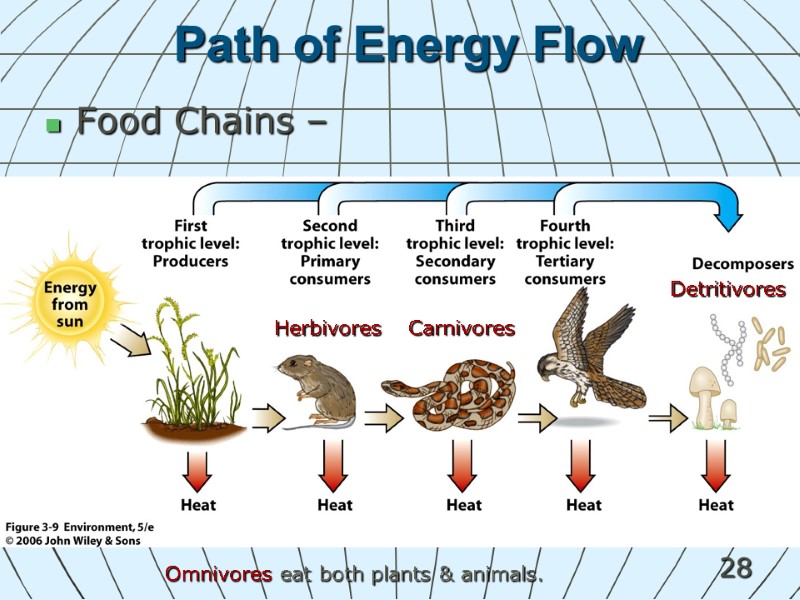
 28 Path of Energy Flow Food Chains – Herbivores Carnivores Omnivores eat both plants & animals. Detritivores
28 Path of Energy Flow Food Chains – Herbivores Carnivores Omnivores eat both plants & animals. Detritivores
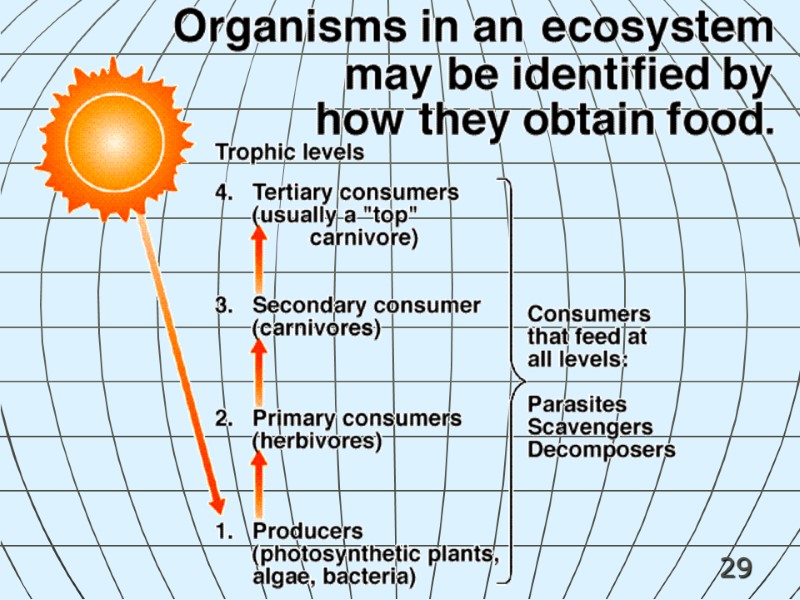
 29
29
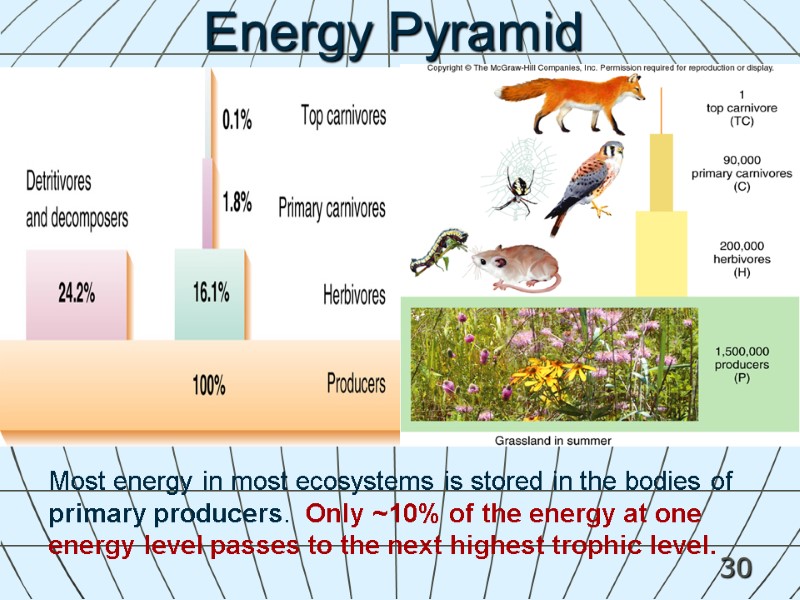
 30 Most energy in most ecosystems is stored in the bodies of primary producers. Only ~10% of the energy at one energy level passes to the next highest trophic level. Energy Pyramid ADD FIG. 2.18
30 Most energy in most ecosystems is stored in the bodies of primary producers. Only ~10% of the energy at one energy level passes to the next highest trophic level. Energy Pyramid ADD FIG. 2.18
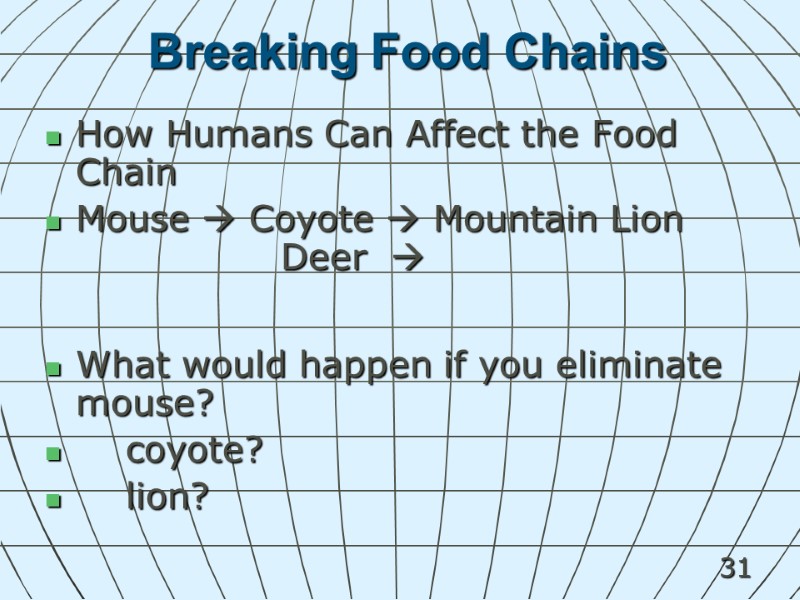
 31 Breaking Food Chains How Humans Can Affect the Food Chain Mouse Coyote Mountain Lion Deer What would happen if you eliminate mouse? coyote? lion?
31 Breaking Food Chains How Humans Can Affect the Food Chain Mouse Coyote Mountain Lion Deer What would happen if you eliminate mouse? coyote? lion?
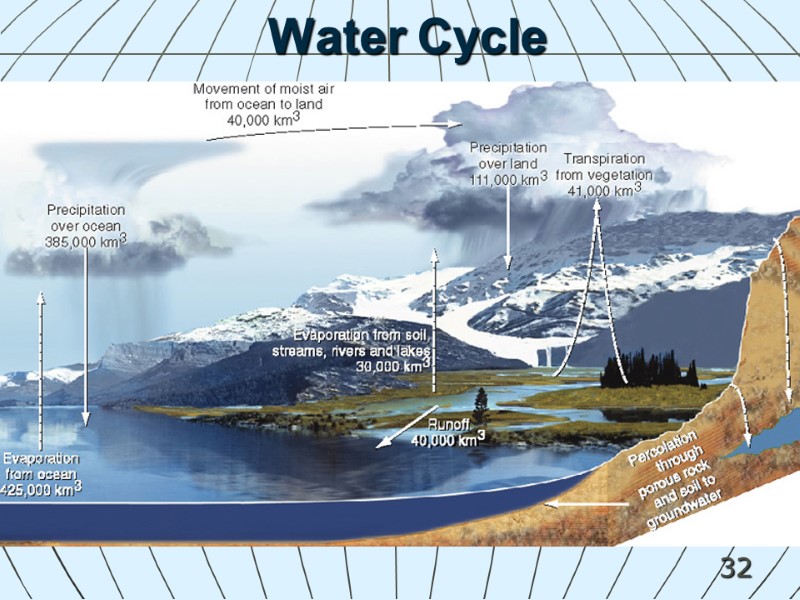
 32 Water Cycle
32 Water Cycle
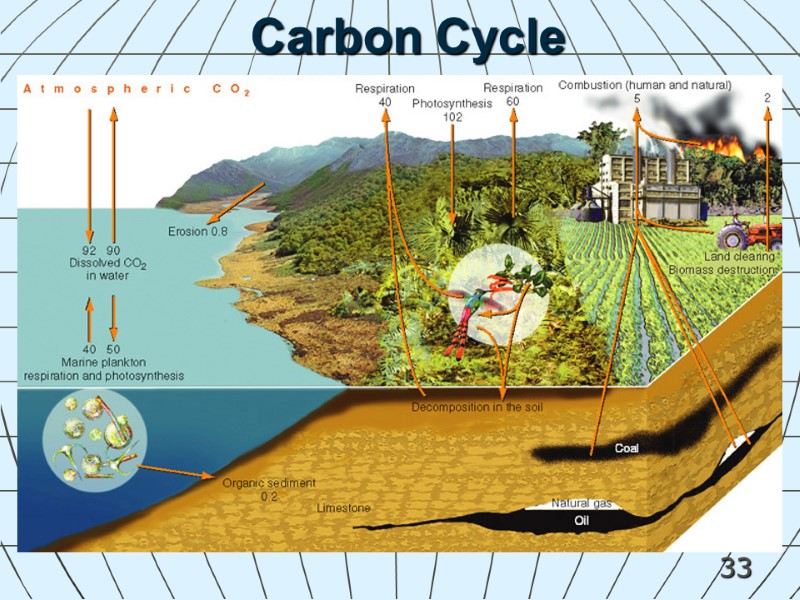
 33 Carbon Cycle
33 Carbon Cycle
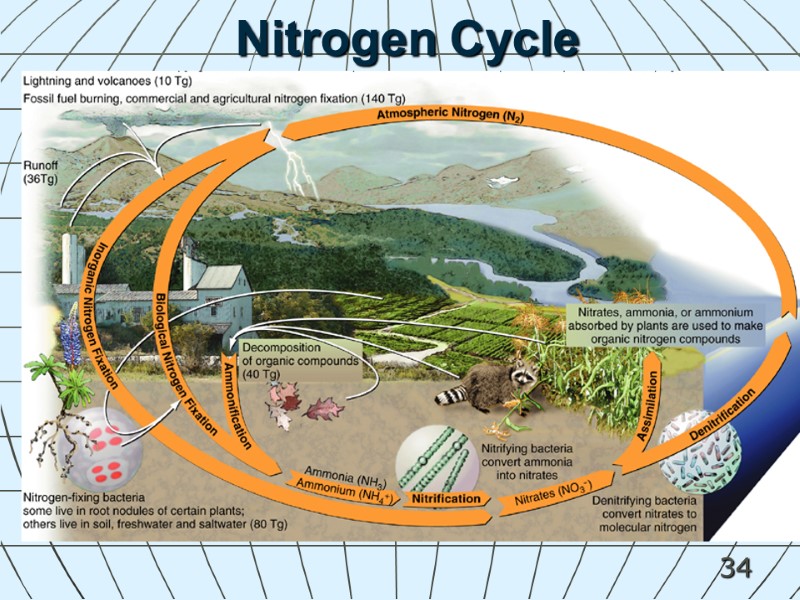
 34 Nitrogen Cycle
34 Nitrogen Cycle
 35 Summary Biological levels of the Environment Energy & Matter: basic principles. 3 states of matter: solid, liquid and gas Kinetic and Potential energy. 4 trophic leveles: producers, herbivores, carnivores, top carnivores. Omnivores, detritivores, decomposers. Only 10% of energy is being transferred from one trophic level to the next one.
35 Summary Biological levels of the Environment Energy & Matter: basic principles. 3 states of matter: solid, liquid and gas Kinetic and Potential energy. 4 trophic leveles: producers, herbivores, carnivores, top carnivores. Omnivores, detritivores, decomposers. Only 10% of energy is being transferred from one trophic level to the next one.

